ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2018) Volume 29, Issue 18
Effect of Ganoderma lucidum polysaccharides on oxidative damage in liver of STZ-diabetic rats
Hüseyin Avni Ero?lu1* and Ebru Beytut2
1Medical Faculty, Çanakkale Onsekiz Mart University, Terzio?lu Campus, Çanakkale, Turkey
2Medical Faculty, Erzincan University, Erzincan, Turkey
- *Corresponding Author:
- Hüseyin Avni Ero?lu
Medical Faculty
Çanakkale Onsekiz Mart University
Terzio?lu Campus, Çanakkale, Turkey
Accepted on August 07, 2018
DOI: 10.4066/biomedicalresearch.29-18-831
Visit for more related articles at Biomedical ResearchIn this study, the effects of exogenic application of Ganoderma lucidum polysaccharide on oxidative stress and hyperlipidemia was determined in diabetics rats. The study included 60 Wistar albino rats aged 4-5 months. Rats were divided into 6 equal groups. The groups were control, Ganoderma lucidum polysaccharide, diabetic, diabetic+60 mg/kg polysaccharides, diabetic+120 mg/kg polysaccharides and diabetic+10 mg/kg polysaccharide. Diabetes was induced by intraperitoneal injection of streptozocine at the dose rate of 50 mg/kg. The rats were fed ad-libitum throughout the experiment. At the end of the study, we took some samples out of total oxidant and total antioxidant levels and measured them with the spectrophotometer; thereafter we used the auto-analyser to measure the levels of total cholesterol, triglycerides, HDL, LDL, VLDL cholesterol and pancreatic amylase. We examined the blood of diabetic fasting rats and the effect was that their glucose level was increased significantly. (p<0.05), nonetheless the dose of 60, 120 and 180 mg/kg of Ganoderma lucidum polysaccharides did not reduce the blood glucose level (p>0.05). With the diabetic rats, the dose of 60, 120 and 180 mg/kg of Ganoderma lucidum polysaccharides rides effected in the increase of serum levels of total cholesterol, triglycerides, VLDL, and HDL cholesterol levels, although it was not a significant change (p>0.05), instead, we observed a statistically significant decrease of LDL cholesterol level (p<0.05). According to this study we concluded that polysaccharides effects in the decrease of hyperlipidaemia with regard to diabetes. During the study we applied Ganoderma lucidum polysaccharides exogenously (60, 120, 180 mg/kg) and it did not affect the level of total antioxidant in diabetes significantly (p>0.05), however with a dose of 180 mg/kg the total oxidant level in pancreas and liver tissues was reduced significantly and has been considered as crucial fact. The collected data supported our view on histopathologic examination. Based on all the information, we used one type of fungus Ganoderma lucidum polysaccharides which did not influence the blood glucose level in diabetes, whereas with regard to LDL and total oxidants it played a significant role in reducing its levels.
Keywords
Type I diabetes, Ganoderma lucidum, Polysaccharide, Oxidative stress, Lipid profile.
Introduction
Diabetes mellitus (DM) is a chronic hyperglycemia resulting from insulin secretion or whole or partial insufficiency of secreted insulin action and is an endocrine and metabolic disease characterized by various complications arising from disorders in carbohydrate, lipid and protein metabolism. Diabetes mellitus is characterized by classical symptoms such as polydipsia, polyuria, and polyphagia and weight loss. Complications such as angiopathy, cardiomyopathy, neuropathy, nephropathy, ketoacidosis, hyperglycemic hyperosmolar non-ketotic coma and atherosclerosis can develop in the later stages of the disease. Every year thousands of people in the world lose their lives due to these complications [1].
For diabetic treatment in recent times, due to side effects of therapeutic agents (oral hypoglycemic medications) there is a serious trend toward herbal medications. The majority of traditional medical plant extracts can be used for diabetes treatment. One of these plants is Ganoderma lucidum which has been used as medical drug for centuries in China, Korea and Japan. Known as the “immortality mushroom”, this mushroom species is called "Reishi" in Japanese and "Ling Zhi" in Chinese. The results of experimental studies have stated that in animals with induced diabetes mellitus, Ganoderma lucidum and components reduce blood glucose levels by a significant amount [2-5].
This effect of Ganoderma lucidum is considered to be occur by stimulating release of insulin by preventing destruction of β cells in the pancreas; preventing elevation of blood glucose levels by reducing or delaying absorption of glucose by the small intestine; and indirectly suppressing production of pyruvate carboxylase, the enzyme responsible for ATP concentration and gluconeogenesis in hepatocytes, and phosphoenolpyruvate carboxykinase (PEPCK) at molecular level causing reduction of glucose production [2,5]. When oxidative injury is assessed, the amino polysaccharide fraction (G009) iron in Ganoderma lucidum inhibits induced lipid peroxidation by making hydroxyl radicals and superoxide anions inactive and additionally is reported to have chemopreventive effect by reducing oxidative DNA damage [6]. A study of mice focused on the positive effect of Ganoderma lucidum polysaccharides on lipid profile [2].
