ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2013) Volume 24, Issue 1
2-Methoxyestradiol-bis-sulphamate: A promising anticancer agent in an esophageal carcinoma (SNO) cell line.
Department of Physiology, University of Pretoria, Private Bag X 323, Arcadia, 0007, South Africa
- *Corresponding Author:
- A.M. Joubert
Department of Physiology
University of Pretoria, Private Bag X 323
Arcadia, 0007, South Africa
Tel: +27 12 319 2246
Fax: +27 12 321 1679
E-mail: annie.joubert@up.ac.za
Accepted date: November 03 2012
2-Methoxyestradiol-bis-sulphamate (2-MeOE2bisMATE) is a bis-sulphamoylated analogue of a biological estradiol metabolite, 2-methoxyestradiol (2ME2) with potential as an anticancer drug. The aim of this in vitro study was to evaluate the effects of 2-MeOE2bisMATE on alpha-tubulin structure, as well as its potential to induce apoptosis in an esophageal carcinoma (SNO) cell line using confocal microscopy, flow cytometry and spectrophotometry. 2-MeOE2bisMATE disrupted the microtubule network of SNO cells, arrested cells in metaphase and induced apoptosis. An increase in the number of cells present in sub-G1, mitochondrial membrane potential reduction and increased caspase 6 activity were observed. This in vitro study demonstrated new insights on the action mechanism of 2 MeOE2bisMATE in esophageal carcinoma (SNO) cells, since these activities have not been studied in esophageal carcinoma cells up to date. Future studies are warranted to further determine which gene and protein expression changes are induced by 2-MeOE2bisMATE in SNO cells.
Keywords
2-Methoxyestradiol-bis-sulphamate, Esophageal cancer, Microtubules, Apoptosis
Introduction
Esophageal cancer is one of the 10th most common cancers in the world [1,2]. In South Africa, squamous esophageal cancer is considered to be more prevalent amongst black males, with the highest incidence being observed in the Transkei region [3-5]. Squamous cell carcinoma of the esophagus presents a significant health problem since the development of the disease is asymptomatic, resulting in late diagnosis and subsequent poor prognosis [6,7]. Therefore, there is a need to develop better diagnostic and therapeutic approaches and thereby decrease the high mortality rate that is associated with this malignancy [6,7].
2-Methoxyestradiol (2ME2) has been studied in the past decades due to its ability to exert antiproliferative and anti-angiogenic properties both in vitro and in vivo [8-12]. 2ME2 is an endogenous metabolite of 17-β-estradiol and it is currently undergoing phase II human clinical trials under the registered name PANZEM® [8]. Although 2ME2 has been shown to be a promising anticancer agent, it has the reduced bioavailability, thus derivatives thereof are currently being synthesized and tested for anticancer activity [13]. 2-MeOE2bisMATE is derived from 2ME2 by the sulphamoylation of 2ME2 at position C3 and position C17. 2-MeOE2bisMATE exerts antiproliferative activity in vitro and in vivo on different cell types [14-19].
In a study conducted by Newman et al. (2008), a concentration of 500nM of 2-MeOE2bisMATE inhibited the proliferation of drug resistant cells (MCF-7DOX) after 72 hours of exposure. MCF-7DOX cells are known to be resistant to various metastatic breast cancer drugs such as taxol and doxorubicin [17]. Visagie et al. 2010 revealed that an exposure of breast cancer cells (MCF-7) to 0.4μM of 2- MeOE2bisMATE for a period of 48 hours resulted in cell numbers up to 47%, while the same dose-time relationship decreased cell numbers to 79% in the non-tumourigenic MCF-12A cell population. The tumorigenic MCF-7 cells were more susceptible to 2-MeOE2bisMATE treatment compared to the normal MCF-12A cells [16,18]. The exact mechanism(s) by which 2-MeOE2bisMATE induces cell death still remains to be determined and has been found to differ according to cell types. The purpose of this in vitro study was to investigate the mechanism of action of 2-MeOE2bisMATE in a South African esophageal cancer cell line (SNO) by exploring its influence on alphatubulin morphology, cell cycle progression, cytotoxicity, mitochondrial membrane potential as well as caspase activity. These activities have not been studied till date in esophageal carcinoma cells. Therefore we planned this study.
Material and Methods
Cell line
The SNO esophageal carcinoma cell line was purchased from Highveld Biological Ltd. (Pty) (Sandringham, South Africa). The cells are described as non-keratinizing squamous epithelial cells.
