ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 21
A novel method for respiratory function measurement in conscious dog
Xinghan Song1*, Fei Liu2, Jun Chen1, Shanping Wang1, Meiling Wen1, Junhua Hu2, Rui Zhang1, Shuyao Zhan1 and Wen Tan2
1School of Bioscience and Bioengineering, South China University of Technology, Guangzhou, Guangdong, PR China
2Institute of Biomedical and Pharmaceutical Sciences, Guangdong University of Technology, Guangzhou, Guangdong, PR China
Accepted on December 28, 2017
Respiratory function measurement in dog has been limited by the side effects of anesthetic drugs. In this context, a method for respiratory function measurement in conscious dog has been developed. A headout body plethysmography has been utilized for measuring the tidal volume; a facemask has been utilized for measuring the airway flow. A wireless-telemetry implant has been placed into the pleural cavity to directly measuring the real pleural pressure. Exposure to the respiratory stimulant 4% CO2, and intravenous administration of the histamine verified that this method was successfully investigated the process of respiratory changes and quantify the airway resistance and dynamic lung compliance. Comparison of the generating process of airway hyper-responsiveness in anaesthetizes dogs and conscious dogs showed that the respiratory function measurement in conscious dogs were more sensitive than in anaesthetizes dogs.
Keywords
Conscious dogs, Respiratory function, Airway resistance, Hyper-responsiveness, Telemetry.
Introduction
According to S7A safety pharmacology, clinical observation of animals is generally not adequate to assess respiratory function in large animals. Therefore, respiratory function measurement in large animal has been widely study to investigate the pharmacodynamic and toxicology of drugs. There are breathing parameters that should be quantified by using appropriate methodologies to measure the respiration function. To evaluate the breathing parameters, both volume and flow changes need to be measured. The volume changes can be evaluated directly by using a plethysmograph chamber, the flow changes can be estimated by a flow meter attached to a tracheal catheter or a facemask. The parameters airway resistance and dynamic compliance are available which are known to be the “gold standard” for detection and quantification of bronchoconstriction and obstruction [1]. To assess the lung resistance and compliance, a pressure-sensitive catheter must be inserted into the anesthetized animal pleural cavity or the esophagus for the measurement of pleural, airway, or transpulmonary pressure [2-5]. Although conscious models are generally preferred in order to avoid alterations of respiratory function but most methods used for respiratory measurement uses anaesthetized animals [6]. The anesthesia animals are easy to control the process of experience, the emotional instability and the physical struggle of animal can be avoided whereas anesthesia has a depressant effect on respiration which decreases the breathing frequency and changes the breathing pattern [5].
In recent years researches are conducting all the respiratory function experiments on conscious animals to overcome the influence of anesthetics in the animal and to obtain more realistic physiological parameters. Many new methods have been developed to evaluate the respiratory function in conscious state, however most methods used for respiratory measurements have limitations. Amdur et al. attempted to evaluate the tidal volume in conscious rat but the volume change data can’t be recorded accurately due to the volume signal interfered by the mouth breathing flow, making this method inaccurate to measure respiratory function for conscious animals. Diamond et al. designed a head-out body plethysmography respiratory function measurement system [5]. This system can well detect the tidal volume signals, the lung pleural pressure has been measured by a chest intubation, and this might cause certain stimulus for the conscious animal during the experience. Besides, the absence of airflow determination made this system unsuitable to estimate the airway resistance.
To better address the physiological data collection, telemetry technology has begun to be applied. It allowed researchers to obtain the physiological parameters such as blood pressure, ventricle pressure, ECG, body temperature etc. In principal, the telemetry implants are placed into the right position by surgical process, after the surgical recovery, the physiological signal can be real-time recorded through the wireless switch controller. Based on the telemetry system, the respiratory function measurement in conscious animal can also be realized. Dennis et al. successfully designed a telemetry-based approach and measured the pleural pressure by installing a telemetry implants (a pressure-sensitive catheter) into the serosal layer of the esophagus within the thoracic cavity of conscious monkey [7]. The volume and flow signal were obtained by a pressure transducer-associated head mask. However, compare to dogs, monkeys are harder to training, the poor behavior related to acceptance the experimental procedures may influence the signal recording process. On the other hand, it is hard to avoid the expansion of gases caused by the temperature change when animal expire the gas from lung to atmosphere. This approach has been limited by the ability to monitor pleural pressure. Although many research indicate that the esophagus pressure partially reflects the pleural pressure, however, due to the insertion and improper placement of an esophageal catheter, the feasibility of obtaining accurate measurements, and the interpretation of the measurements is still hardly used in the clinical arena.