Streptozotocin is an N-nitroso derivative of D-glucosamine used in experimental animal studies to generate human-like diabetes in rats. It is thought that oxidizing substances cause diabetes by selectively destroying the Langerhans cells [7]. Streptozotocin is a metabolite of a microorganism named Streptomyces achromogenes found in the soil and was first isolated in 1960. It was first identified with antibiotic, antitumoral and carcinogenic properties and was described as a diabetogenic agent in dogs, cats and rats in 1963. The active substance in streptozotocin contains a methylnitrozole side chain attached to the C-2 position of 2-deoxy-D-glucose [8].
This study was designed to determine the effect of Ganoderma lucidum on the oxidative stress occurring in STZ-induced diabetes in rat’s by evaluation of biochemical parameters.
Materials and Methods
The study was approved by the Local Ethics Committee of Kafkas University for Animal Experimentation (KAÜ- HADYEK, code: 2011-40). A total of 60 male Wistar Albino rats aged 4-5-months-old, weighing 275-345 g, were allocated into 6 equal groups with 10 in each group. Animals were fed ad-libitum and housed at 25 ± 2°C temperature, with 60%-65% humidity and 12 h light and 12 h dark conditions and kept in cages cleaned daily (5 animals per cage). Feed was given in special steel vessels and water was supplied as normal tap water in stainless steel walled bottles. Applications were initiated after 1 week of adaptation and all applications were carried out between 17:00 and 18:00 every day. The groups were as follows;
Control group (n=10)
Animals were fed ad-libitum with normal diet and given saline by oral gavage during the study in order to establish the same standard as other groups.
Ganoderma lucidum control group (n=10)
Animals were orally fed 180 mg/kg Ganoderma lucidum only. This dose was chosen to obtain the most effective antioxidant activity.
Diabetic control group (n=10)
50 mg/kg Streptozotocin (STZ) dissolved in 50 ml citric acid plus 40 ml disodium hydrogen phosphate buffer solution (pH: 4.5) was administered intra-peritoneally (i.p.). Additionally, animals were given saline with oral gavage during the study period. Fasting blood glucose levels were determined after fasting animals for 8 h before STZ injection and 3 d after STZ injection. Animals with fasting blood glucose values measured as 250 mg/dL were accepted as type I diabetic.
Diabetes plus 60 mg/kg Ganoderma lucidum polysaccharide group (n=10)
Diabetic rats were orally given 60 mg/kg Ganoderma lucidum polysaccharide dissolved in 15% DMSO for 21 d.
Diabetes plus 120 mg/kg Ganoderma lucidum polysaccharide group (n=10)
Diabetic rats orally received 120 mg/kg Ganoderma lucidum polysaccharide dissolved in 15% DMSO for 21 d.
Diabetes plus 180 mg/kg Ganoderma lucidum polysaccharide group (n=10)
Diabetic rats orally received 180 mg/kg Ganoderma lucidum polysaccharide dissolved in 15% DMSO for 21 d.
Preparation of Ganoderma lucidum extract
Dried mushrooms were obtained from Agroma Mushroom Breeding (Denizli/Turkey) where Ganoderma lucidum is permitted to be dried since 29.12.2011 with the registration number TR-20-K-0000062 according to Food and Feed Law No. 5996 by the Ministry of Food, Agriculture and Livestock, Lt;/RTI and gt. Dried Ganoderma lucidum mushrooms (6 kg) were ground and milled in 120 L hot water for 14 h at 60°C in a shaking water bath, then the mixture was filtered through filter paper and the remaining t volume, determined as 60 L, was treated with 90 L of ethanol in order to obtain polysaccharides. This mixture was kept at +4°C for 4 h. It was then centrifuged at 3000 rpm for 5 min to allow the precipitation of high molecular weight components. Then, the supernatant was removed and ethanol was added at a ratio of 1/3 (v/v, solution/ethanol) and stored at +4°C for 4 h. The liquid was removed by centrifugation at 3000 rpm for 5 min. The remaining solid was washed with ethanol, acetone and diethyl ether, respectively, and chemicals used for washing were removed by evaporation. Ganoderma lucidum polysaccharides in the form of brownish powder were obtained [9]. In order to determine the presence of polysaccharide in the obtained extract, the Molisch assay and the hydrolysis test for polysaccharides were carried out as described [10]. These tests were positive.