Compound and reagents
2-MeOE2bisMATE is not commercially available and the compound was synthesized by Prof. Vleggaar from the Department of Chemistry (University of Pretoria, Pretoria, South Africa). All required reagents of cell culture analytical grade were obtained from Sigma (St. Louis, United States of America) unless otherwise specified. BCA protein assay kit was obtained from Thermo Scientific (Johannesburg, South Africa). Mitocapture mitochondrial apoptosis detection kit, alpha-tubulin antibody, alexafluor 488, 4’,6-diamidino-2-phenylindole (DAPI), Caspase 6 & 8 colorimetric assay kits, Annexin V fluorescein isothiocyanate (FITC) kit and the lactate dehydrogenase cytotoxicity kit were purchased from BIOCOM biotech (Pty) Ltd. (Clubview, South Africa).
Cell culture
Cells were propagated in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heatinactivated fetal calf serum (FCS) and 100U/ml penicillin G, 100μg/ml streptomycin and 250μg/l fungizone. Cells were cultured in 25 cm2 tissue culture flasks at 37°C in a humidified atmosphere containing 5% CO2 and left overnight for attachment. A stock solution of 2- MeOE2bisMATE with a concentration of 2.0x10-3M was prepared in dimethyl sulphoxide (DMSO) and stored at - 20°C. 2-MeOE2bisMATE was added to the cells at a final concentration of 0.4μM for a period of 24 hours. A concentration of 0.4μM and an exposure period of 24 hours were chosen since it was observed in our previous study that it is in this range of 2-MeOE2bisMATE which significantly decreased cell growth of SNO cells [20]. Control samples included cells propagated in growth medium. The vehicle-treated control included cells treated with the same volume of DMSO used to expose cells with 2- MeOE2bisMATE. The DMSO content of the final dilutions never exceeded 0.05% (v/v). Positive controls for induction of apoptosis included cells which were exposed to Actinomycin D (0.1μg/ml).
Confocal microscopy- alpha (α)-tubulin assay
The α-tubulin detection assay was utilised to determine the effects of 2-MeOE2bisMATE on tubulin structure of SNO cells. Exponentially growing SNO cells were seeded at 250 000 cells per well in 6-well plates on coverslips. After 24 hours of attachment, cells were exposed to 2- MeOE2bisMATE and to appropriate controls for a period of 24 hours. The experiment was terminated by fixing the cells in 10% formalin (containing 2mM EGTA in phosphate buffered saline (PBS)) for 10 minutes and then permeabilizing them in ice-cold 97% methanol (containing 2mM EGTA), which was dissolved by adding a few drops of 1mM sodium hydroxide at -20°C for 10 minutes. After 10 minutes of incubation, the coverslips were washed in PBS (5 minutes) between successive 1 hour incubations with each of the following: mouse monoclonal antibody against human α-tubulin (Clone 2-28-33; 1:1000), biotinconjugated anti-mouse IgG (Fab-specific, developed in goat) in FITC-conjugate diluent as secondary antibody (1:15) and ExtrAvidin-FITC conjugates (1:200 in FITCconjugate diluent). Following three 5 minute washes with PBS, the coverslips were stained with 4',6-diamidino-2- phenylindole (a nucleic stain) that produces blue fluorescence and were mounted with a glycerol-based mounting fluid and cells were then examined with a ZEISS, LSM 510 Meta confocal microscope at the Electron Microscopy unit at the University of Pretoria (Pretoria, South Africa).
Cell cycle progression
Flow cytometry was used to identify the distribution of cells during various phases of the cell cycle after exposure to 2-MeOE2bisMATE. SNO cells were seeded at 500 000 per 25cm2 flask. Cells were incubated for a period of 24 hours to allow for attachment, subsequently the medium was discarded and cells were exposed to 2- MeOE2bisMATE and appropriate controls were included as defined previously. After 24 hours of incubation, cells were trypsinized and resuspended in 1ml of growth medium. Cells with a density of 500 000 were centrifuged for 5 min at 300×g. The supernatant was discarded and cells were resuspended in 200μl of ice-cold PBS containing 0.1% FCS. In order to avoid cell clumping 4ml of ice-cold 70% ethanol was added in a drop wise manner while vortexing. Samples were stored at 4°C for 24 hours and cells were subsequently pelleted by centrifuging at 300×g for 5 minutes. The supernatant was removed and the cells were resuspended in 1ml of PBS containing 40μg/ml propidium iodide (PI) and 100μg/ml RNAse A. The solution was incubated for 45 minutes in an incubator (5% CO2, 37°C). The fluorescence of propidium iodide (measuring relative DNA content per cell) was measured with a fluorescence activated cell sorting (FACS) FC500 System flow cytometer (Beckman Coulter South Africa (Pty) Ltd.) equipped with an air-cooled argon laser excited at 488nm. Data from at least 30 000 cells were analyzed with CXP software (Beckman Coulter South Africa (Pty) Ltd). For accuracy, the data from cell debris (particles smaller than apoptotic bodies) and clumps of two or more cells were excluded from further analysis. Cell cycle distributions were calculated with Cyflogic 1.2.1 (Perttu Terho & Cyflo Ltd) by assigning relative DNA content per cell to sub-G1, G1, S and G2/M fractions. Data obtained from the fluorescence channel for red monomers (Fl3 Lin) were represented as histograms on the x-axis.
