ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 7
A new strategy for repairing large bone defects using an interventional micro-circulation system
1Department of Rehabilitation, the Second Affiliated Hospital of Nanchang University, Regeneration and Rehabilitation Research Centre for Bone and Nerve of Nanchang University, Nanchang, Jiangxi Province, PR China
2Department of Orthopaedics, the First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, PR China
- *Corresponding Authors:
- Jianghua Dai
Department of Rehabilitation
the Second Affiliated Hospital of Nanchang University
PR China - Jun Luo
Department of Rehabilitation
the Second Affiliated Hospital of Nanchang University
PR China
Accepted date: December 28, 2016
Repairing large bone defects remains a difficult clinical problem because of the variability in the defects. The traditional methods, such as autograft, allograft bone and biological filler material transplantation are still facing difficulties in clinical application nowadays. Tissue engineering technology has the potential to solve this problem, and it has thus become a popular research topic. However, ideal solutions for engineering large pieces of bone tissue with vascularization and other key technical problems have not been found to date. Using conventional repair approaches for large bone defects also faces enormous challenges. In order to circumvent these difficulties, we proposed and established a new method called the interventional micro-circulatory system (IMCS) for repairing large-segment bone defects in situ. On the one hand, the system provides nutrition and removes inflammatory cytokines, oxygen free radicals, and toxic metabolites to improve the ischemic injury microenvironment; on the other hand, seed cells are supplied dynamically, and their biological behavior ability such as the migration, proliferation, differentiation, and directional distribution are promoted. We demonstrate the repair of large bone defects in an animal model using this system. Compared with conventional reconstruction methods, this strategy has the potential to provide a new approach to clinical stem cell transplantation for the treatment of large bone defects.
Keywords
SDF-1/CXCR4 axis, BMSCs, Segmental defects, Intervention microcirculation system, Stem cell transplantation
Introduction
Large-segment bone defects are commonly seen in clinical practice. They are caused by different types of trauma, infections, congenital malformations, and cancers, and their restoration remains a challenge. In recent years, some progress has been made in the approach to treat large-segment bone defects by using seed cells implanted into a scaffold containing a complex of bone morphogenetic proteins to construct a tissue-engineered bone graft [1,2]. However, the defect area is characterized by ischemia and hypoxia, the presence of inflammatory cytokines, oxygen free radical accumulation, and a scar-forming microenvironment, which greatly limits seed cell survival, homing, proliferation, and differentiation and ultimately limits bone regeneration. To aid in finding clinical solutions, large animals can be used as segmental bone defect models and to develop clinical treatment targets. Unfortunately, after implanting a large volume of tissue-engineered bone (TEB) in vivo, the implanted cells rely mainly on the host tissue fluid and blood to provide nutrients, and their survival rate is low even when the thickness of the new tissue is less than 0.5 mm, suggesting that implantation of a large segment of TEB seed in vivo makes it difficult for cells to obtain nutrition by diffusion [3,4].
A serious issue in tissue engineering is the inability to maintain large grafts of living cells upon transfer from in vitro to in vivo conditions. Most cells more than few hundred micrometers from the nearest capillary will die, mainly because of diffusion limitations. Engineering bone constructs in vitro with a pre-existing vascular component is a major challenge [5]. One study found that [6,7] autologous bone with a vascular network of blood vessels possessing physiological functions and containing the seed cells could promote bone formation and improve function when transplanted into the bone defect region. However, the occurrence of secondary damage has limited its application. Zhang et al. [8] reported that seed cells carrying pro-angiogenic factors can promote large bone vascularization, while a prerequisite for the survival of seed cells is early nutrition and blood supply, namely, applying TEB to the damaged area to repair large bone defects have no choice but to overcome the problem of the lack of blood supply and provided a nutritional microenvironment early in the repair process. In addition, the lack of nutrition in the damage zone and accumulation of inflammatory cytokines, oxygen free radicals, and high concentrations of potassium ions generates traumatic hematoma products that are toxic to cells, negatively affecting cell survival [9] and limiting seed cell survival and bone regeneration. Therefore, there is a need to develop an approach that will improve the nutrition network in the ischemic injury microenvironment, increase the number of seed cells for transplantation, and promote their survival, proliferation, and differentiation to achieve a regenerative effect in large bone defects.