In this study, we develop a method for measuring respiratory function (including tidal volume, flow, respiratory rate, airway resistance and dynamic compliance) and cardiac function (ECG, heart rate) continuously in conscious and in anesthesia dog. Secondly, to compare the difference between generating process of airway hyper-responsiveness in anaesthetizes dogs and conscious dogs. This approach utilizes a head-out body plethysmography for measuring the tidal volume, a facemask for measuring the airway flow. A wireless-telemetry implant has been placed into the pleural cavity to directly measuring the real pleural pressure. This method also allow for monitoring of cardiac electrophysiology signals in conscious dogs. To verify the reliability and sensibility of our method, two approaches has been considered. First, an anoxia conscious dog’s model was developed by inhaling CO2. Air containing elevated CO2 was used to produce a sustained increase in respiration within seconds after exposure [8]. Second, a histamine-induced dog’s airway hyper-responsiveness and Salbutamol induce anti-airway hyper-responsiveness models has been chosen. Histamine causes bronchoconstriction both directly, by stimulating H1 receptors on airway smooth muscle, and indirectly, through vagal reflex this evaluates airway hyper-responsiveness in clinical research [9]. Salbutamol has been widely used as a short-acting β2-adrenoceptor agonist (SABA), it induces anti-airway hyper-responsiveness. SABA was reported to inhibit ACh release from the vagus nerve terminals at a concentration less than that needed to relax airway smooth muscle. Our method will successfully investigate and compare the airway resistance and dynamic lung compliance change during the presence of airway hyperresponsiveness symptom in anaesthetises dogs and conscious dogs.
Materials and Methods
Animals
The dogs used in this study were approximately 7-8 months of age and weighed approximately 5-6 kg. The dogs were individually housing in stainless steel cages in a controlled environment set to maintain a temperature range of 22-25°C and a relative humidity of 30-70%. The room was maintained on a 12 h light/dark cycle. Each dog was feed 300 g extruded feed and enough water twice per day (8:30~10:30 am and 5:30 to 7:30 pm). All animals used in this study were cared for and used humanely in accordance with the China's experimental animal welfare regulations. All the testing programs of animals were submitted to the Center of Animal Welfare Committee in the School of Bioscience and Bioengineering, South China University of Technology (Guangzhou, China).
Compound formulation and dosing
A gas mixture containing 4% CO2, 21% O2 and 75% N2 was administered by inhalation using a facemask placed on the snout. Histamine (Sigma, USA) was prepared at 0.08 mg/kg in sterile water for injection. Dogs were given an intravenous bolus dose of 0.08 mg/kg at a dose volume of 0.25 ml/kg. 0.5 ml ventolin respiratory solution (Ventolin, UK, 2.5 mg of salbutamol) that be diluted to a final volume of 2 ml with sterile normal saline was administered by inhalation using a facemask placed on the snout. Pentobarbital (Sigma, USA) was prepared at 40 mg/kg in sterile water for intraperitoneal injection 40 mg/kg. Dogs were given an intraperitoneal injection bolus dose of 40mg/kg at a dose volume of 0.20 ml/kg.
Parameters
The intrathoracic pressure data and ECG data were obtained from animals by the telemetry system, the flow rate was measured by the signal converter on respiratory masks and the tidal volume was recorded by the integral of the flow rate using the plethysmograph box. The airway resistance Raw and dynamic lung compliance Cdyn were calculated as the equations describe below.
Raw=ΔPR/f
Cdyn=VT/ΔPR
Where PR is the pleural pressure (cmH2O); f is the flow rate (L/min); VT is tidal volume (cmH2O).
Surgical procedure
The anesthesia was induced with pentobarbital sodium (40 mg/kg) and maintained with isoflurane (1-3%) delivered in oxygen. To monitor the animal’s physiological states during the surgery, the ECG signal was recorded by Powerlab System (ADInstruments, Australia). Disinfected the skin with medical iodine and medical alcohol, and inserted the central venous catheter along with the jugular vein of animal, where we can injected the ceftriaxone and glucose to the animal during the surgery and injected the histamine stimulus to the animal during the experiments.