Annexin V-fluorescein isothiocyanate (FITC)
Phosphatidylserine (PS) is a phospholipid which was predominantly observed on the inner surface of the plasma membrane facing the cytosol. Cells undergoing apoptosis lose their phospholipid asymmetry of the plasma membrane and expose PS by translocating it to the outer layer of the plasma membrane. This occurs in the early phases of apoptosis during which the cell membrane remains intact [21,22]. Exponentially growing SNO cells were seeded at 500 000 cells per 25cm2 flask. Cells were incubated for 24 hours to allow for attachment, the medium was discarded and the cells were exposed to either 2- MeOE2bisMATE or previously defined controls. After exposure, the cells were trypsinized, 500 000 cells were resuspended in 1ml of 1x Binding Buffer and then centrifuged at 300×g for 10 minutes. The supernatant was removed and the cells were resuspended in 100μl of the 1x Binding Buffer. Subsequently, 10μl of Annexin V-FITC was added and incubated for 15 minutes in the dark at room temperature. After incubation, cells were washed with 1ml of the 1x Binding Buffer and then centrifuged at 300×g for 10 minutes. The supernatant was carefully pipetted off and the cells were resuspended in 500μl of the 1x Binding Buffer solution. Prior to analysis, 12.5μl of the propidium iodide was added and mixed into the solution. Propidium iodide fluorescence and annexin V-FITC fluorescence were measured with a FACS FC500 System flow cytometer (Beckman Coulter South Africa (Pty) Ltd) equipped with an air-cooled argon laser excited at 488nm. Data from at least 30 000 cells were analysed with CXP software(Beckman Coulter South Africa (Pty) Ltd. Information gained was analyzed using Cyflogic 1.2.1 software, data from fluorescence channel for green monomers (Fl1 log) and fluorescence channel for red monomers (Fl3 log) were represented as dot plots on the y- and x-axis, respectively.
Caspase 6 & 8 colorimetric assays
Caspase 6 & 8 colorimetric assays were conducted to observe whether 2-MeOE2bisMATE has an effect on the caspase 6 & 8 activities of SNO cells. Exponentially growing SNO cells were seeded at 1000 000 cells per 25cm2 flask. After a 24 hours incubation period at 37°C, cells were exposed to 2-MeOE2bisMATE. Cells were trypsinized and then centrifuged at 3 000rpm for 3 minutes. Pellets were then resuspended in 50μl of cell lysis buffer and incubated on ice for 10 minutes. After incubation the cells were centrifuged at 14 000rpm for 1 minute. After determining the protein concentration using the BCA protein assay (Thermo Scientific, Johannesburg, SA) the supernatant was transferred into 50μl of 2X reaction buffer containing 10mM dithiothreitol and 5μl of 4mM VEID-pNA (caspase 6 specific substrate), Ac-IETD-pNA (caspase 8 specific substrate) was added to the supernatants, and the reaction mixtures were incubated at 37°C for 2 hours in the dark. The absobances were determined at 405nm using an ELx800 Universal Microplate Reader available from Bio-Tek Instruments Inc. (Vermont, United States of America).
Mitochondrial membrane potential
One of the earliest intracellular events that occur following the induction of apoptosis is the disruption of the mitochondrial transmembrane potential [23]. The 5,5’,6,6’- tetrachloro-1,1’,3,3’- tetraethylbenzimidazolylcarbocyanine iodide is a fluorescent lipophilic cationic reagent that can be used to detect the loss of the mitochondrial membrane potential; therefore this assay will allow distinguishing between apoptotic cells and viable cells based on changes in the mitochondrial membrane potential. Exponentially growing SNO cells were seeded at 500 000 cells per 25cm2 flask. After 24 hours of attachment, the medium was discarded and cells were exposed to 2-MeOE2bisMATE. Cells were subsequently trypsinized and centrifuged at 13 000xg for 5 minutes. Cells (500 000 per 25cm2) were resuspended in 500μl of the diluted MitoCapture solution, then incubated for 20 minutes in an incubator (5% CO2, 37°C) and centrifuged at 500xg. The supernatant was discarded, cells were resuspended in 500μl of pre-warmed incubation buffer and samples were analysed using a flow cytometer (fluorescence channel for green monomers (FL1 log) (Beckman Coulter South Africa (Pty) Ltd). Generated data was analysed with Cyflogic 1.2.1 software.