Hypothesis
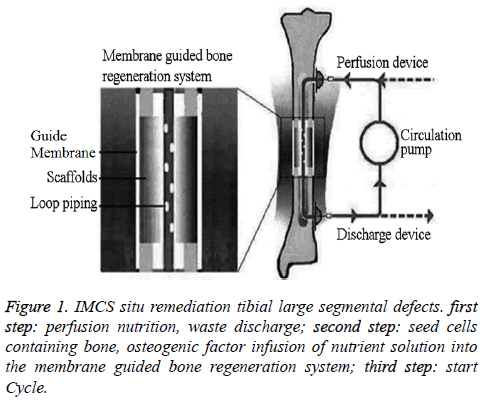
The in vitro system components include the perfusion delivery and discharge devices consisting of two subcutaneous implantable drug delivery systems, discharge tubes, and needles. This device has good long-term biocompatibility when implanted in the body to form a "guided dosing channel," and the circulating pump delivers nutrition to the bone microenvironment and removes metabolic waste. The circulation pump is composed of micro-electromechanical system (MEMS). The MEMS is portable because of its small size, and it can be adjusted to re-administer a drug or to adjust the infusion rate [10]; the use of an MEMS in an in vitro cell culture system ensures suitable culture conditions for BMSCs and the long-term maintenance of the proliferation and differentiation of the cultured seed cells [11]. These characteristics of the MEMS make possible the fine control of the infusion time and dose of nutrient solution through the circulating pump. In addition, the MEMS can control the waste that is discharged from the established microcirculation membrane-guided bone regeneration system. The circulation pump infuses the nutrient solution into the body through the entry injection site, and the metabolic wastes are removed from the body through the exit site. The nutrient perfusion liquid circulates through the system at a pressure greater than the ambient pressure, causing the liquid nutrients to move from the area of higher pressure in the tubing to the area of lower pressure in the pores of the scaffold; on the contrary, the negative pressure within the circulation tubing causes the hydraulic pressure in the pores of the film-guided scaffold of the bone metabolic regeneration system to be higher than the pressure in the circulation tubing, causing the metabolic waste fluid to move into the circulation tubing namely discharged device to be removed from the body. This continuous and dynamic action of the circulating pump system can not only fully deliver nutrients into scaffolds but also remove metabolic waste products from scaffold, thereby improving the regenerated TEB microenvironment and promoting seed cell survival (Figure 1).
Seed Cells
CXCR4 is a seven-transmembrane domain G protein-coupled membrane receptor and is widely expressed on the surface of mononuclear cells, stromal cells, and CD34+ cells. SDF-1 and CXCR4 constitute the SDF-1/CXCR4 axis, which plays an important regulatory role in the tissue injury and bone repair process. During bone regeneration and repair, the secretion of SDF-1 is increased; CXCR4+ stem cells, which are involved in bone repair and reconstruction, move along the SDF-1 concentration gradient to reach the site of the injury. Primary BMSCs expressing a high level CXCR4 will be used as seed cells. Along with the continuously releasing of SDF-1 factorCXCR4+-BMSC can be attracted to home to the region of bone defectand along the SDF-1 concentration gradientgradually distributed to the bone formation site [12,13]. Studies have shown that the repair of large bone defects requires transplants that can ensure the long-term migration and colonization ability of seed cells. CXCR4- expressing BMSCs can meet this requirement, which is a key factor in the success of the repair of the bone defect [14].