To get the pleural pressures, the transducer (ITS, USA) were placed in the 7th intercostals space and made a small incision through the inter-costals muscle and pleural membrane, the silastic washer were placed on the overlying muscles. Placing surgery sutures under the washer to close the opening in the intercostals muscles. Suture the muscle layers with continuous sutures; suture the skin with subcuticular sutures.
To get the ECG signal, the ECG implantation (ITS, USA) was placed in the subcutaneous tissue, the positive electrode was placed in the subcutaneous tissue near the left lower limbs and the negative electrode was placed in the subcutaneous tissue near right side of the chest. Suture the muscle layers with continuous sutures; suture the skin with subcuticular sutures.
The battery and electronic module (ITS, USA) were placed on the back symmetrically, and the transmit antenna were taken from the implant site up towards the spine and guarantee the antenna wouldn’t be entwined.
Surgical recovery
After surgery, the animal were given water instead of food in the first day, given liquid food in the second day and given extruded feed later. The dogs were given conventional antiinfective therapy, such as the cefradine (NCPC, China) (1 g: 100 million units, once per day and for one week at least). Disinfect the wound daily and smear lomefloxacin hydrochloride cream (TPG, China) or erythromycin ointment (Hengjian, China) twice per day. The ibuprofen sustained release capsules (GSK, UK) were given 0.3 g per day to the dogs to relieve the pain. It takes 5-7 d for animals to recover. After 7 d of recovery, the dogs were allowed to start the 1 w prior of training. In the week prior to the measurements, the animals were trained for 5 d in order to increasing time periods to get accustomed to the plethysmograph. (Lung function measurements in rodents in safety pharmacology studies) Dogs were restrained in a head-out plethysmography, and to wearing the mask used for respiratory measurements. Each training was maintained for 40 min per day.
Respiratory parameters measurements
The dog is placed in lie prostrate position in a head-out body plethysmography (Figure 1). The tidal volume is measured as the integral of flow through a calibrated pneumotachograph (AD Instruments, Australia) connected with the head-out body plethysmograph and caused by the thoracic movements of the animal. The flow rate is measured through a calibrated pneumotachograph (AD Instruments, Australia) which connected to a respiratory mask. The pleural pressures are measured through a telemetry implanted pressure transducer (ITS, USA).
Data analysis
All data was acquired from ADInstruments LabChart 7 Pro system and all programming was performed using SAS (SAS Institute, Cary, NC). Group means and the Standard Error of the Mean (S.E.M.) are presented for all quantitative data (n=6).
Results
Verification of Pleural pressure signal
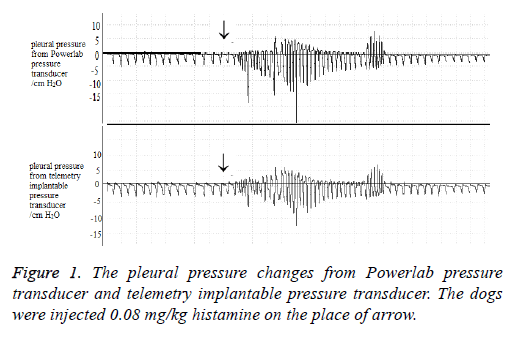
Flow and volume waveforms were obtained in conscious, restrained dogs simultaneously using a face mask equipped with a pneumotachograph. The pleural pressure changes and the ECG signal were measured by the telemetry implantable transducer. The results of the bronchoconstrictor response to histamine are shown in Figure 1.
The pleural pressure changes were measured by the telemetry implantable pressure transducer. To investigate the reliability of this transducer, chest tube was inserted into the thoracic cavity then reference pressure signals were recorded by a Powerlab pressure transducer. Figure 2 compare the pleural pressure changes with utilize the telemetry transducer and the Powerlab transducer in a same anesthetic dog. The pleural pressure was both below 0 cm H2O, when challenge with 0.08 mg/kg histamine (i.v) to the dog, the pleural pressure would change above and below 0 cm H2O. Pleural pressure wave form from telemetry transducer was similar with the pleural pressure from Powerlab transducer (a chest tube inside the dog’s chest). This result proved that the pleural pressure changes signal recorded by telemetry implantable pressure transducer was reliable.