Lactate dehydrogenase cytotoxicity assay
Lactate dehydrogenase (LDH) is a cytosolic enzyme which is present in all cell types and it is rapidly released into the cell culture medium upon cell death (late apoptosis or necrosis) by leaking through damaged plasma membranes. The activity of LDH in culture medium can be used as an indicator of cell membrane integrity and as measurement of the cytotoxicity of a drug. Absorbance is directly proportional to the concentration of LDH in the cell culture medium [24]. Exponentially growing SNO cells were seeded in 96-well tissue culture plates at a cell density of 5 000 cells per well. Cells were incubated at 37°C for a period of 24 hours to allow for attachment. After incubation, the medium was discarded and cells were exposed to 2-MeOE2bisMATE, including previously defined controls respectively. After exposure, the plate was shaken gently and the supernatant was centrifuged at 600xg for 10 minutes. Thereafter, 10μl of the supernatant was transferred to an optically clear 96-well plate. Subsequently, 100μl of the LDH Reaction Mix was added to each well, mixed and incubated for two hours at room temperature. The absorbance of all controls was measured with a plate reader equipped with a 450nm filter with a reference wavelength of 630nm. The absorbance of the samples were analysed using an ELx800 Universal Microplate Reader available from Bio-Tek Instruments Inc. (Vermont, United States of America).
Statistical analysis
Quantitative data was provided by means of flow cytometry (cell cycle progression, annexin V-FITC and mitochondrial membrane potential assay) and spectrophotometry (caspase 6&8 and LDH assay). Confocal microscopy supplied qualitative data. The ANOVA students’t-test was used to determine the analytical variation in experimental procedures and biological variations within each experiment. A P-value of <0.05 was regarded as statistically significant. Means are presented in bar charts, with T-bars referring to standard deviations. For flow cytometric data no less than 30 000 events were counted for each sample and three independent experiments were conducted and data produced was analysed using Cyflogic 1.2.1 (Perttu Terho & Cyflo Ltd).
Results
Confocal microscopy- alpha (α)-tubulin assay
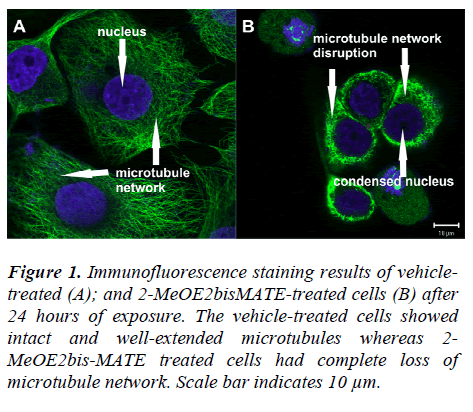
Tubulin polymerization dynamics within the cell are critical for the process of mitosis and are frequently targeted by agents that induce mitotic arrest. To determine whether the observed antitumour effect of 2-MeOE2bisMATE on SNO cells may be a consequence of alterations in gross microtubule structure, the morphology of tubulin networks was examined by immunofluorescence staining using an antibody against α-tubulin. Vehicle-treated cells showed intact and well-extended microtubules (Figure 1A);
Figure 1: Immunofluorescence staining results of vehicletreated (A); and 2-MeOE2bisMATE-treated cells (B) after 24 hours of exposure. The vehicle-treated cells showed intact and well-extended microtubules whereas 2-MeOE2bis-MATE treated cells had complete loss of microtubule network. Scale bar indicates 10 μm.
whereas 2-MeOE2bisMATE treated cells had a complete loss of microtubule network (Figure 1B).
Cell cycle progression
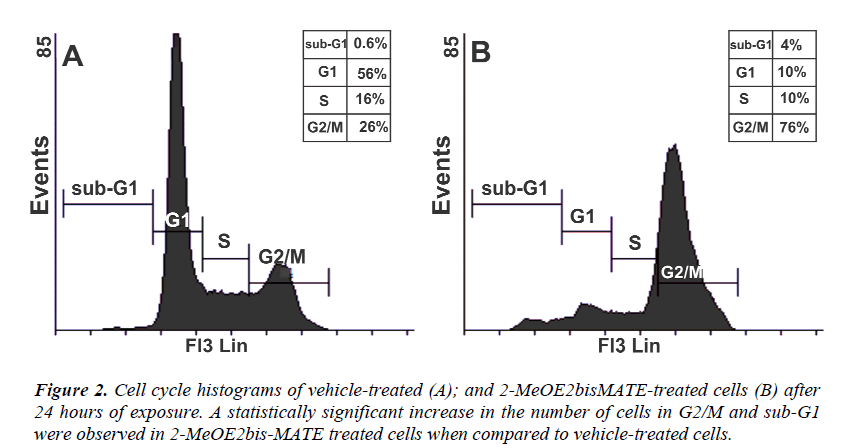
Flow cytometry was used to analyse the effects of 2- MeOE2bisMATE on cell cycle progression. The results revealed a significant increase in the number of 2- MeOE2bisMATE-treated cells (Figure 2B) present in G2/M (metaphase block, 76%) and sub-G1 (apoptosis fraction, 4%) population when compared to vehicletreated cells (Figure 2A) present in G2/M (26%) and sub- G1 (0.6%) respectively.