Discussion
The structure of the membrane-guided bone regeneration system (Figure 1) consists of the circulation loop tubing, scaffold, and directing film. The circulation tubing is located in the central tunnel of columnar coral porite scaffold with interconnected three-dimensional pore, creating a pathway to deliver nutrients and remove waste products as well as a BMSC migration channel. The scaffold is made from natural coral porites because this material has a similar porous structure as cancellous bone, is composed of calcium carbonate, and all of the pores are interconnected. The scaffold is highly porous and permeable, which favors nutrient and metabolite exchange, and has high biocompatibility; in addition, the scaffold is more easily degraded than ceramic stents, and the degradation rate can be matched with the bone tissue regeneration rate. Therefore, this scaffold material favors nutrition delivery and metabolic waste removal and provides an ideal place for BMSCs to migrate, proliferate, and differentiate [15-17].
The guide film is a composite derived from the stromal cell factor-1 chitosan/collagen carrier membrane. As reported in the literature [18-20], the G protein-coupled transmembrane receptors of SDF-1 are activated to induce CXCR4+-BMSCs to migrate to the site of bone formation. The chitosan/collagen carrier has controllable biocompatibility and biodegradability and can be used as an SDF-1-releasing carrier, which can delay the removal of SDF-1 and extend the directed migration of CXCR4+-BMSCs [21]. Therefore, the SDF-1 complex, chitosan, and collagen are combined to construct the SDF-1/ chitosan/collagen composite film, which has the ability for the slow controlled release of SDF-1 to ensure the long-term directed migration of CXCR4+-BMSCs. However, under normal circumstances, this kind of membrane was only used to form a mechanical barrier that can prevent the rapid ingrowth of tissue cells (such as fibroblasts), fibrosis from scarring while provide a space for guided bone regeneration by ensuring seed cell differentiation and proliferation [22], the Off-the shelf membrane does not attract CXCR4+-BMSCs through directional migration and distribution. Thus, the developed SDF-1/chitosan/collagen composite film has both a barrier function and, through the use of the SDF-1/CXCR4 axis to recruit stem cells, the ability to actively regulate CXCR4+- BMSC directional distribution in the bone formation site.
Osteogenic Factors
After implantation, the directed differentiation of bone seed cells requires inducing factors in the microenvironment, for example BMP-2 is a member of the transforming growth factor superfamily and plays a significant role in bone formation. BMP-2 binds to type I and type II transmembrane serines as a dimer or to hydroxybutyrate isomers of the threonine kinase receptor protein as a tetramer to trigger the nuclear and osteoblast-specific transcription factor Runx2/Cbfa1/Osf2/ AML3 interaction and positive regulation of gene expression in bone cells. The BMP-2 protein plays a vital role in the migration of bone progenitor cells, mesenchymal cell proliferation, differentiation of cartilage- and bone-derived cells, vascular ingrowth, and remodeling.
The initial reaction is the release of BMP-2 from the matrix after the fracture and the homing of primitive mesenchymal cells that also secrete BMP-2. BMP-2 is expressed in the early cartilage stage of bone formation and continues to induce mesenchymal differentiation into cartilage cells and osteogenic differentiation until woven bone formation. Tsuji et al. [23] stated that in the absence of BMP-2, the bone fracture healing process is blocked, and although other osteogenic stimuli exist, they cannot compensate for the function of BMP-2. BMP-2 is essential to start the process of bone repair. Hosogane et al. [24] demonstrated that BMP-2-induced BMSC osteogenic differentiation through the SDF-1/CXCR4 axis also played an important role, as it promoted the osteogenic potential of BMSCs. Otsuru et al. [25] found that the SDF-1/CXCR4 axis caused bone marrow-derived precursor cells to migrate to the bone formation site, with the full ability to differentiate into bone following the induction by BMP-2. Therefore, an interventional micro-circulatory system (IMCS) is used to pump BMP-2 into the bone formation site. The SDF-1/CXCR4 axis can further enhance BMP-2 to induce BMSC to differentiate into bone ability to differentiate into bone.
