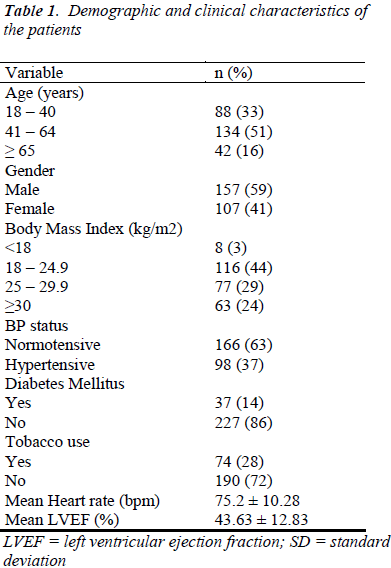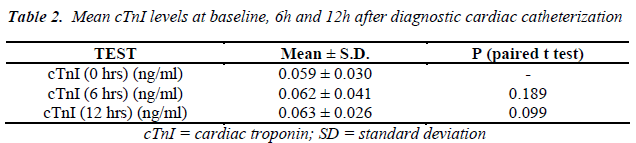ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2015) Volume 26, Issue 4
Analysis of Troponin I to determine myocardial injury following diagnostic cardiac catheterization
1Department of Cardiology, The First Affiliated Hospital of Anhui Medical University, Hefei, 230032, China
2Department of Cardiology, the First People’s Hospital of Anqing, Anqing 246003, China
3Department of Cardiovascular Medicine, the First Affiliated Hospital of Anhui Medical University, Hefei 230032,China
- *Corresponding Author:
- Ai-Ling Wang
Department of Cardiology
The First Affiliated Hospital of Anhui Medical University
No. 218 Jixi Road, Hefei
Anhui 230032 , China
Accepted July 24 2015
An increased level of serum cardiac troponin I is a specific biomarker for myocardial injury. The objective of this study was to evaluate the changes in cardiac troponin I levels to determine myocardial injury following diagnostic cardiac catheterization. A prospective cohort study was carried out between July 1 2006 and March 31 2012 among patients who underwent diagnostic cardiac catheterization in a tertiary hospital. Blood samples were collected at baseline and at 6- and 12- hours of the procedure to measure any changes in cardiac troponin I levels. A total of 264 adult patients; consisting of 157 males and 107 females, who underwent diagnostic cardiac catheterization were chosen for the study. Out of them 42 (16%) had a positive family history of coronary artery disease (CAD). Before the procedure, the mean cardiac troponin I level was 0.059 ± 0.030 ng/mL. However, the mean cardiac troponin I level after 6 hours of catheterization was 0.062 ± 0.041 ng/mL (P = 0.189) and was 0.063 ± 0.026 ng/mL (P = 0.099) after 12 hours. Average cardiac troponin I levels at 6- and 12- hours following diagnostic cardiac catheterization did not differ according to patients’ demographic or clinical characteristics (P >0.05). No in-cathlab complications and major adverse cardiac events were observed after onemonth. There were no significant changes in cardiac troponin I levels before or at 6- and 12- hours after diagnostic cardiac catheterization. This study, therefore, suggests that diagnostic cardiac catheterization does not appear to be associated with substantial subclinical myocardial injury.
Keywords
Troponin-I; catheterization; myocardial injury; diagnosis
Introduction
Cardiac troponin I is a sensitive and specific marker for detecting myocardial damage. This biomarker is normally not found in the circulation. Injured cardiac muscles release troponin from the contractile proteins into the blood stream and thus an increased level of serum cardiac troponin I may suggest myocardial injury [1-3]. Elevated serum cardiac troponin I levels have been reported to occur in 5% to 40% of patients undergoing percutaneous coronary intervention [4-6]. Two recent meta-analyses indicates that cardiac troponin I or cardiac troponin T elevation after elective percutaneous coronary intervention is indicative of an increase in long-term allcause mortality as well as the composite adverse events of all-cause mortality and acute coronary syndromes [7-8].
Although the safety of diagnostic cardiac catheterization has been demonstrated in several large cohort studies which showed very rare clinical complications following the procedure [9-12], very few studies have investigated for any evidence of subclinical myocardial injury resulting from the procedure. In a pediatric population, Kannankeril et al. documented elevations in serum cardiac troponin I levels occurring in 79% of cases immediately after the procedure, and in 86% of them six hours post-diagnostic cardiac catheterization [13]. However, these elevations in serum cardiac troponin I have not yet been shown to be predictive of future adverse cardiac events [14]. The objective of this study was to determine the occurrence of myocardial injury due to diagnostic cardiac catheterization procedure in adults by evaluating the elevation in serum cardiac troponin I levels.
Materials and Methods
Subjects
Using a cohort study design, we prospectively studied patients presenting to our hospital between July 1 2006 and March 31 2012; who underwent diagnostic cardiac catheterization. Patients whose pre-procedure serum cardiac troponin I levels were >0.15 ng/mL indicative of myocardial infarction, as well as pregnant or lactating mothers were excluded from the study. The study fully complied with the Declaration of Helsinki and the study protocol was approved by the hospital institutional review board. All patients gave a written informed consent to participate in the study.
Blood sample collection and serum cardiac troponin I measurement
Before the patients underwent diagnostic cardiac catheterization, blood samples were collected for the baseline profile. