ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2014) Volume 25, Issue 2
A preliminary study of CD150 expression in responders and non- responders to the Hepatitis B vaccine.
1Department of Infectious Disease, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, China
2Guangdong International Travel Healthcare Center, Tianhe District, Guangzhou 510635, China
3Department of Infectious Diseases, The Second People’s Hospital of North Guangdong, Shaoguan, Guangdong 512028,China
- *Corresponding Author:
- Chao-Shuang Lin
Department of Infectious Disease
The Third Affiliated Hospital of Sun Yat-sen University
Guangzhou 510630, China
Accepted date: November 24 2013
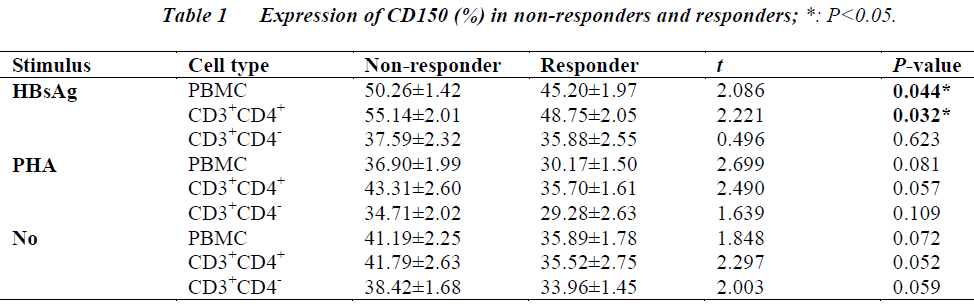
The aim was to study the association of CD150 expression in peripheral blood mononuclear cells (PBMCs) with response to hepatitis B (HB) vaccination. Heparinized blood drawn from non-responders and responders was used to obtain PBMCs. Out of 460 adult healthy males and non-pregnant females, 27 subjects who were negative for HB markers were defined as non-responders (15 males and 12 females, aged 21-47 years). Among subjects who were anti-HB positive, 27 subjects were randomly chosen as responders (16 males and 11 females, aged 20-48 years). The isolated PBMCs were cultured and induced with recombinant HB surface antigen (rHBsAg) or phytohaemaglutinin (PHA). The expression of CD150 was then analyzed using flow cytometry. The levels of CD150 in both PBMC (t = 2.086, P = 0.044) and CD3+CD4+ cells (t = 2.221, P = 0.032) in non-responders to the hepatitis B vaccine were found to be significantly higher than those in the responders when the cells were induced with rHBsAg, while the level of CD150 in CD3+CD4- cells in non-responders were not significantly different from the responders. However, no significant difference was found in the level of CD150 in CD3+CD4+ cells or CD3+CD4- cells between non-responders and responders when the cells were induced with PHA. Therefore, CD150 may directly induce the proliferation of CD4+ and play a role in non-response to HB vaccination.
Keywords
CD150; CD3+CD4+ cells; Hepatitis B vaccine; non-response; peripheral blood mononuclear cells (PBMCs)
Introduction
Hepatitis B (HB) is an infectious disease that causes epidemics in many parts of Asia. The vaccine for HB is regarded as a safe, effective preventative for people living in regions where the virus is endemic, or for patients within high-risk population. However, 5%-10% of subjects (i.e., non-responders) do not develop protective surface antibodies after completing the full series of the standard HB immunization. Non-responders therefore remain susceptible to infection by the hepatitis B virus (HBV) [1].
There may be multiple reasons for non-response to immunization, because the underlying mechanisms are complex. It is well established that genetic factors play a critical role in the induction of the anti-hepatitis B surface (anti-HBs) response that prevents or controls HBV infections. It has also been suggested that human leukocyte antigen phenotypes are related to nonresponse [2,3]. Other factors affecting immunity, such as, failure of antigen presentation or a lack of T helper cell response to the HB surface antigen (HBsAg) are also implicated [4-6]. In vitro experiments have shown that non-responders have poor peripheral blood mononuclear cell (PBMC) proliferative responses and decreased expression of cell surface molecules such as CD69, CD25 and CD40 in T cells [7].
