ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 11
Aesthetic characteristics of the orthodontic wire with silver plating
Takashi Usui*, Sinjiro Miyake, Toshio Iwata, Takero Otsuka, So Koizumi, Nobukazu Shirakawa and Toshitsugu Kawata
Orthodontic Division, Department of Oral Interdisciplinary Medicine, Graduate School of Dentistry, Kanagawa Dental University, 82 Inaokacho, Yokosukashi, Kanagawa, Japan
- *Corresponding Author:
- Takashi Usui
Orthodontic Division, Department of Oral Interdisciplinary Medicine
Graduate School of Dentistry, Kanagawa Dental University, Japan
Accepted date: March 28, 2017
Introduction: The aesthetics of orthodontic appliances represents one of the main concerns of patients receiving orthodontic treatment. A large number of orthodontic appliances have been composed of silver and have a metallic color. The aim of this study is to prepare esthetic orthodontic wires using silver electroplating. The color of the aesthetic wires was assessed using a devised colorimetric measurement method as described later.
Materials and Methods: A silver-plated wire that we have developed and six brands of esthetic arch wires were assessed. These wires were measured three times using a colorimeter color system 1. Lightness (L*), 2. Hue (a* • b*), 3. Chroma (C*), were evaluated. The colors of these wires need to be compared with the natural tooth color. VITA shade A1 (VA1) as evaluation criteria was measured.
Result: 1. In the L* value, the silver-plated wire was the closest to VA1 at 70.5 than (p<0.01). 2. Among the a* values, epoxy coating wire was the closest to VA1 at -1.3. 3. Among the b* and C* values, the silver-plated wire was the most approximate at 8.4 to VA1 (p<0.01).
Discussion: The silver-plated wire was closest to the natural tooth color, lightness, chroma, hue. In the future, we are working on development of a more aesthetic wire.
Keywords
Orthodontic wire, Color matching, Silver-platting, Aesthetic material.
Introduction
The aesthetics of orthodontic appliances represents one of the main concerns for patients receiving orthodontic treatment. A large number of orthodontic appliances are composed of silver and have a metallic color [1]. The demand for orthodontic appliances having better aesthetics is increasing, and recent trends in orthodontic treatments have led to the development of orthodontic appliances with acceptable aesthetics and sufficient clinical performance for patients and clinicians [2]. Orthodontic appliances that incorporate plastic and ceramic materials have been developed for the market [3]. Additionally teflon and epoxy resin-coated arch wires have been introduced [4].
However, the color differences between orthodontic materials and teeth negatively affect aesthetics. White-coated wires are of different types, and the color of these wires varies depending on clinical applications. In addition, it is unclear whether the color of these wires matches the color of teeth. Few studies have been conducted on the color of arch wires. Therefore, we believe that there is an increasing demand for developing orthodontic appliances having a high aesthetic appearance.
Wires used in orthodontic appliances are coated using different methods including the sputtering, plating, and printing methods. Among these methods, we focused on the electroplating method in this study. Electroplating is a process that uses an electric current to reduce dissolved metal cations so that they form a coherent and uniform coating on an electrode [5]. Silver is known to show superior performance in reproducing white color in arch wires during electroplating [6]. The plating is used widely at present, most of which are alloy plating. The alloy plating can get various kinds of favorable colors depending on the composition, and its application range as the decoration plating is very wide. In addition, there is corrosion resistance plating, wear resistant plating, and plating for soldering by being easy to melt [7]. Alloy plating is precipitated at the same time with two or more kinds of metals and non-metals in the electroplating or other methods to make the alloy coating film. We used an electroplating treatment. Electroplating method can be applied to a substrate of complex shape, and it is possible to perform mass production. Electroplating treatment is the method that the metal ions in the plating bath is deposited on the target metal surface as a metal atom, and formed a crystal lattice by diffusing its surface.
In the present study, we focus on the difference in color between an archwire and a natural tooth, as it is one of the aesthetic issues associated with orthodontic devices, as described above. Therefore, the aim of this study is to develop aesthetic orthodontic arch wires by using silver electroplating. The color of the aesthetic arch wires is assessed using a devised colorimetric measurement method, which will be described later.