Obtaining and preparing blood samples
At the end of the experimental stages, animals were placed under anesthesia with 0.4 ml/kg pentobarbital sodium and intra-cardiac blood samples were obtained. To obtain serum from a portion of the blood samples, they were placed in gel tubes; the remaining portion was placed in heparin tubes to remove plasma. Blood samples in gel tubes were immediately placed in a cooled centrifuge at +4°C with 3000 rpm for 10 min to obtain serum samples. Blood samples in heparin tubes were centrifuged at +4°C with 3000 rpm for 10 min to separate plasma. The prepared serum and plasma samples were stored in a freezer at -20°C until analysis.
Obtaining and preparing tissue samples
After taking blood samples, liver and pancreas tissue were removed. All of the pancreas tissue and a portion of the liver tissue were fixated in 10% buffered formalin solution for histopathology investigation. The remaining tissue samples were washed in lactate ringer solution and wrapped in polyethylene bags and labelled before being stored in a freezer at -20°C until analysis.
Immediately before analyses, in accordance with the procedure for the kits used, an amount of liver tissue sample was weighed and 9 times this weight of phosphate buffer solution was added (1/10 ratio) and the sample was homogenized with a homogenator. Later the homogenized tissue samples had homogenates separated with the aid of a centrifuge and stored in polyethylene tubes.
Biochemical analysis
Blood glucose levels were determined with a Yasee glucometer (GLM-76, Taiwan). Serum lipids (total cholesterol, HDLcholesterol, triglyceride) and pancreatic amylase levels were measured using commercial kits (Abbott diagnostic). LDL and HDL levels were calculated using the Friedewald formula. Total oxidant and antioxidant capacities were determined using a commercial kit (Rel Assay, Gaziantep).
Histopathological examinations
Tissue samples of liver and pancreas were fixed and the paraffin blocks were prepared by routine tissue monitoring. For histopathologic examinations, sections taken from paraffin blocks were examined microscopically by staining with hematoxylin and eosin. Slides were evaluated for degenerative and necrotic changes.
Statistical analysis
Data were analyzed with the one-way analysis of variance (ANOVA) and Tukey test using the SPSS 18 package program. Correlation analysis was also performed to determine the level of association between HDL-LDL, TOS-TAS and TOS-fasting blood glucose levels. Values of p<0.05 were considered statistically significant.
Results
Blood glucose levels increased gradually in the diabetic control group (Table 1); but this was only significant on the 3-7th, 3-10th, 3-15th, 3-20th (p<0.001) and 7-20th d (p˂0.05). In the group receiving 60 mg of Ganoderma lucidum, 3-7 d with 10-15 (p<0.05) compared to days 3 and 7 (p<0.001) on day 10 and day 10 and day 15 on day 20, although the blood glucose levels of the days were similar among them. Although the increase in fasting glucose levels of 120 mg Ganoderma lucidum compared to day 3 and day 10 and day 15 of the 10th day was not significant (p>0.05), this increase was not significant (p>0.05) 3 (p˂0.001) and 7 (p˂0.01) d. Blood glucose levels at days 3, 7 and 10 and days 7, 10 and 15 of 180 mg Ganoderma lucidum group were similar to each other. The increase in day 3 was statistically significant compared to day 15 and day 7 increase 20 d (p˂0.05).
| Glucose (mg/dl) | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| 0 d | 86.57 ± 2.23 | 88.50 ± 6.89 | 88.70 ± 5.76 | 83.60 ± 5.02 | 82.60 ± 5.30 | 84.50 ± 6.69 |
| 3 d | 90.00 ± 1.63b | 97.00 ± 3.52b | 314.40 ± 35.98a | 321.50 ± 33.81a | 320.60 ± 41.88a | 336.70 ± 42.99a |
| 7 d | 101.33 ± 3.14b | 99.17 ± 5.49b | 354.40 ± 27.40a | 344.00 ± 25.76a | 335.67 ± 20.41a | 357.11 ± 44.30a |
| 10 d | 104.57 ± 4.28b | 100.00 ± 5.55b | 375.60 ± 42.01a | 407.33 ± 36.27a | 373.00 ± 37.06a | 368.00 ± 67.21a |
| 15 d | 103.14 ± 6.77b | 85.17 ± 13.67b | 388.89 ± 32.31a | 408.00 ± 25.49a | 377.11 ± 52.17a | 409.78 ± 19.16a |
| 20 d | 106.00 ± 6.27c | 94.17 ± 12.16c | 400.78 ± 18.89b | 460.25 ± 29.68a* | 401.75 ± 50.31b* | 421.22 ± 23.11ab |
Different letters on the same line show statistical significance; 0 d: p>0.05; 3 d: a-b: p<0.001; 7 d: a-b: p<0.001; 10 d: a-b: p<0.001; 15 d: a-b: p<0.001; 20 d: a-c, a-b, ab-c, a*-c, b*-c, a*-b: p<0.001, a*-b*: p<0.01.
Table 1. Changes in fasting blood glucose levels of control and trial groups.