In a recent investigation of the response of health care workers to the HB vaccine, CD150 (also known as the signaling lymphocytic activation molecule, or SLAM) was identified as an expression marker upregulated upon vaccination. It was also shown by gene chip analysis that CD150 has a suppressive regulatory cell function on all activated T cells [8]. The cellular proliferation response to specific or non-specific antigens is significantly associated with enhanced surface expression of CD150 in non-responders. Thus, it might be possible to use CD150 as a potential predictive marker for non-responsiveness [9].
The SLAM family of human genes consists of seven related members of the immunoglobulin superfamily. These include CD150, CD244 (also known as 2B4), CD84, CD229 (formerly Ly-9), CD48, 19A and the B-lymphocyte activator macrophage expressed (BLAME). This set of genes, related to CD2 and CD58 on Chromosome (Chr) 1p98, are found clustered together in the human genome on Chr 1q22-q23. These genes are expressed to varying degrees in subsets of immune cells (T, B, natural killer, and myeloid cells) and may function as ligands or receptors. Four of these family members (CD150, CD244, CD84, CD229) contain conserved tyrosine motifs in their cytoplasmic tails that enable them to bind intracellular signaling molecules SAP and EAT-2 [10]. SLAM and SLAM-associated protein (SAP) families influence the functions of conventional lymphocytes, including the secretion of certain cytokines by CD4+ T cells, and these molecules can mediate B cell help, CD8+ T cell proliferation and cytokine production, NK cell-mediated cytotoxicity, and B cell antibody production. These unique functional properties appear to be facilitated by the ability of SLAM-related receptors to serve as self-ligands during homotypic interactions between immune cells [11].
To investigate the possible association of CD150 with response to the HB vaccine in the Chinese population, we analyzed and compared its expression in responders and non-responders. We further analyzed the expression of CD150 on PBMCs when the cells were induced with phytohaemaglutinin (PHA) or recombinant HBsAg (rHBsAg) in vitro.
Materials and Methods
Subjects
Volunteers were enrolled by the Department of Infection at the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou from September 2007 to June 2010. All volunteers satisfied the following criteria: adults with a well-documented history of HB vaccination within the past two years (completing at least one standard immunization schedule); negative serologies for HB virus; and healthy, with normal liver function. Following standard boosting schedules (20μg yeast recombinant HB vaccine, 0, 1, 6 months), anti-HBs were determined seven to twelve months after inoculation (Abbott kits, IMx AUSAB). Out of 460 adult healthy males and nonpregnant females, 27 subjects who were negative for HB markers (HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc) were defined as non-responders (15 males and 12 females, aged 21-47 years). Among subjects who were anti-HBs positive, 27 subjects were randomly enrolled as responders (16 males and 11 females, aged 20-48 years). The study was approved by the hospital research Ethics Committee. Signed informed consent was obtained from all participants in this study.
Isolation of PBMCs
A total of 18 ml of venous blood was drawn from each patient, divided into three sterile tubes, and subsequently sent for preparation of PBMCs within 4 h. PBMCs were isolated using the density gradient centrifugation method. The blood was diluted with phosphate buffered saline (PBS) (1:1 volume ratio), slowly added onto the surface of lymphocyte separation medium (5 ml/each tube), and subsequently centrifuged at 700 g for 20 min. The buffy interface layer was collected and washed with sterile PBS several times. The isolated PBMCs were used for both flow cytometric analysis of CD150 expression, and further cell culture using induction with PHA or rHBsAg.
Culture of PBMCs with PHA or rHBsAg
PBMCs were cultured with Roswell Park Memorial Institute (RPMI)-1640 medium containing 10% fetal calf serum (FCS), and stimulated with either phytohemagglutinin (PHA, 1μg/ml) or the purified yeast HB antigen (rHBsAg, 3μg/ml) at 37°C under 5% CO2 and 95% relative humidity for 48 h.
Flow cytometry assay
Following 48 h of cell culture, cells were washed with PBS twice, re-suspended in PBS and adjusted to a concentration of 1x106/ml. Fluorescein isothiocyanate (FITC)-conjugated anti-human CD4 monoclonal antibody, phosphatidylethanolamine (PE), PE-conjugated anti-human CD150, and allophycocyanin (APC)–conjugated anti-human CD3 (each 20 μl) were added to 100 μl of cell suspension and incubated at 20 °C for 30 min. A total of 10 μl of mouse IgG1 (kappa) was added to 100 μl of cell suspension as a control. Cells from each tube were washed with 1 ml PBS. Following centrifugation at 300 g for 5 min, cells were resuspended in 500 μl of PBS. A three-color fluorescence flow cytometry (BD-FACScalibur) assay was performed after setting the gates for the forward scattering and side scattering lights and the compensation of fluorescence for each channel. Cell Quest software was used to analyze the expression of CD150 in the PBMCs, CD3+CD4+ and CD3+CD4- cells.
Statistical analysis
Statistical analysis (bilateral t-test) was performed using the SPSS 16.0 statistical package. A P-value P≦0.05 was assumed to indicate a significant difference.
Results
Flow cytometry assays revealed that the levels of CD150 in both PBMC (t = 2.086, P = 0.044) and CD3+CD4+ cells (t = 2.221, P = 0.032) in non-responders to the hepatitis B vaccine were significantly higher than those in the responders when the cells were induced with rHBsAg. While the level of CD150 in CD3+CD4- cells in non-responders were not significantly different from the responders. There were no significant differences in the levels of CD150 in CD3+CD4+ cells or CD3+CD4- cells between the non-responders and responders when the cells were induced with PHA. (Table 1).
Discussion
HB virus infection remains a major public health problem worldwide. The efficacy of HB vaccines for preventing disease is well known. However, 5–10% of patients who receive the vaccine are unable to achieve a protective level of antibodies (>10 U/L) after vaccination. The lack of response to HB vaccination remains a problem for individuals who are at risk of HB infection. The mechanism responsible for the non-response to HB vaccination is associated with the dysfunction or dysregulation of T lymphocytes, B lymphocytes, and APCs in non-responding individuals. No consensus about the mechanism has been established, and the results presented by different investigators are controversial, indicating that further studies are needed [3,12-16].
CD150, a lymphocyte activation signaling molecule, was recently reported to be significantly upregulated in non-responders, compared with high responders. This finding prompted us to investigate whether this also occurs in the Chinese population. In our preliminary study of 54 subjects who received HB vaccination (27 non-responders, 27 responders), the CD150 level in CD3+CD4+ cells in the non-responders was significantly higher than that in the responders. This preliminary result seems to be consistent with a previous report showing the up-regulation of CD150 in non-reponders to HB vaccination in Indian health care workers [9]. To probe the possible mechanism underlying non-response to HB vaccination, we induced PBMCs, isolated from patients, with PHA or rHBsAg in vitro, and compared the expression of CD150 between the cells of responders and non-responders. We observed an altered expression pattern of CD150 in the rHBsAg-induced PBMCs of non-responders. The expression of CD150 on rHBsAg-induced PBMCs or rHBsAg-induced CD3+CD4+ cells in non-responders was significantly higher than that in the responders (P≦0.05). And there were no significant differences in the levels of CD150 in PHA-induced PBMCs or PHA-induced CD3+CD4+ cells or CD3+CD4- cells between the non-responders and responders. The mechanism underlying the altered CD150 expression patterns before and after treatment with PHA or rHBsAg in this study was not determined due to the limited size of samples collected.
The functional role of CD150 in the development of the response to HB vaccination has not yet been evaluated. CD150 is constitutively expressed on T cells, a proportion of B cells, and rapidly induced on naive T cells after activation. CD150 can enhance antigen-specific proliferation and cytokine production by CD4+ T cells, strongly upregulate the production of interferon-γ, and directly induce the proliferation of CD4+ T-cell clones and preactivated T cells, even in the absence of any other stimuli, and without CD28 involvement [17]. Future investigations are needed to determine what position CD150 holds in the complicated regulation of the immune response, and in particular, to the response to vaccination. In the future, a more detailed analysis of the CD150 expression spectrum on subtypes of PBMCs in a large number of patients will be necessary in order to cast light on the mechanism of non-response to HB vaccination, and to further elucidate the potential role of CD150 in the immune response.
Conclusion
Our preliminary study on a small number of patients suggests that CD150 may play a role in the response to the HB vaccine. However, the results emphasize the need for a larger sample population in order to study the association between the expression spectrum of CD150 with respect to responders and non-responders, and to elucidate the role of CD150 in the immune response.
Source of Funding
The study was supported by Natural Science Fund from Government Agency, Guangdong province, China (Grant number: 10151008901000011) and The Natural Science Fund from Government Agency, China (Grant number: 81172889).