Materials and Methods
Wire samples
Typical alloy plating which can provide aesthetic properties to the metal are Silver alloy, gold, rhodium, platinum, and palladium alloy. In them, silver alloy can be brought close to the appearance to the natural color of teeth by adjusting the alloy composition. The method is plating treatment. Silver plating wire was created by the electroplating process. The film thickness was set to 5 μm. It can be seen that silver plating wire is nearly the same dimension of non-coated stainless steel wire. Thus silver plating wire was created based on non-coated stainless steel wire while keeping its dimension. We developed a silver-plated wire and assessed its performance in reproducing a white color in arch wires by comparing it with six brands of aesthetic arch wires in the present study. The brands or wire names, cross-sectional sizes, compositions, and coating surfaces are listed in Table 1, as described by the manufacturers (examples shown in Figure 1).
| Wire name and manufacturer | Section size | Composition | Coating surface |
|---|---|---|---|
| Silver | 0.016 × 0.016 | Stainless steel | All?surfaces |
| Ever White | 0.016 × 0.016 | NiTi | All surfaces |
| Nano White, DensplySankin, Tokyo, Japan | 0.016 × 0.016 | NiTi | All surfaces |
| White Stainless wire,TOMY, Tokyo, Japan | 0.016 × 0.016 | Stainless steel | Labial |
| Epoxy coating, archwire, ORTHIKA, Tokyo, Japan | 0.016 × 0.016 | Stainless steel | All?surface |
| Gum metal, Rocky mountain Morita, Tokyo, Japan | 0.016 × 0.022 | β-Titanium | No coating |
| SS | 0.016 × 0.016 | Stainless steel | No coating |
Table 1: Description of tested arch wires.
Tooth color setting
The A1 of the shade guide (VITA) was used as a reference for white color as the color of teeth is different for different people. The shade guide was employed to determine the color of teeth in prosthetic and restorative treatments. Sixteen variations of color exist between A1 and D4 [8]. In VITA, A1 is the brightest color and is hereafter referred to as VA1.
Color measurements
In order to measure arch wires with small diameters and accurately determine their color using colorimetry, samples having minimum widths of 3 mm are required. Therefore, in the present study, the technique described by da Silva et al. was used with slight modifications [9]. Six wire segments were fixed tightly at both ends by using utility wax, as shown in Figure 2, and the colorimeter (Shade eye, Shofu, Kyoto, Japan) used in this study is shown in Figure 3. Colorimetric measurements were performed by contacting the measurement tip of the optical sensor to the sample. To measure the reference white color, the instrument tip was placed perpendicular to and in complete contact with the labial surface of the tooth [10]. Measurement values were obtained by averaging the values of the wire samples and VA1; all of which were measured three times. Color parameters were expressed using the Commission International de l’Eclairage (CIE) L*a*b* color space system. As the assessment of visual color is subjective, quantitative color systems with rectangular coordinates that allow an objective assessment of visual color were used in the present study. The three axes in this threedimensional color space were L*, a*, and b*. The L*-axis denotes the brightness, which increases as the value of L* increases. The value of a* represents the degree of redness (+a*) or greenness (-a*) of an object. The value of b* represents the degree of yellowness (+b*) or blueness (-b*) of an object. The value of C*, which represents the vividness of color, is calculated using C*=((a*)2+(b*)2)1/2 [11]. The color difference between VA1 and each wire (ΔE*ab) was calculated according to
ΔE*ab=((ΔL*)2+(Δa*)2+(Δb*)2)1/2 .
Furthermore, in order to relate the extent of color difference to a clinical environment, the data were converted to National Bureau of Standards (NBS) units by using the following formula: NBS units=ΔE*ab × 0.92. The definition of color changes quantified by NBS units was used [12] (Table 2).
| NBS Units | Definitions color differences | |
| 0.0-0.5 | Trace | Extremely slight change |
| 0.5-1.5 | Slight | Slight change |
| 1.5-3.0 | Noticeable | Perceivable change |
| 3.0-6.0 | Appreciable | Marked change |
| 6.0-12.0 | Much | Extremely marked change |
| 12.0+ | Very much | Change to other color |
Table 2: Color differences according to the National Bureau Standards.
Statistical analysis
We examined the experimental results by using an analysis of variance and Tukey Kramer’s multiple comparison tests in order to identify differences among the different values with the significance level set at p=0.01.