The Relationship between the IMCS and Large Bone Regeneration
SDF-1 can induce many endogenous stem cells to migrate to the damaged area and regenerate the damaged bone tissue [27,28], and the SDF-1/CXCR4 axis of the induced stem cells plays a key role in homing to bone formation area and promoting directed differentiation. The guided bone regeneration membrane was placed in the periosteum defect site, where it controlled the slow release of SDF-1, similar to the outer membrane of embryonic cartilage, and the live bone graft membrane efficiently expressed SDF-1 to attract a sufficient number of CXCR4+-BMSCs through directed migration to the bone formation site to regenerate bone. In addition, the study demonstrated that SDF-1 and BMP-2 synergistically promote osteogenic potential of BMSCs. Thus, the use of the IMCS combined with the SDF-1/CXCR4 axis can promote stem cell to home as to recruit a sufficient number of CXCR4+-BMSCs to the bone formation site; in addition, the SDF-1/CXCR4 axis was used to promote the BMP-2- induced osteoblast action of CXCR4+-BMSCs to regenerate and repair large bone defects. We will apply IMCS to repair goat tibial defect model in vivo and determine its effectiveness in inducing bone repair.
Conclusion
Based on the literature and the results of previous studies, we established an IMCS composed of the following: (1) the combined capabilities of the delivery of nutrients and the removal of waste to improve the ischemic injury microenvironment; (2) membrane-guided bone regeneration that did not cause scarring and safely created an osteoinduction microenvironment; and (3) the addition of a sufficient number of bone seed cells, inducing factors of BMP-2 and (4) the use of the SDF-1/CXCR4 axis to attract stem cells to the bone formation site and something important, under genuine stress stimulus ,the morphological repair and functional reconstruction of large bone defect form the nature of the case be promoted. Compared with conventional reconstruction methods, this strategy is a potential new approach to treating large bone defects using clinical stem cell transplantation.
Acknowledgement
This work was supported by a National Natural Science Foundation Project (No.81160184), Natural science foundation of Jiangxi province (No. 20114 BAB 205044), Science and technology support project of Jiangxi province (No.2008), the support project of Jiangxi province Education Department (No. GJJ08140) and the support project of Jiangxi province health department (No. 20083052).
References
- Ueha T, Akahane M, Shimizu T, Uchihara Y, Morita Y, Nitta N, Kido A, Inagaki Y, Kawate K, Tanaka Y. Utility of tricalcium phosphate and osteogenic matrix cell sheet constructs for bone defect reconstruction. World J Stem Cells 2015; 7: 873-882.
- Gómez-Barrena E, Rosset P, Müller I, Giordano R, Bunu C, Layrolle P, Konttinen YT, Luyten FP. Bone regeneration: stem cell therapies and clinical studies in orthopaedics and traumatology. J Cell Mol Med 2011; 15: 1266-1286.
- Zhao L, Zhao JL, Wan L, Wang SK. The study of the feasibility of segmental bone defect repair with tissue-engineered bone membrane: a qualitative observation. Strategies Trauma Limb Reconstr 2008; 3: 57-64.
- Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res 2014; 9: 18.
- Laflamme C, Rouabhia M. A medical device for prefabrication of large bone grafts in modern medicine. Med Hypotheses 2011; 76: 489-491.
- Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 2012; 40: 363-408.
- Temple JP, Yeager K, Bhumiratana S, Vunjak-Novakovic G, Grayson WL. Bioreactor cultivation of anatomically shaped human bone grafts. Methods Mol Biol 2014; 1202: 57-78.
- Zhang X, Xie C, Lin AS, Ito H, Awad H, Lieberman JR, Rubery PT, Schwarz EM, O'Keefe RJ, Guldberg RE. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res 2005; 20: 2124-2137.