Concentrations of total cholesterol, HDL, LDL, VLDL, TG and amylase activity differed among the groups (Table 2). Serum total cholesterol concentrations were significantly higher in group III and treatment groups (IV, V, VI) when compared to control groups (I, II) (p<0.05). Similarly, serum TG levels were significantly higher in group III and treatment groups (IV, V, VI) when compared to control groups (I, II) but treatment with Ganoderma reduced the TG concentration. As the dose of Ganoderma increased the concentration of TG proportionally decreased (p<0.05). Ganoderma treatment did not significantly decrease the concentrations of HDL and VLDL in diabetic animals as the concentrations of HDL and VLDL in groups III-VI were markedly higher than those of control groups (I, II) (p<0.05). Serum LDL levels in group III were significantly higher than those of control groups (I, II) and Ganoderma treated groups (IV, V, VI) (p<0.05). Pancreatic amylase activity greatly declined in the diabetic group (I) (p<0.05) and Ganoderma treatment did not alter this declined activity when compared to control groups (I, II) as the activity remained lower despite treatment.
| I | II | III | IV | V | VI | |
|---|---|---|---|---|---|---|
| TG | 83.60 ± 16.09c | 93.00 ± 17.96bc | 133.40 ± 26.48ab | 120.86 ± 25.56abc | 135.40 ± 17.81a | 114.89 ± 26.96abc |
| Chol. | 57.40 ± 3.36c | 65.50 ± 9.20bc | 77.78 ± 11.84ab | 77.00 ± 4.78ab | 77.20 ± 8.79ab | 82.11 ± 8.42a |
| HDL | 23.60 ± 0.55a | 23.50 ± 3.73a | 37.75 ± 5.26b | 35.50 ± 3.12b | 36.80 ± 1.92b | 39.44 ± 4.56b |
| LDL | 17.08 ± 5.01b | 21.60 ± 10.50b | 35.80 ± 13.33a | 17.83 ± 6.60b | 13.76 ± 3.19b* | 18.33 ± 5.46b |
| VLDL | 16.72 ± 3.22b | 18.60 ± 3.59ab | 26.68 ± 5.30a | 24.17 ± 5.11ab | 25.24 ± 4.69ab | 22.98 ± 5.39ab |
| Amilase | 2846.00 ± 102.40a | 2670.40 ± 381.60a | 1667.00 ± 362.27b | 1597.38 ± 210.87b | 1887.80 ± 57.07b | 1742.60 ± 277.25b |
Different letters on the same line show statistical significance. Total cholesterol: a-c: p<0.001; ab-c, a-bc: p<0.01; Triglyceride: a-c: p<0.001; a-bc: p<0.01; ab-c: p<0.05; HDL: a-b: p<0.001; VLDL: a-b: p<0.05; LDL: a-b*: p<0.001; a-b: p<0.01; Amylase: a-b: p<0001.
Table 2. Concentration of serum total cholesterol, HDL, LDL, VLDL, TG and activity of pancreatic amylase.
While there was a statistically insignificant reduction in serum total oxidant levels in the diabetic control group (p>0.05), there was no significant changes determined in the 60, 120 and 180 mg/kg Ganoderma lucidum groups compared to the diabetic control group (p>0.05). There was no significant difference identified between the control, Ganoderma lucidum control and diabetic control groups along with the 60, 120 and 180 mg/kg Ganoderma lucidum groups in terms of erythrocyte total oxidant levels (p>0.05). In the Ganoderma lucidum control group the total oxidant levels in liver tissue showed a statistically unimportant reduction compared to the control group (p>0.05), while the diabetic control group had a nonsignificant increase in liver total oxidant levels compared to the control group (p>0.05). Additionally, the high total oxidant levels in the diabetic control group reduced in the 60, 120 and 180 mg/kg Ganoderma lucidum groups; however, this reduction was only statistically significant for the 180 mg/kg group (p<0.05). In the Ganoderma lucidum control group, total oxidant levels in pancreas tissue showed an insignificant increase compared to the control group; however, this increase was significant in the diabetic control group and 60 mg/kg and 180 mg/kg Ganoderma lucidum groups at p<0.001 level. Additionally, in the 120 mg/kg group, this increase was identified to be significant at p<0.05 level. The total oxidant levels of serum, erythrocyte, liver and pancreas samples in the groups are given in Table 3.
| TOS | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| Serum | 10.92 ± 3.20a | 6.96 ± 1.98ab | 7.96 ± 3.65ab | 7.43 ± 1.04ab | 8.16 ± 2.53ab | 5.21 ± 0.46b |
| Erythrocyte | 122.03 ± 8.10 | 121.35 ± 6.42 | 119.81 ± 6.64 | 112.31 ± 9.65 | 115.71 ± 9.56 | 116.68 ± 11.28 |
| Liver | 31.72 ± 7.41ab | 26.20 ± 8.69b | 43.07 ± 3.74a | 31.94 ± 5.31ab | 36.81 ± 7.19ab | 30.51 ± 7.99b* |
| Pancreas | 9.11 ± 1.53b | 12.48 ± 1.72ab | 16.26 ± 1.32a | 10.49 ± 1.54b | 12.10 ± 1.52b* | 11.46 ± 3.64b |
Different letters on the same line show statistical significance; Serum: a-b: p<0.01; Erythrocyte: p>0.05; Liver: a-b: p<0.01, a-b*: p<0.05; Pancreas: a-b: p<0.001, a-b*: p<0.05.