Results
The color measurement results of each wire are shown in Table 3. For the reference VA1, the lightness (L*), red-green hue (a*), and yellow-blue hue (b*) were obtained as 72.3, -1.2, and 8.0, respectively. We then compared the numerical value of each wire sample. The lightness (L*) of the silver-plated wire was 70.5, which was the closest to VA1 than other groups (0.01>p). The red-green hue (a*) for Epoxy Coating wire was the closest to VA1 at -1.3 than other groups (0.01>p). The yellow-blue hue (b*) and chroma (C*) of the silver-plated wire were approximately 8.4, which was the closest to VA1 than other groups (0.01>p).
| VA1 | Silver | Ever white | Nano white | TOMY | ORTHIKA | Gummetal | SS | |
| L* (Lightness) | 72.3 | 70.5 | 75.8 | 81.1 | 60.9 | 66.4 | 49.1 | 55.7 |
| a* (Hue) | -1.2 | 0.2 | -5 | -0.5 | 1.6 | -1.3 | 1 | 0.5 |
| b* (Hue) | 8 | 8.4 | 9 | 3.7 | 2.7 | 10.6 | 3.3 | 3 |
| C* (Chroma) | 8.1 | 8.4 | 10.3 | 3.7 | 3.1 | 10.7 | 3.4 | 3 |
Table 3: L*a*b* color space. The Lightness (L*) and the yellow-blue hue (b*) and chroma (C*) of the silver-plated wire was the closest to VA1.
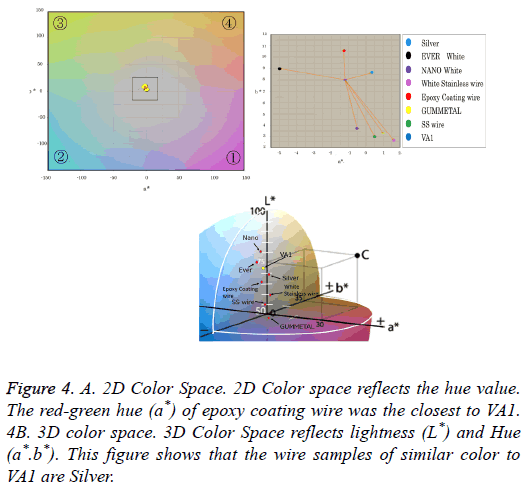
A chromaticity diagram is shown in Figure 4A; the diagram does not display the value of L* because it is a twodimensional diagram. The blue dot at the center of the figure denotes the value of VA1. The red-green hue (a*) of epoxy coating wire was the closest to VA1, followed by Nano White and the silver-plated wire, respectively. Three-dimensional color spaces are shown in Figure 4B. In this figure, the coordinates of the seven wire samples and VA1 are displayed. The 3D figure shows that the wire samples of similar color to VA1 are Silver, Ever White, and epoxy coating wire. Color differences (ΔE*ab) were observed between the wire samples; the values of VA1 and NBS were also different (Table 4). The color differences represent numerical differences between two colors. The color differences were calculated using a color difference formula that corrects differences between measured values and visual evaluations, i.e., a weakness of the L*a*b* color system. Weighting (SL, SC, and SH) and parametric (KL, KC, and KH) coefficients were corrected based on the differences in brightness (ΔL*), chroma (ΔC*), and hue (ΔH*). A low value indicates a low color difference from VA1. ΔE*ab values for Silver were significantly lower than other groups (0.01>p) (Table 4). The NBS values for Silver were significantly lower than other groups (0.01>p) (Table 4).
| Wire name | ΔE*ab | NBS units |
|---|---|---|
| Silver | 2.32 ± 0.17 | 2.13 ± 0.15 |
| Ever white | 5.26 ± 0.34 | 4.84 ± 0.31 |
| ORTHIKA | 6.49 ± 0.27 | 5.97 ± 0.25 |
| Nano white | 9.82 ± 0.42 | 9.03 ± 0.39 |
| TOMY | 12.88 ± 0.28 | 11.85 ± 0.25 |
| SS | 17.42 ± 0.17 | 16.03 ± 0.15 |
| Gummetal | 23.77 ± 0.51 | 21.87 ± 0.47 |
Table 4: ΔE* value were converted to National Bureau of Standards (NBS) units by the equation: NBS units=ΔE* × 0.92.