Annexin V-FITC.
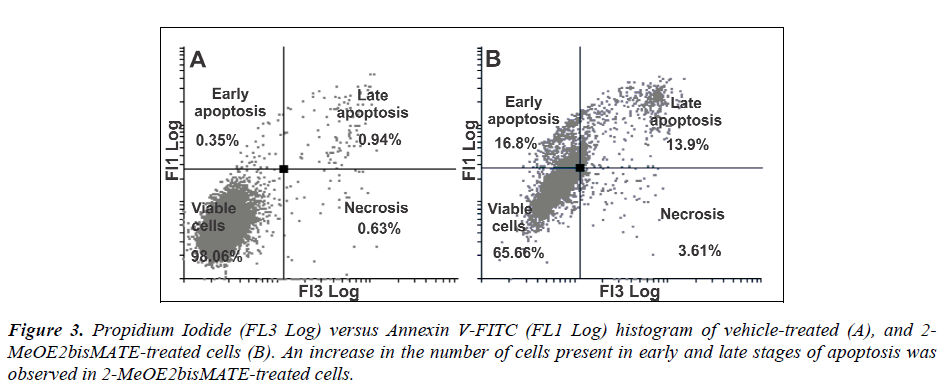
Apoptosis induction by 2-MeOE2bisMATE in SNO cells was confirmed by annexin V-FITC assay. During early phases of apoptosis, cells lose their phospholipid asymmetry of the plasma membrane and expose phosphatidylserine (PS) by translocating it to the outer layer of the plasma membrane. Annexin V (phospholipid-binding protein) preferentially binds to the negatively-charged phospholipids like PS in the presence of Ca2+. When compared to vehicle-treated cells, 2-MeOE2bisMATE-treated cells showed an increase in the number of cells present in early and late stages of apoptosis (16.8% and 13.9% respectively) (Figure 3A and 3B).
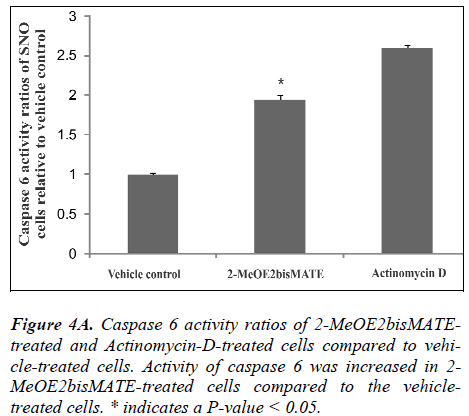
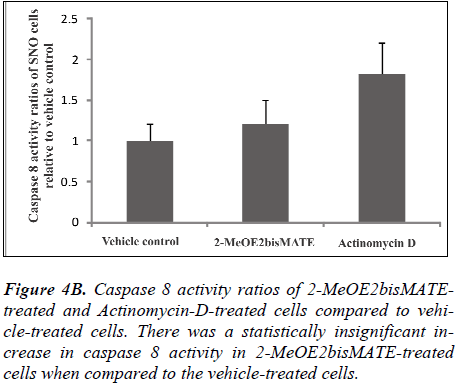
Caspase 6 and 8 colorimetric assays. To further understand the mechanism of action by which 2- MeOE2bisMATE induces apoptosis in SNO cells, caspase 6 and 8 colorimetric assays were conducted. Figure 4A represents caspase 6 activity ratios of 2-MeOE2bisMATEtreated and actinomycin D-treated cells (positive control for apoptosis induction) compared to vehicle-treated cells. Figure 4B represents caspase 8 activity ratios of 2- MeOE2bisMATE-treated and actinomycin D-treated cells compared to vehicle-treated cells. Ratios were calculated from the averages of caspase activities obtained from independent experiments of triplicate samples (n=3). Activity of the executioner caspase (caspase 6) was statistically increased in 2-MeOE2bisMATE-treated cells compared to the vehicle-treated cells (Figure 4A). The activity of initiator caspase (caspase 8) showed a slight increase in 2- MeOE2bisMATE-treated cell, however, not statistically significant (Figure 4B).
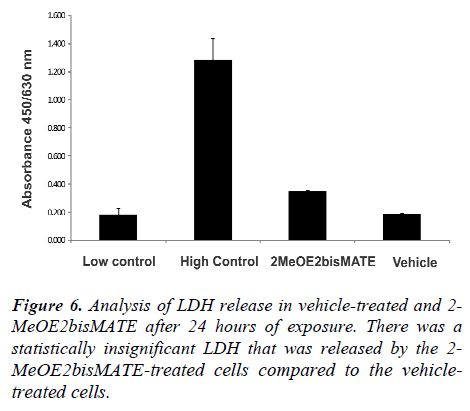
Lactate dehydrogenase assay (LDH). Figure 6 shows the results of LDH release by SNO cells after 24 hours of exposure. Low and high controls were included as specified in the manufacturer’s instructions. The low control refers to cells propagated in growth medium only, while the high control refers to cells propagated in growth medium with cell lysis solution added to the cells 15 minutes before termination. Results revealed a slight increase in LDH released by the 2-MeOE2bisMATE-treated cells (not statistically significant) compared to the vehicle-treated cells (Figure 6). LDH activity was represented by the absorbance at 450 nm with a reference absorbance of 630.