Conflicts of Interest
No editorial or financial conflict of interest exists
References
- Alper CA. The human immune response to hepatitis B surface antigen. Exp Clin Immunogenet 1995; 12: 171-181.
- Alper CA, Kruskall MS, Marcus-Bagley D, Craven DE, Katz AJ, Brink SJ, Dienstag JL, Awdeh Z, Yunis EJ. Genetic prediction of nonresponse to hepatitis B vaccine. N Engl J Med 1989; 321: 708-712.
- Pol S, Legendre C, Mattlinger B, Berthelot P, Kreis H. Genetic basis of nonresponse to hepatitis B vaccine in hemodialyzed patients. J Hepatol 1990; 11: 385-387.
- Vingerhoets J, Vanham G, Kestens L, Penne G, Leroux-Roels G, Gigase P. Deficient T-cell responses in non-responders to hepatitis B vaccination: absence of TH1 cytokine production. Immunol Lett 1994; 39: 163-168.
- Salazar M, Deulofeut H, Granja C, Deulofeut R, Yunis DE, Marcus-Bagley D, Awdeh Z, Alper CA, Yunis EJ. Normal HBsAg presentation and T-cell defect in the immune reponse of nonresponders. Immunogenetics 1995; 41: 366-374.
- Hsu HY, Chang MH, Hsieh RP, Ni YH, Chi WK. Humoral and cellular immune responses to hepatitis B vaccination in hepatitis B surface antigen-carrier children who cleared serum-hepatitis B surface antigen. Hepatology 1996; 24: 1355-1360.
- Goncalves L, Albarran B, Salmen S, Borges L, Fields H, Montes H, Soyano A, Diaz Y, Berrueta L. The nonresponse to hepatitis B vaccination is associated with impaired lymphocyte activation. Virology 2004; 326: 20-28.
- Browning MB, Woodliff JE, Konkol MC, Pati NT, Ghosh S, Truitt RL, Johnson BD. The T cell activation marker CD150 can be used to identify alloantigen-activated CD4(+)25+ regulatory T cells. Cell Immunol 2004; 227: 129-139.
- Pati NT, Sukriti, Hissar S, Agrawal K, Rani R, Sarin SK. Decrease in CD4+ T lymphocyte proliferation responses and enhanced CD150 cell expression in health care workers non-responsive to HBV vaccine. Vaccine 2007; 25: 1848-1855.
- Fraser CC, Howie D, Morra M, Qiu Y, Murphy C, Shen Q, Gutierrez-Ramos JC, Coyle A, Kingsbury GA, Terhorst C. Identification and characterization of SF2000 and SF2001, two new members of the immune receptor SLAM/CD2 family. Immunogenetics 2002; 53:843-850.
- Veillette A, Dong Z, Latour S. Consequence of the SLAM-SAP signaling pathway in innate-like and conventional lymphocytes. Immunity 2007; 27: 698-710.
- Jafarzadeh A, Shokri F. The antibody response to HBs antigen is regulated by coordinated Th1 and Th2 cytokine production in healthy neonates. Clin Exp Immunol 2003; 131: 451-456.
- Walsh PT, Tayloe DK, Turka LA. Tregs and transplantation tolerance.J Clin Invest 2004; 114: 1398-1403.
- Wataya M, Sano T, Kamikawaji N, Tana T, Yamamoto K, Sasazuki T. Comparative analysis of HLA restriction and cytokine production in hepatitis B surface antigen-specific T cells from low- and high-antibody responders in vaccinated humans. J Hum Genet 2001; 46:197-206.
- Chedid MG, Deulofeut H, Yunis DE, Lara-Marquez ML, Salazar M, Deulofeut R, Awdeh Z, Alper CA, Yunis EJ. Defect in Th1-like cells of nonresponders to hepatitis B vaccine. Hum Immunol 1997; 58: 42-51.
- Livingston BD, Alexander J, Crimi C, Oseroff C, Celis E, Daly K, Guidotti LG, Chisari FV, Fikes J, Chesnut RW, Sette A. Altered helper T lymphocyte function associated with chronic hepatitis B virus infection and its role in response to therapeutic vaccination in human. J Immunol 1999; 162: 3088-3095.
- Cocks BG, Chang CC, Carballido JM, Yssel H, de Vries JE, Aversa G. A novel receptor involved in T-cell activation. Nature 1995; 376: 260-263.