Effect of CO2 inhalation on ventilator parameters
Conscious dogs were exposed to a gas mixture containing 4% CO2, 21% O2, and 75% N2 for 10 min then the gas mixture was switched back to normal air (21% O2 and 79% N2). The physiological parameters of conscious dogs in this experiment are showed as Table 1, when given the CO2 inhalation, the heart rate decrease from 132 BPM to 116 BPM, the pleura pressure increase from 8 cmH2O to 13 cmH2O, the flow rate increase from 230 ml/s to 373 ml/s, the tidal volume increase from 13 ml to 19 ml, the breath rate increase from 26 BPM to 34 BPM. The flow rate, tidal volume, and breath rate has a significant increase, while the heart rate has a significant decrease. On the other hand, the airway resistance and the lung compliance have no significant change.
| Before inhaling CO2 | During inhaling CO2 | |
|---|---|---|
| Heart Rate (BPM) | 131.87 ± 9.70 | 115.58 ± 4.50* |
| Pleural pressure (cm H2O) | 7.63 ± 2.00 | 12.87 ± 4.04* |
| Flow rate (ml/s) | 230 ± 41 | 373 ± 49* |
| Tidal volume (ml) | 13 ± 2 | 18.9 ± 3.5* |
| Breath rate (BPM) | 25.66 ± 3.22 | 33.50 ± 4.99* |
| Airway Resistance (cm H2O/L × s) | 35.02 ± 13.58 | 35.93 ± 15.29 |
| Lung compliance (ml/cm H2O) | 1.7 ± 0.7 | 1.6 ± 0.45 |
*represents significant difference between before inhaling CO2 and during inhaling CO2. Each value is the mean of 6 dogs and error bars are x̄ ± S.E.M
Table 1. The physiological parameters before and after inhaling CO2 (n=6).
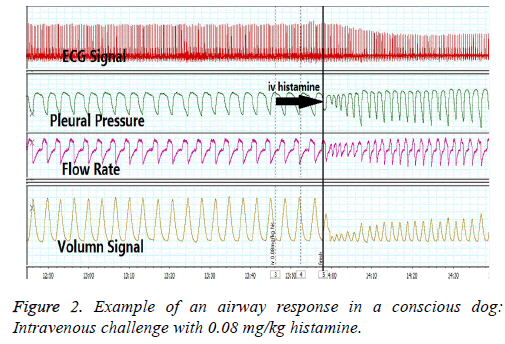
Airway response in conscious dog
The time-matched data collection of airway response in conscious dog shown in Figure 2. Flow and volume waveforms were obtained in conscious, restrained dogs simultaneously using a face mask equipped with a pneumotachograph. The pleural pressure changes and the ECG signal were measured by the telemetry implantable transducer. The airway hyperresponsiveness was induced by intravenous of 0.08 mg/kg histamine.
The physiological parameters change before and after histamine challenge is showed as Table 2. When challenge with histamine, the heart rate decrease from 126 BPM to 98 BPM, pleural pressure increase from 8.6 cm H2O to 10.6 cm H2O, flow rate decrease from 410 ml/s to 290 ml/s, tidal volume decrease from 70 ml/s to 60 ml/s, breath rate decrease from 28 BPM to 22 BPM. On the other hand, airway resistance has significant increase from 61 cm H2O/L × s to 167 cm H2O/L × s, lung compliance decrease from 9 ml/cm H2O to 6 ml/cm H2O.
| Before saline | After saline | Before histamine | After histamine | Before ventolin | After ventolin | |
|---|---|---|---|---|---|---|
| Heart rate (BPM) | 118 ± 30 | 118 ± 32 | 126 ± 33 | 98 ± 42 | 109 ± 34 | 141 ± 22* |
| Pleural pressure (cm H2O) | 8.70 ± 4.45 | 8.89 ± 4.74 | 8.57 ± 4.08 | 10.58 ± 4.66 | 8.16 ± 2.84 | 8.07 ± 1.23 |
| Flow rate (ml/s) | 340 ± 150 | 340 ± 130 | 410 ± 130 | 290 ± 160 | 119 ± 36 | 129 ± 30* |
| Tidal volume (ml) | 54 ± 16 | 58 ± 20 | 70 ± 30 | 60 ± 12 | 56 ± 8 | 68 ± 15 |
| Breath rate (BPM) | 26 ± 12 | 26 ± 12 | 28 ± 10 | 22 ± 11 | 24 ± 9 | 27 ± 7 |
| Airway resistance (cm H2O/L × s) | 72 ± 33 | 73 ± 37 | 61 ± 30 | 167 ± 105* | 77 ± 39 | 66 ± 20 |
| Lung Compliance (ml/cm H2O) | 7 ± 2 | 7 ± 2 | 9 ± 3 | 6 ± 2 | 8 ± 3 | 8 ± 1 |
*represents significant difference (p<0.05) between before and after challenge with histamine. Each value is the mean of 6 dogs and error bars are x̄ ± S.E.M.