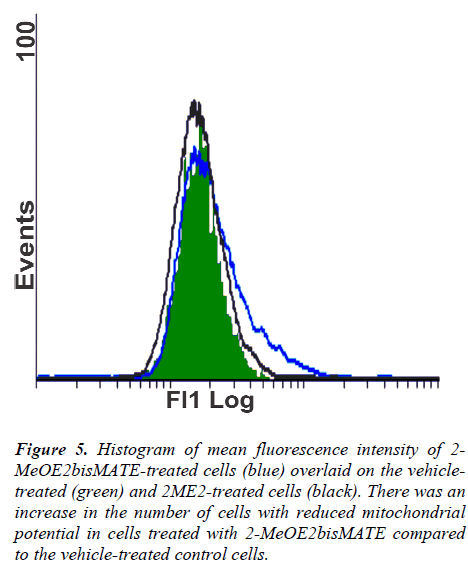
Figure 5: Histogram of mean fluorescence intensity of 2-MeOE2bisMATE-treated cells (blue) overlaid on the vehicletreated (green) and 2ME2-treated cells (black). There was an increase in the number of cells with reduced mitochondrial potential in cells treated with 2-MeOE2bisMATE compared to the vehicle-treated control cells.
Discussion
Since the exact mechanism of action of 2- MeOE2bisMATE is not known and varies according to cell type, the in vitro effects of 2-MeOE2bisMATE on α- tubulin morphology and apoptosis induction were investigated in SNO esophageal carcinoma cells. Studies conducted on 2ME2, the parental molecule of 2-MeOE2bisMATE, have reported that 2ME2 interacts with microtubules, which may result in the destruction of the tubulin network with subsequent cell cycle arrest [25]. The α-tubulin detection assay was utilized to determine the effects of 2-MeOE2bisMATE on the tubulin structure of SNO cells. Immunofluorescence staining results revealed that 2-MeOE2bisMATE disrupts the morphology of microtubules in esophageal carcinoma cells. Thus, these results confirm that the new derivatives and 2ME2 share the same intracellular target molecule. To further investigate and understand the mechanism of action of 2- MeOE2bisMATE in SNO cells, flow cytometry studies were conducted to analyse the effects of 2- MeOE2bisMATE on cell cycle progression. Results showed that the treatment of SNO cells with 2- MeOE2bisMATE caused a G2/M arrest, as well as an increase in the sub-G1 peak. The latter is indicative of the presence of apoptotic cells. The annexin V-FITC assay further confirmed the induction of apoptosis by 2- MeOE2bisMATE; shown by the increase in the number of cells in early apoptosis (16.8%) and late apoptosis (13.9%) when compared to the control. These results were consistent with those of Day et al. [15] which showed that 1μM of 2-MeOE2bisMATE induced cell death of two prostate cancer cell lines (androgen-responsive prostate carcinoma (LNCaP), and androgen-independent prostate carcinoma cells (PC3)), as well as an ovarian cancer cell line (A2780) [15]. It was observed that 2-MeOE2bisMATE caused an increase in the number of prostate and ovarian cells in the G2/M and sub-G1 phases within 24 hours of exposure to the drug [15]. Another study revealed that 24 hours exposure of adult human dermal fibroblasts to 0.1μM 2-MeOE2bisMATE induced G2/M arrest [26]. Data collected in our laboratory demonstrated that the exposure of a non-tumorigenic breast cancer cell line (MCF-12A) and a tumorigenic breast cancer cell line (MCF-7) to 2-MeOE2bisMATE resulted in an increase in the number of cells in apoptosis [16,27]. Since 2-MeOE2bisMATE induces apoptosis in SNO cells, its effects on caspase 6 and 8 activities and on mitochondrial membrane potential were assessed. It is postulated that a decrease in mitochondrial membrane potential results in the opening of the mitochondrial permeability transition pore, which may lead to the release of pro-apoptotic proteins such as cytochrome c, leading to the intrinsic pathway of apoptosis induction [28-30]. The extrinsic pathway, on the other hand, involves receptor binding to ligand, followed by activation of initiator caspases. Caspase 8, one of the initiator caspases is involved in the extrinsic pathway. Caspase 6 is one of the executioner caspases and it is involved in both the extrinsic and intrinsic pathways. Results of mitochondrial membrane potential revealed an increase in the number of 2- MeOE2bisMATE-treated cells that were undergoing a reduction in their mitochondrial membrane potential compared to the vehicle control. Foster et al. (2008) demonstrated that 500nM of 2-MeOE2bisMATE caused depolarization of the inner mitochondrial membrane potential in MDA-MB-231 and HUVECs after 72 hours of exposure [14]. The caspase 6 results showed a statistically significant increase within SNO cells that were exposed to 2-MeOE2bisMATE, although, the caspase 8 activity results showed a slight increase in caspase 8 activity in 2- MeOE2bisMATE-treated cells, it was not statistically significant. Wood et al. 2004 demonstrated no effect of 2- MeOE2bisMATE on caspase 8 activation in CAL51 breast cancer cells [31]. Our result suggests that mitochondria are involved in the process of 2- MeOE2bisMATE-induced apoptosis as shown in previous studies using 2ME2 [28,29,32,33]. Since the caspase 8 activity was not significantly increased, it seems that the intrinsic pathway arising from the mitochondria may be a potential route underlying 2-MeOE2bisMATE-induced apoptosis in the current study.Since our studies suggests that 2-MeOE2bisMATE has potential anticancer effects on SNO cells, the LDH assay was conducted to study the cytotoxic effects of 2-MeOE2bisMATE in SNO cells. LDH is a stable cytosolic enzyme that is readily released in the early stage of cellular necrosis, but only in the late stage of apoptosis [24]. Results showed that 2- MeOE2bisMATE had a minimal effect on LDH release, which demonstrates that 2-MeOE2bisMATE does not severely affect the integrity of the cell’s plasma membrane. This further confirms the previous result that 2- MeOE2bisMATE causes the induction of apoptosis and not of necrosis (due to the fact that the cell’s membrane integrity was not severely damaged).
Conclusion
This in vitro study revealed novel insights on the action mechanism of 2-MeOE2bisMATE in esophageal carcinoma (SNO) cells. Future studies are anticipated to further determine which gene and protein expression changes are induced by 2-MeOE2bisMATE in SNO cells. The identification of 2MeOE2bisMATE as a potential anticancer drug warrants it as a possible candidate for further investigation as a chemotherapeutic agent against esophageal cancer.
Acknowledgements
This research was supported by grants awarded to Professor AM Joubert (University of Pretoria) from the National Research Foundation (NRF), Cancer Association of South Africa (AK246), the Medical Research Council (AG374, AK076) and Research Committee of the University of Pretoria (Pretoria, South Africa). Flow cytometry and confocal microscopy were conducted at the Department of Pharmacology and at the Electron Microscopy Unit, respectively (University of Pretoria, Pretoria, South Africa). Thandi Mqoco, Sumari Marais and Annie Joubert contriuted the research.
References
- Zhao Y, Wang R, Fan D. The molecular mechanisms of oesophageal cancer. Excli J 2006; 5: 79-92.
- Boone J, Livestro DP, Elias SG, et al. International survey on esophageal cancer: part II staging and neoadjuvant therapy. Dis Esophagus 2009; 22: 203-210.
- Dlamini Z, Bhoola K. Esophageal cancer in African blacks of Kwazulu natal, South Africa: an epidemiological brief. Ethn Dis 2005; 15: 786-789.
- Sumeruk R, Segal I, Te Winkel W. et al. Oesophageal cancer in three regions of South Africa. SAMJ 1992; 81: 91-93.
- Du Plessis L, Dietzsch E, Van Gele M, et al. Mapping of Novel Regions of DNA Gain and Loss by Comparative Genomic Hybridization in Esophageal Carcinoma in the Black and Colored Populations of South Africa. Cancer Res 1999; 59: 1877-1883.
- Hendricks D, Parker MI. Oesophageal Cancer in Africa. Life 2002; 53: 263-268.
- Essack M, Radovanovic A, Schaefer U, et al. DDEC: Dragon database of genes implicated in esophageal cancer. BMC Cancer 2009; 9: 219-225.
- Stander BA, Marais S, Vorster CJJ, et al. In vitro effects of 2-methoxyestradiol on morphology, cell cycle progression, cell death and gene expression changes in the tumorigenic MCF-7 breast epithelial cell line. J Steroid Biochem Mol Biol 2010; 119: 149-160.
- Fukui M, Zhu BT. Mechanism of 2-methoxyestradiolinduced apoptosis and growth arrest in human breast cancer cells. Mol Carcinog 2009; 48: 66-78.
- Fotsis T, Zhang Y, Pepper MS, et al. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature 2004; 368: 237-239.
- Van Zijl C, Lottering ML, Steffens F, et al. In vitro effects of 2-methoxyestradiol on MCF-12A and MCF-7 cell growth, morphology and mitotic spindle formation. Cell Biochem Funct 2008; 26: 632-642.