Table 3. Total oxidant levels of serum, erythrocyte, liver and pancreas samples (μmol H2O2 Equiv/L). TOS
The total antioxidant levels in serum samples were determined to be significantly high in the Ganoderma lucidum control group compared to the control group (p<0.001). The total antioxidant levels in the diabetes control group were identified to increase compared to the control group at p<0.001 level. In the groups administered 120 mg/kg and 180 mg/kg Ganoderma lucidum the total antioxidant levels were not different to the diabetic control group (p>0.05); however, in the group administered 60 mg/kg dose of Ganoderma lucidum there was a significant reduction determined (p<0.05). The liver tissue of the Ganoderma lucidum control group had a significant decrease in total antioxidant levels compared to the control group (p>0.01); however, it was observed the reduction in total antioxidant levels in the diabetic control group was not significant (p>0.05). There were no significant differences identified in erythrocyte total antioxidant levels between the control, Ganoderma lucidum control and diabetic control with the 60, 120 and 180 mg/kg Ganoderma lucidum groups (p>0.05). The total antioxidant levels in liver tissue in the Ganoderma lucidum control group showed a significant reduction compared to the control group (p<0.01); however, the reducing in total antioxidant levels in the diabetic control group was not significant (p>0.05). Compared with the diabetic control group, the other groups were not identified to have a significant variation in total antioxidant levels (p>0.05). In pancreas tissue, there were no statistical differences determined for total antioxidant levels between the groups (p>0.05). The total antioxidant levels in serum, erythrocyte, liver and pancreas samples from the groups are given in Table 4.
| TAS | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| Serum | 0.83 ± 0.05b | 0.99 ± 0.05a | 0.97 ± 0.09a* | 0.86 ± 0.03b* | 1.00 ± 0.04a | 0.99 ± 0.04a |
| Erythrocyte | 0.30 ± 0.06 | 0.39 ± 0.06 | 0.31 ± 0.03 | 0.30 ± 0.03 | 0.36 ± 0.09 | 0.39 ± 0.06 |
| Liver | 0.81 ± 0.08a | 0.68 ± 0.04b | 0.77 ± 0.04ab | 0.82 ± 0.08a | 0.70 ± 0.05b | 0.74 ± 0.05ab |
| Pancreas | 0.58 ± 0.31 | 0.74 ± 0.12 | 0.71 ± 0.13 | 0.75 ± 0.08 | 0.66 ± 0.28 | 0.55 ± 0.09 |
Different letters on the same line show statistical significance; Serum: a-b, a-b*, a*-b: p<0.001; a-b*: p<0.01; a*-b*: p<0.05; Erythrocyte: p>0.05; Liver: a-b: p<0.01; Pancreas: p>0.05.
Table 4. Total antioxidant levels of serum, erythrocyte, liver and pancreas samples (mmol Trolox Equiv/L).
Correlation analyses identified a significant positive correlation between Ganoderma lucidum control group serum TOS and TAS values at p<0.01 level. Additionally, in the 60 mg/kg Ganoderma lucidum group, there was a positive correlation in liver tissue at p<0.01 level. However, there was no statistical correlation identified between HDL and LDL cholesterol levels and TOS and fasting blood glucose levels in all groups (p>0.05).
Histopathology
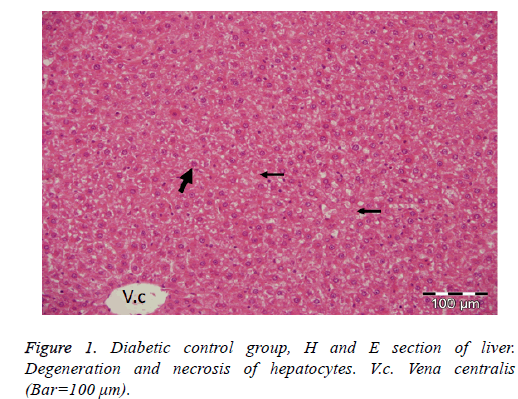
In the diabetic control group; degenerative and necrotic changes were observed in general. Acute cell swelling and hydropic degeneration in hepatocytes and vacuolar degeneration of some hepatocytes were detected. The cytoplasm of degenerative cells was observed to be lightly colored and granular. Necrotic changes were observed in single or small areas, especially in hepatocytes in the acinar and midzonal regions. Dilatation of the synuclein in the liver and slight activation increase in the star cells of Kuppfer were also detected (Figure 1).