The airway hyper-responsiveness protection effect was performed by inhaled of Ventolin, a known β2 agonist bronchodilator. The physiological parameters change before and after inhaled of ventolin are showed as Table 2. Compare the Histamine challenge group with inhaled ventolin group. When inhaled ventolin, heart rate increase from 109 BPM to 141 BPM, Pleural pressure decrease from 8.2 cm H2O to 8.1 cm H2O, flow rate increase from 119 ml/s to 129 ml/s, tidal volume increase from 56 ml to 68 ml, breath rate increase from 24 BPM to 27 BPM, airway resistance decrease from 77 cm H2O/L × s to 66 cm H2O/L × s.
Comparison of conscious and anesthesia dog
To evaluate the difference respiratory function between conscious and anesthesia dog, Ventilation and airway resistance changes were recorded during the histamine induce airway hyper-responsiveness in both conscious and anesthesia dogs. 40 mg/kg pentobarbital sodium was haven to the dogs to induce anesthesia.
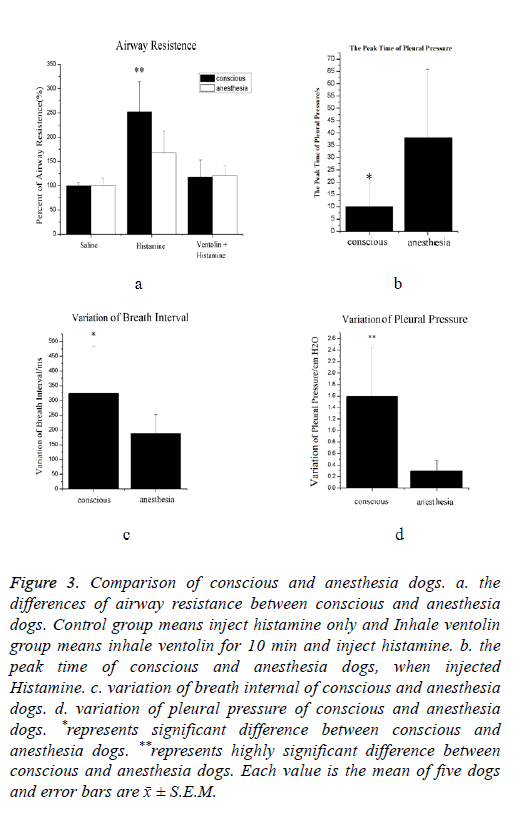
As show in Figure 3a, when challenge with histamine, airway resistance increased both in conscious and anesthesia dogs. The mean value for the conscious and anesthesia animal were 250% and 120% of the mean control value, respectively. There are highly significant differences between conscious and anesthesia animal in this group, percentage increase in conscious animals are 2 times higher than in anesthesia animals. In ventolin histamine group, no significant differences between conscious and anesthesia have been observed.
Figure 3: Comparison of conscious and anesthesia dogs. a. the differences of airway resistance between conscious and anesthesia dogs. Control group means inject histamine only and Inhale ventolin group means inhale ventolin for 10 min and inject histamine. b. the peak time of conscious and anesthesia dogs, when injected Histamine. c. variation of breath internal of conscious and anesthesia dogs. d. variation of pleural pressure of conscious and anesthesia dogs. *represents significant difference between conscious and anesthesia dogs. **represents highly significant difference between conscious and anesthesia dogs. Each value is the mean of five dogs and error bars are x̄ ± S.E.M.
The peak time means the time of animal’s peak-to-peak value of pleural pressure become largest when the animal was injected histamine (Figure 3b). When injected histamine, the mean value for the conscious and anesthesia dogs were 10 s and 38 s respectively. It could be noticed that the peak time of conscious dogs is shorter than that in anesthesia dogs. There are significant differences between conscious and anesthesia dogs.
The variation of breath internal represents the regularity of respiratory movement. The higher value recorded, the more disorders breathing have been observed. As show in Figure 3c, the mean value for the conscious and anesthesia dogs were 325 and 185 respectively. There are significant differences between conscious and anesthesia dogs.