- Thaver V, Lottering M, Van Papendorp D, et al. In vitro effects of 2-methoxyestradiol on cell numbers, morphology, cell cycle progression, and apoptosis induction in esophageal carcinoma cells. Cell Biochem Funct 2009; 24(4): 205-210.
- Stander A, Joubert F, Joubert A. Docking, Synthesis, and in vitro Evaluation of Antimitotic Estrone Analogs. Chem Biol Drug Des 2011; 77: 173-181.
- Foster PA, Ho YT, Newman SP, et al. 2-MeOE2bisMATE and 2-EtE2bisMATE induce cell cycle arrest and apoptosis in breast cancer xenografts as shown by a novel ex vivo technique. Breast Cancer Res Treat 2008; 111: 251-260.
- Day JM, Newman SP, Comninos A, et al. The effects of 2-substituted oestrogen sulphamates on the growth of prostate and ovarian cancer cells. J Steroid Biochem Mol Biol 2003; 84: 317-325.
- Visagie MH, Joubert AM. The in vitro effects of 2-methoxyestradiol-bis-sulphamate on cell numbers, membrane integrity and cell morphology, and the possible induction of apoptosis and autophagy in a nontumorigenic breast epithelial cell line. Cell Mol Biol 2010; 15(4): 564-581.
- Newman SP, Foster PA, Stengel C, et al. STX140 is efficacious in vitro and in vivo in taxane-resistant breast carcinoma cells. Clin Cancer Res 2008; 14: 597-606.
- Visagie MH, Joubert AM. The in vitro effects of 2-methoxyestradiol-bissulphamate on cell numbers, membrane integrity and cell morphology, and the possible induction of apoptosis and autophagy in a nontumorigenic breast epithelial cell line. Cellular & molecular biology letters 2011; 375: 343-352.
- Utsumi T, Leese MP, Chander SK, et al. The effects of 2-methoxyoestrogen sulphamates on the in vitro and in vivo proliferation of breast cancer cells. J Steroid Biochem Mol Biol 2005; 94: 219-227.
- Mqoco T, Marais S, Joubert A. Influence of estradiol analogue on cell growth, morphology and death in esophageal carcinoma cells. Biocell 2010; 34: 113-120.
- Vermes I, Haanen C, Steffens-Nakken H, et al. A novel assay for apoptosis - flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 1995; 184: 39-40.
- Zhang G, Gurtu V, Kain SR, et al. Early Detection of Apoptosis Using a Fluorescent Conjugate of Annexin V. BioTechniques 1997; 23: 525-531.
- Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (_ψm) in apoptosis; an update. Apoptosis 2003; 8: 115-128.
- Fotakis G, Timbrell JA. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett 2006; 160: 171-177.
- Kamath K, Okouneva T, Larson G, et al. 2-Methoxyestradiol suppresses microtubule dynamics and arrests mitosis without depolymerising microtubules. Mol Cancer Ther 2006; 5: 2225-2233.
- Ho YT, Newman SP, Purohit A, et al. The effects of 2- methoxy oestrogens and their sulphamoylated derivatives in conjunction with TNF-α on endothelial and fibroblast cell growth, morphology and apoptosis. J Steroid Biochem Mol Biol 2003; 86: 189-196.
- Vorster C, Joubert A. In vitro effects of 2-methoxyestradiol-bis-sulphamate on cell growth, morphology and cell cycle dynamics in the MCF-7 breast adenocarcinoma cell line. Biocell 2010; 34: 71-79.
- Dobos J, Timar J, Bocsi J, et al. In vitro and in vivo antitumor effect of 2-methoxyestradiol on human melanoma. Int J Cancer 2004; 112: 771-776.
- Ghosh R, Ganapathy M, Alworth WL, et al. Combination of 2-methoxyestradiol (2-ME2) and eugenol for apoptosis induction synergistically in androgen independent prostate cancer cells. J Steroid Biochem Mol Biol 2009; 113: 25-35.
- Salido M, Gonzalez JL, Vilches J. Loss of mitochondrial membrane potential is inhibited by bombesin in etoposide-induced apoptosis in PC-3 prostate carcinoma cells. Mol Cancer Ther 2007; 6: 1292-1299.
- Wood L, Leese MP, Mouzakiti A, et al. 2-MeOE2bisMATE induces caspase-dependent apoptosis in CAL51 breast cancer cells and overcomes resistance to TRAIL via cooperative activation of caspases. Apoptosis 2004; 9: 323-332.
- Chang YF, Hsu YC, Hung HF, et al. Quercetin induces oxidative stress and potentiates the apoptotic action of 2-methoxyestradiol in human hepatoma cells. Nutr Cancer 2009; 61: 735-745.
- Zhang X, Huang H, Xu Z, et al. 2-Methoxyestradiol blocks cell-cycle progression at the G2/M phase and induces apoptosis in human acute T lymphoblastic leukemia CEM cells. Acta Biochim Biophys Sinica 2010; 42: 615-622.