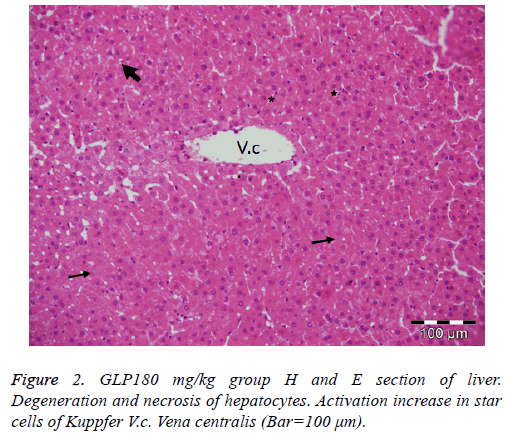
Administration of 60 mg of Ganoderma lucidum to the diabetic group did not alter the severity of degenerative and necrotic changes when compared to the diabetic control group. In the groups given 120 mg and 180 mg of Ganoderma lucidum, acute cell swelling, hydropic degeneration and necrotic changes were determined in the liver cells, but the severity of the lesions was less than in the diabetic control group (Figure 2).
Discussion
Diabetes mellitus is a chronic metabolic disease requiring continuous medical care in which the organism does not benefit from carbohydrates, fats and proteins due to insulin deficiency [11]. According to the World Health Organization (WHO), the number of people with diabetes is estimated to double by 2030 from 171 million in 2000 [12]. The persistent hyperglycemia observed also increases the formation of free radicals, primarily reactive oxygen species (ROS), by glucose auto-oxidation and protein glycosylation in all tissues. The increase in ROS can be due to the increase in reactive oxygen species, as well as the decrease in the antioxidants CAT, GSH and SOD [13]. Increased polyol pathway activity due to persistent hyperglycemia increases the occurrence of oxidative damage in tissues causing damage to tissue antioxidant defense systems [14].
A number of experimental studies have been carried out on diabetes using extracts from both fruit stem of Ganoderma lucidum as well as different components [2,15,16]. As a result of these studies, Ganoderma lucidum and its components decreased the number of oxidants and blood glucose level by increasing total anti-oxidant level. It was stated that the antioxidant properties of Ganoderma lucidum polysaccharides protect pancreatic beta cells by preventing oxidative damage. Thus, stimulation of insulin secretion from beta cells of the pancreas is also maintained. It has also been reported that Ganoderma lucidum polysaccharides slow the absorption of glucose from the small intestines, keeping the blood glucose level at a certain level and indirectly controlling insulin secretion [5].
Many studies have shown that blood glucose levels increase in experimental diabetic rats [15,17,18]. Mohammed et al. administered extracts of Ganoderma lucidum diluted in water as a single dose of 250, 500, 1000 mg/kg intraperitoneally to Wistar albino rats with diabetes induced by alloxan [3]. They reported a statistically significant decrease in the blood glucose levels of the groups administered doses of 500 and 1000 mg/kg [3], whereas a change in the blood glucose levels of rats fed the 250 mg/kg dose was not detectable compared to the diabetes control group [18]. Li et al. reported that Ganoderma lucidum polysaccharides administered with streptozotocin-induced diets in basal diets at doses of 50 and 150 mg/kg for 28 d reduced blood glucose levels relative to the diabetes control group, but that this reduction was statistically significant only in the 150 mg/kg group [2]. Similarly, Seto et al. also investigated genetically diabetic rats administered 3, 30 and 300 mg/kg doses of water-extracted Ganoderma lucidum for 4 weeks with oral gavage in type 2 diabetic mice [4]. They reported a reduction in the blood glucose levels of the group given Ganoderma lucidum only at a dose of 300 mg/kg [4]. Jia et al. reported that Ganoderma lucidum polysaccharides were given orally at doses of 60, 120, and 180 mg/kg to Wistar albino rats with experimental diabetes mellitus induced by streptozotocin administration [15]. All of the administered doses reduced the blood glucose levels statistically compared to the diabetic control group [15]. However, in our study, it was observed that different doses of Ganoderma lucidum (60, 120 and 180 mg/kg) elevated fasting blood glucose levels compared to the diabetic and non-diabetic control groups. These findings are in accordance with those of Jia et al. [15], Li et al. [2] and Seto et al. [4]. The likely reason as to why our results differ from other studies may be due to the broad spectrum of biological effects of G. lucidum and its composition including alkaloids, amino acids, peptides, inorganic elements, steroids, fatty acids, polysaccharides, triterpenes and organic acids [19].