The variation of pleural pressure reflects the change of pleural pressure. As show in Figure 3d, the mean value for the conscious and anesthesia dogs were 1.6 and 0.3 respectively. The change of intrathoracic pressure of conscious dogs is more obvious than anesthesia dogs. There are significant differences between conscious and anesthesia dogs.
Discussion
Although the similarity of large animals respiratory system are generally closer to humans, respiratory function measurements in conscious models are still commonly performed in rodents due to lack of a viable alternative in large animals [10].
Therefore conscious model in large animals and their evaluation approaches need to be further develop.
The purpose of this study is to establish a novel method for measuring respiratory function continuously either in conscious or anesthesia dog and to compare its respiratory function. This method is based on facemask, head-out body plethysmography and pleural pressure telemetry signals determination.
Airway resistance is a critical parameter for assessing bronchoconstriction. For the measurement of airway resistance by pleural pressure, a pressure sensitive probe has to be inserted into the pleural cavity [3,11,12]. Because of the pain associated with this process, these procedures have to be operated in anesthetized animals [7].
An approach that was developed for measuring pleural pressure chronically in conscious monkey this involved the surgical implantation of a fluid-filled polyurethane catheter attached to a telemetry-based pressure transducer into the esophagus [7]. Esophageal pressure changes can only partially reflect the pleural pressure changes and also component of the cardiac beat overlaps with that of esophageal pressure signal [13-16], therefore, accurate measurement of pleural pressure by esophageal pressure changes has been limited.
The advantages of assessment of bronchoconstriction in conscious animals using telemetry have long been recognized [17]. The integrate telemetry pressure transducer provide the stable pleural pressure signals in either conscious or anesthesia dogs. The pleural pressure signals from integrate telemetry system was similar with the signal from the chest tube in the thoracic cavity.
Inhalation of air containing elevated CO2 was used to produce a sustained increase in respiration within seconds after exposure [8] When the dogs inhaled CO2, the breath rate, flow rate, tidal volume and pleural pressure all have increased and the heart rate has decrease. When exposed to air again, all the signals recover in about 3 min. This is expected response based on the findings and the known effects of elevated CO2 on respiration. CO2 has a strong stimulation to breathe and maintain normal activities of the medulla oblongata respiratory center. When dogs start to inhaled CO2, the oxygen partial pressure in alveolar decreased, it would increase CO2 concentration in the cerebrospinal fluid and produce more H+ and strengthen the ventilation activity. The results proved that our respiratory function measurement system can successfully reflect the sober hypoxia reaction. Inhaling CO2 can’t cause contraction of smooth muscle and stricture of airway, thus, no obviously increase of airway resistance. This respiratory function measurement system can distinguish the different between airway hyper-responsiveness reaction and hypoxia reaction.
Histamine has been widely used for bronchial hyperreactivity experiences. Histamine stimulate the H1 receptors and contract the bronchial smooth muscle, cause edema of airway mucosal, bronchospasm, increase airway resistance and decrease the lung compliance [18]. When dog was administrated with Histamine, there were expected results base on the know effect of histamine. On the contrary, the inert control (saline) did not produce any statistically significant change in respiratory parameters. Ventolin (the active drug is Salbutamol sulfate) as a β2 receptor agonist can smooth the airway by inhibiting the release of histamine, relieving the increase of airway resistance [19]. There were expected results base on the know effect of Ventolin. These results proved our respiratory function measurement approach can accurately evaluate the pharmacodynamic effects of Ventolin nebulizer. Both CO2 inhalation experience and histamine-induced airway hyperresponsiveness reflected the reliability of our respiratory function measurement system.
It is widely accepted that, most anesthetics, analgesics, and sedatives can influence ventilatory reflexes, respiratory drive, and airway reactivity, hence non-clinical studies evaluating the effects of drugs on respiratory function should utilize conscious animal models [6,20]. To the authors' knowledge, utilize a same respiratory function measurement system to compare the difference between generating process of airway hyper-responsiveness in anesthesia state and conscious state in dogs and to compare the difference therapeutic effect of ventolin between anesthesia and conscious dogs have not previously been reported.
The histamine induces airway hyper-responsiveness in conscious dogs showed the breathing disorders. Breathe interval and pleural pressure Irregular phenomenon has been found in breath interval and pleural pressure, these suggesting the intervention of some compensatory phenomenon. In contrast, no breathing disorders have been observed in anesthesia dogs. Pentobarbital inhibited the reaction of sympathetic nerve; lead too many subtle breathing neuroregulation couldn’t be reflected.