- Kruyt MC, Dhert WJ, Oner FC, van Blitterswijk CA, Verbout AJ, de Bruijn JD. Analysis of ectopic and orthotopic bone formation in cell-based tissue-engineered constructs in goats. Biomaterials 2007; 28: 1798-1805.
- Elman NM, Upadhyay UM. Medical applications of implantable drug delivery microdevices based on MEMS (Micro-Electro-Mechanical-Systems). Curr Pharm Biotechnol 2010; 11: 398-403.
- Corbin EA, Dorvel BR, Millet LJ, King WP, Bashir R. Micro-patterning of mammalian cells on suspended MEMS resonant sensors for long-term growth measurements. Lab Chip 2014; 14: 1401-1404.
- Dupont KM, Sharma K, Stevens HY, Boerckel JD, García AJ, Guldberg RE. Human stem cell delivery for treatment of large segmental bone defects. Proc Natl Acad Sci U S A. 2010;107:3305-3310.
- Helmy KY, Patel SA, Silverio K, Pliner L, Rameshwar P. Stem cells and regenerative medicine: accomplishments to date and future promise. Ther Deliv 2010; 1: 693-705.
- Ho CY, Sanghani A, Hua J, Coathup M, Kalia P, Blunn G. Mesenchymal stem cells with increased stromal cell-derived factor 1 expression enhanced fracturehealing. Tissue Eng Part A 2015; 21: 594-602.
- Wang Y, Bi X, Zhou H, Deng Y, Sun J, Xiao C, Gu P, Fan X. Repair of orbital bone defects in canines using grafts of enriched autologous bone marrow stromal cells. J Transl Med 2014; 12: 123.
- Yuan J, Zhang WJ, Liu G, Wei M, Qi ZL, Liu W, Cui L, Cao YL. Repair of canine mandibular bone defects with bone marrow stromal cells and coral. Tissue Eng Part A 2010; 16: 1385-1394.
- Dong QS, Lin C, Shang HT, Wu W, Chen FL, Ji XT, Liu YP, Zhang JR, Mao TQ. Modified approach to construct a vascularized coral bone in rabbit using an arteriovenous loop. J Reconstr Microsurg 2010; 26: 95-102.
- Fong EL, Chan CK, Goodman SB. Stem cell homing in musculoskeletal injury. Biomaterials 2011; 32: 395-409.
- Lau TT, Wang DA. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther 2011; 11: 189-197.
- Granero-Moltó F, Weis JA, Miga MI, Landis B, Myers TJ, O'Rear L, Longobardi L, Jansen ED, Mortlock DP, Spagnoli A. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 2009; 27: 1887-1898.
- Zhang Y, Shi B, Li C, Wang Y, Chen Y, Zhang W, Luo T, Cheng X. The synergetic bone-forming effects of combinations of growth factors expressed by adenovirus vectors on chitosan/collagen scaffolds. J Control Release 2009; 136: 172-178.
- Thoma DS, Halg GA, Dard MM, Seibl R, Hammerle CH, Jung RE. Evaluation of a new biodegradable membrane to prevent gingival ingrowth into mandibular bone defects in minipigs. Clin Oral Implants Res 2009; 20: 7-16.
- Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet 2006; 38: 1424-1429.
- Hosogane N, Huang Z, Rawlins BA, Liu X, Boachie-Adjei O, Boskey AL, Zhu W. Stromal derived factor-1 regulates bone morphogenetic protein 2-induced osteogenic differentiation of primary mesenchymal stem cells. Int J Biochem Cell Biol 2010; 42: 1132-1141.
- Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells 2008; 26: 223-234.
- Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T, Nakamura T. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum 2009; 60: 813-823.
- Sundelacruz S, Kaplan DL. Stem cell and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin Cell Dev Biol 2009; 20: 646-655.
- Dawson JI, Oreffo RO. Bridging the regeneration gap: stem cells, biomaterials and clinical translation in bone tissue engineering. Arch Biochem Biophys 2008; 473: 124-131.