In diabetes, contrary to the increase in triglyceride amounts, HDL cholesterol levels fall causing an increase in LDL cholesterol [2]. Studies have found contradictory results indicating total cholesterol levels increase in diabetes [20], both total cholesterol and triglyceride levels increase [21] and there is a reduction in triglyceride levels with no change in total cholesterol levels [22]. Ramakrishna et al. noted that in type 1 diabetes total cholesterol, triglyceride, LDL and VLDL cholesterol levels clearly increased, while HDL cholesterol levels reduced [23]. Meng et al. studied Sprague Dawley rats with diabetes induced using streptozotocin and stated that total cholesterol and triglyceride levels significantly increased [24]. They stated that there was no change in the lipid profile with administration of Ganoderma lucidum at doses of 200, 400 and 800 mg/kg [24]. Li et al. induced diabetes with streptozotocin in mice and sated that increased LDL, total cholesterol and triglyceride levels reduced with 50 and 150 mg/kg dose of Ganoderma lucidum [2]. Additionally, HDL cholesterol levels significantly increased [2]. In our study, there were significant increases in total cholesterol, triglyceride, and VLDL, LDL and HDL levels in the diabetic control group. In this way, our findings (apart from HDL cholesterol) comply with results from Ramakrishna et al. [23]. The reason for the increase in serum triglyceride and total cholesterol levels in the diabetic control group may be the activation of the hormone-susceptible lipase enzyme by insufficiency or deficiency of insulin along with increasing mobilization of free fatty acids in peripheral stores. However, in groups administered Ganoderma lucidum polysaccharides, total cholesterol, triglycerides, VLDL and HDL cholesterol levels were no significantly different compared to the diabetic control group, while LDL cholesterol levels were statistically reduced compared to the diabetic control group. The fall in LDL cholesterol levels may be due to Ganoderma lucidum slowing the synthesis of cholesterol in the liver.
In our study there was a statistically significant positive correlation determined between the total oxidant levels and total antioxidant levels in serum samples in the control group and in liver samples in the group administered 60 mg/kg dose of Ganoderma lucidum. Tabur et al. stated there was a positive correlation between total oxidant levels and antioxidant levels in individuals with non-diabetic metabolic syndrome and obese people [25]. In our study, there was no correlation determined between TOS and TAS levels in tissue samples from other groups. Additionally, there was no statistically significant correlation observed between HDL cholesterol and LDL cholesterol levels and between TOS and fasting blood glucose levels.
In trials involving diabetics experimentally induced with STZ, hydropic and vacuolar degeneration, sinusoidal dilatation, necrosis, increased activation in astrocytes, and hypertrophy in cells were found in the livers of animals [16,26,27]. In parallel with our studies, acute cell swelling, hydropic degeneration, vacuolar degeneration in some hepatocytes, and necrotic changes in hepatocytes in the acinar and mid-zonal regions were found in the diabetic control group of our study. In addition, dissociation in liver cells and slight activation increase in star cells of Kuppfer were detected (Figure 1). However, some studies have not detected severe fat degeneration in liver cells [27]. It was concluded that the probable cause may have been differences in the animal species used and the experimental set-up.
The findings of the present study confirm the previous results and additional suggest that Ganoderma lucidum polysaccharides would be quite effective in ameliorating the histopathological changes caused by diabetes in liver tissues.
Conflict of Interest
The authors declare that there is no conflict of interest.
Role of Funding Source
This study was funded by Kafkas University (Project number: 2012-VF-25).
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed Consent
This article does not contain any studies with human participants.
References
- Atalay M, Laaksonen DE. Diabetes, oxidative stress and physical exercise. J Sports Sci Med 2002; 1: 1-14.
- Li F, Zhang Y, Zhong Z. Anti-hyperglycemic effect of Ganoderma lucidum polysaccharides on streptozotosin-induced diabetic mice. Int J Mol Sci 2011; 12: 6135-6145.
- Mohammed A, Adelaiye AB, Abubakar MS, Abdurahman EM. Effects of aqueous extract of Ganoderma lucidum on blood glucose levels of normoglycemic and alloxan induced diabetic Wistar rats. J Med Plants Res 2007; 1: 034-037.
- Seto SW, Lam TY, Tam HL, Au AL, Chan SW, Wu JH, Yu PH, Leung GP, Ngai SM, Yeung JH. Novel hypoglycemic effects of Ganoderma lucidum water-extract in obese/diabetic (+db/+db) mice. Phytomedicine 2009; 16: 426-436.
- Zhang H, Lin Z. Hypoglycemic effect of Ganoderma lucidum polysaccharides. Acta Pharmacol Sin 2004; 25: 191-195.
- Lee JM, Kwon H, Jeong H. Inhibition of lipid peroxidation and oxidative damage by Ganoderma lucidum. Phytother Res 2001; 15: 245-249.