Our study established a novel method for measuring respiratory function. All our results verified the reliability of this method. The S7A guideline recommends the use of conscious models for safety pharmacology evaluations. Our results suggest that the conscious model is more sensitive than the anesthetized model for respiratory and cardiac electrophysiology effect when administration of the same drugs at the same dose to both models. Our respiratory monitoring in dogs is conducted with methodologies that require restraining. The restraining method may not suitable for long time evaluation of respiratory function [1], further study is needed to improve this methodology.
References
- Hoymann HG. Invasive and noninvasive lung function measurements in rodents. J Pharmacol Toxicol Meth 2007; 55: 16-26.
- Hoymann HG. Lung function measurements in rodents in safety pharmacology studies. Front Pharmacol 2012; 3: 156.
- Amdur MO, Mead J. Mechanics of respiration in unanesthetized guinea pigs. Am J Physiol 1958; 192: 364-368.
- Davidson JT, Wasserman K, Lillington GA, Schmidt RW. Effect of aging on respiratory mechanics and gas exchange in rabbits. J Appl Physiol 1966; 21: 837-842.
- Diamond L, ODonnell M. Pulmonary mechanics in normal rats. Journal of Applied Physiol Resp Env Exerc Physiol 1977; 43: 942.
- Murphy DJ. Comprehensive non-clinical respiratory evaluation of promising new drugs. Toxicol Appl Pharmacol 2005; 207: 414.
- Murphy DJ, Renninger JP, Coatney RW. A novel method for chronic measurement of respiratory function in the conscious monkey. J Pharmacol Toxicol Meth 2001; 46: 13-20.
- Saupe KW, Smith CA, Henderson KS, Dempsey JA. Respiratory muscle recruitment during selective central and peripheral chemoreceptor stimulation in awake dogs. J Physiol 1992; 448: 613.
- Matsumoto K, Aizawa H, Fukuyama S, Yoshida M, Komori M, Takata S, Koto H, Inoue H. Low-dose salbutamol suppresses airway responsiveness to histamine but not methacholine in subjects with asthma. Resp Investig 2013; 51: 158-165.
- Kearney K, Metea M, Gleason T, Edwards T, Atterson P. Evaluation of respiratory function in freely moving Beagle dogs using implanted impedance technology. J Pharmacol Toxicol Meth 2010; 62: 119-126.
- Santing RE, Meurs H, Tw VDM, Remie R, Oosterom WC, Brouwer F, Zaagsma J. A novel method to assess airway function parameters in chronically instrumented, unrestrained guinea-pigs. Pulm Pharmacol 1992; 5: 265-272.
- Weissberg RM, Marian J, Bradshaw J. Respiratory and cardiovascular effects of prostaglandins in the conscious guinea pig. J Pharmacol Exp Ther 1976; 198: 197.
- Cheng YP, Wu HD, Jan GJ, Wang CY. Removal of cardiac beat artifact in esophageal pressure measurement via a modified adaptive noise cancellation scheme. Ann Biomed Eng 2001; 29: 236-243.
- Gillespie DJ, Lai YL, Hyatt RE. Comparison of esophageal and pleural pressures in the anesthetized dog. J Appl Physiol 1973; 35: 709-713.
- Koo KW, Leith DE, Sherter CB, Snider GL. Respiratory mechanics in normal hamsters. J Appl Physiol 1976; 40: 936-942.
- Palecek F. Measurement of ventilatory mechanics in the rat. J Appl Physiol 1969; 27: 149-156.
- Chong BT, Agrawal DK, Romero FA, Townley RG. Measurement of bronchoconstriction using whole-body plethysmograph: comparison of freely moving versus restrained guinea pigs. J Pharmacol Toxicol Meth 1998; 39: 163-168.
- Hamzeh-Gooshchi N, Tamaddonfard E, Farshid AA. Effects of microinjection of histamine into the anterior cingulate cortex on pain-related behaviors induced by formalin in rats. Pharmacol Rep 2015; 67: 593-599.
- Opt Holt TB. Intratracheal albuterol: a potential intervention for the asthma toolbox. Resp Care 2015; 60: 756.
- Amdur MO, Mead J. Mechanics of respiration in unanesthetized guinea pigs. Am J Physiol 1958; 192: 364-368.