Discussion
Interest in color research in the field of dentistry has increased significantly over the past several decades [13]. Silver-plated wires are considered to approximate the color of natural teeth. Silver easily reproduces a particularly brilliant white color in electroplating. In the present study, silver was used as the reference color for natural teeth. Therefore, if the coating color was too dark or too bright, it contrasted with natural teeth. Therefore, in this study, we developed an arch wire by using the electroplating method.
Color differences were assessed by determining the ΔE*ab values of the wires by using the CIE Lab system. The CIE L*a*b* color space is currently one of the most popular and widely used systems for measurement of color, and it is appropriate for determining small color differences [7]. The advantage of this system is that color differences may be expressed in units related to visual perception. A large number of studies have been conducted on threshold levels for color differences in orthodontic appliances that are visually perceptible or clinically acceptable, and these threshold levels have been assessed using various standards. Douglas et al. [14] reported that the predicted value at which 50% of dentists perceived a color difference was 2.6 ΔE* units. In this case, the experimental values obtained for the silver-plated wire used in this study was lower than 2.3 ΔE* units. In addition to using ΔE* values, the extent of color differences between the wires and VA1 was assessed in NBS units. Based on the levels of color differences, the silver-plated wire showed a perceivable color difference in terms of NBS units. However, among the wires used in this experiment, the ΔE*ab of the silver-plated wire was the lowest. In the statistical analysis, the ΔE*ab of the silver-plated wire was significantly lower than those of all other wires. Ever White and Epoxy Coating were statistically similar, as were Nano White and whitestainlesswire.
Hue (a*, b*) may be determined to some extent even when wires are coated with white. The hue of the wires were measured in this experiment, with the minimum and maximum values of a*-excluding the Ever White and whitestainlesswirebeing -5 and 1.6, respectively. The maximum and minimum values of b* were 10.6 and 2.7, respectively. Hue (a*, b*) is considered to a certain extent while reproducing a white color in arch wires. However, the minimum and maximum values of lightness were 60.9 and 81.1, respectively. Among the values measured in this experiment, the value of L* is considered to markedly influence color difference, as the formula to calculate color difference is given by ΔE*ab=((ΔL*)2+(Δa*)2+(Δb*)2)1/2 [15]. Therefore, the lightness (L*) is most important for reducing color differences with respect to VA1.
We did not perform a subjective assessment by self-perception in the present study. Gerlach [16,17] investigated the selfperception of tooth whitening by bleaching and compared the results with objective measurements of changes in tooth color in a study using 50 subjects. The subjective assessment by selfperception with regard to tooth whitening was investigated by distributing a questionnaire among the subjects, and changes in tooth color were objectively measured using a digital image analysis system. The data were fitted into a probability model, and the obtained findings revealed that subjective responses to improvements in whiteness and satisfaction correlated with changes in the yellow-blue hue (b*) but not the lightness (L*) or red-green hue (a*). Thus, Db* (reduction in yellowness) is of primary perceptual importance to users of vital tooth bleaching products [18].
The color properties of wires and teeth are important for enabling orthodontists to achieve better results in aesthetics. The color of teeth differs for different patients. Previous studies measured the color of the maxillary central incisors of subjects. The obtained findings showed that the color parameters (L*, a*, b*) of natural teeth in subjects ranged from 51.1 to 73 for L*, from -0.9 to 5.4 for a*, and from -0.2 to 18.7 for b* [19-23]. Orthodontists need to carefully evaluate color especially that of the maxillary anterior teeth, prior to installing aesthetic wires because they are the most visible. This study only measured the color of wires compared to a single reference. In clinical practice, the harmonization of color for arch wires and brackets used for teeth is important in order to achieve the best aesthetics.
In the present study, we investigated silver-plated wire. The value of color difference between VA1 and the wire, ΔE*ab, was 2.3, which was the closest to the color of teeth. In NBS units, the color difference between the silver-plated wire and VA1 was “perceptible”. This value was the smallest among all the wires examined. However, in order to approximate tooth color, it is desirable for the value to be between 0 and 1.5 in terms of NBS units. In the statistical analysis, the values of ΔE*ab of the silver-plated wire were compared with those of six different brands of arch wires, and these values were approximately equal to the values of ΔE*ab for tooth color. This result shows that the silver-plated wire that we have developed is aesthetically more pleasing than the other wires examined. Based on the results of the present study, silver appears to be the most aesthetic coating material for orthodontic wires.