- Altan N, Dinçel AS, Koca C. Diabetes mellitus and oxidative stress. Turk J Biochem 2006; 31: 51-56.
- Bell RH Jr, Hye RJ. Animal models of diabetes mellitus: physiology and pathology. J Surg Res 1983; 35: 433-460.
- Pang X, Chen Z, Gao X, Liu W, Slavin M, Yao W, Yu LL. Potential of a novel polysaccharide preparation (GLPP) from Anhui-grown Ganoderma lucidum in tumor treatment and immuno stimulation. J Food Sci 2007; 72: 435-442.
- Mert N. Veteriner Klinik Biyokimya. Uludağ Üniversitesi Güçlendirme Vakfı Yayın 1997; 12: 15-26.
- Dinççağ N, Diabetes mellitus. Tanı ve Tedavisinde Güncel Durum, İç Hastalıkları Dergisi 2011; 18: 181-223.
- Wild SH, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 2569.
- Lipinski B. Pathophysiology of oxidative stress in diabetes mellitus. J Diabet Complications 2001; 15: 203-210.
- Turk Z. Glycations and complications of diabetes. Diabetol Croat 2001; 30: 49-54.
- Jia J, Zhang X, Hua Y, Wua Y, Wang Q, Lib N, Guo Q, Dong X. Evaluation of in vivo antioxidant activities of Ganoderma lucidum polysaccharides in STZ-diabetic rats. Food Chem 2009; 115: 32-36.
- Sahin K, Onderci M, Tuzcu M, Ustundag B, Cikim G, Ozercan IH, Sriramoju V, Juturu V, Komorowski JR. Effect of chromium on carbohydrate and lipid metabolism in a rat model of type 2 diabetes mellitus: the fat-fed, streptozotosin-treated rat. Metabolism 2007; 56: 1233-1240.
- Maritim AC, Sanders RA, Watkins JB, Effect of α-lipoic acid on biomarkers of oxidative stress in streptozotosin-induced diabetic rats. J Nutr Biochem 2003; 14: 288-294.
- Melhem MF, Craven PA, Deruberdis FR. Effects of dietary supplementation of a-lipoic acid on early glomerular injury in diabetes mellitus. J Am Soc Nephrol 2001; 12: 124-133.
- Cao Y, Wu S, Dai Y. Species clarification of the prize medicinal Ganoderma mushroom “Lingzhi”. Fungal Diversity 2012; 56: 49-62.
- Feillet-Coudray C, Rock E, Coudray C, Grzelkowska K, Azais-Braesco V, Dardevet D, Mazur A. Lipit peroxidation and antioxidant status in experimental diabetes. Clinica Chimica Acta 1999; 284: 31-43.
- Kashiba M, Oka J, Ichikawa R, Kasahara E, Inayama T, Kageyama A, Kageyama H, Osaka T, Umegaki K, Matsumoto A, Ishikawa T, Nishikimi M, Inoue M, Inoue S. Impaired ascorbic acid metabolism in streptozotosin-induced diabetic rats. Free Radic Biol Med 2002; 33: 1221-1230.
- Vessby J, Basu S, Mohsen R, Berne C, Vessby B. Oxidative stress and antioxidant status in type 1 diabetes mellitus. J Int Med 2002; 251: 69-76.
- Ramakrishna V, Jailkhani R. Evaluation of oxidative stress in insülin dependent diabetes mellitus (IDDM) patients. Diagnos Paathol 2007; 2: 22.
- Meng G, Zhu H, Yang S, Wu F, Zheng H, Chen E, Xu J. Attenuating effects of Ganoderma lucidum polysaccharides on myocardial collagen cross-linking relates to advanced glycation end product and antioxidant enzymes in high-fat-diet and streptozotosin-induced diabetic rats. Carbohydrate Polymers 2011; 84: 180-185.
- Tabur S, Torun AN, Sabuncu T, Turan MN, Celik H, Ocak AR, Aksoy N. Non-diabetic metabolic syndrome and obesity do not affect serum paraoxonase and arylesterase activities but do affect oxidative stress and inflammation. Euro J Endocrinol 2010; 162: 535-541.
- Guven A, Yavuz O, Cam M, Ercan F, Bukan N, Comunoglu C, Gokce F. Effects of melatonin on streptozotosin-induced diabetic liver injury in rats. Acta Histochem 2006; 108: 85-93.
- Güneş HV, Değirmenci Ş, Aydın M, Bozan B, Aral E, Tunalier Z, Üstüner C, Erçakır M, Başer KHC, Başaran A. The effects of rumex patientia L. and Urtica dioica L. on some blood and urine parameters, and liver and kidney histology in diabetic rats. Turk J Med Sci 1999; 29: 227-232.