Conflicts of Interest
The authors have no conflicts of interest relevant to this article.
References
- da Silva DL, Mattos CT, de Araujo MV, de Oliveira Ruellas AC. Color stability and fluorescence of different orthodontic esthetic arch wires. Angle Orthod 2013; 83: 127-132.
- Ryu SH, Lim BS, Kwak EJ, Lee GJ, Choi S, Park KH. Surface ultrastructure and mechanical properties of three different white-coated NiTi arch wires. Scanning 2015; 9999: 1-8.
- Kawata T, Kohno S, Kaku M. Development of a new White metal orthodontic appliance with a spattering method. Chu Shikoku Ortho Soci 2007; 19: 67-71.
- Rongo R, Ametrano G, Gloria A, Spagnuolo G, Galeotti A, Paduano S. Effects of intraoral aging on surface properties of coated nickel-titanium arch wires. Angle Ortho 2014; 84: 665-672.
- Homma T. Plating Technologies. J InstElectrPackag 2003; 6: 616-624.
- Pan LC. Bright silver plating from the cyanide bath. A preliminary note. J Electro Soci 1931; 59: 329-335.
- Parker EA. Electroplating of gold alloys plating. Plating 1951; 38: 1134-1140.
- Prabu PS, Prabu NM, Kumar M, Abhirami M. Pannaikadu. Shade variance in ceramic restoration and shade tab: An in vitro study. J Pharm Bio Sci 2012; 2: 139-141.
- Lagouvardos P, Fougia A, Diamantopoulou S. Repeatability and interdevice reliability of two portable color selection devices in matching and measuring tooth color. J Pro Dent 2009; 101: 40-45.
- Toshihiro I, Yasuhiro T, Naomi M, Masaru Y, Kazutaka K. Color stability of laboratory glass-fiber-reinforced plastics for esthetic orthodontic wires. Korean J Orthod 2015; 45: 130-135.
- OBrien WJ, Hemmendinger H, Boenke KM, Linger JB, Groh CL. Color distribution of three regions of extracted human teeth. Dent Mater 1997; 13: 179-185.
- Mutlu-Sagesen L, Ergun G, Ozkan Y, Semiz M. Color stability of a dental composite after immersion in various media. Lamia Dent Mater J 2005; 24: 382-390.
- Stephen JC, Richard DT, Rade DP. Dental color matching instruments and systems. J Dent 2010; 38: 2-16.
- Douglas R, Steinhauer T, Wee S. Intraoral determination of the tolerance of dentists for perceptibility and acceptability of shade mismatch. J Pro Dent 2007; 97: 200-208.
- Filho H, Maia L, Araújo M, Eliast C. Colour stability of aesthetic brackets: ceramic and plastic. Aust Ortho J 2013; 29: 13-20.
- Gerlach R, Baker M, Silver el P. Objective and subjective Whitening response of two self-directed bleaching systems. A J Dent 2002; 15: 7-12.
- Gegauff AG, Rosenstiel SF, Langhout KJ, Johnston WM. Evaluating tooth color change from carbamide peroxide gel. J Am Dent Assoc 1993; 124: 65-72.
- Joiner A, Hopkinson I, Deng Y, Westland S. A review of tooth colour and whiteness. J Dent 2008; 36: 2-7.
- Rubino M, Barcia JA, Jimenez L, Romero J. Colour measurement of human teeth and evaluation of a colour guide. Color Res Appl 1994; 19: 19-22.
- Zhao Y, Zhu J. In vivo color measurement of 410 maxillary anterior teeth. Chin J Dent Res 1998; 1: 49-51.
- Odioso L, Gibb R, Gerlach R. Impact of demographic, behavioural, and dental care utilization parameters on tooth color and personal satisfaction. Compendium Continuing Edu Dent 2000; 29: 35-41.
- Russell MD, Gulfraz M, Moss BW. In vivo measurement of colour changes in natural teeth. J Oral Rehabil 2000; 27: 786-792.
- Hasegawa A, Motonomi A, Ikeda I, Kawsilver U. Color of natural tooth crown in Japanese people. Color Res Appl 2000; 25: 43-48.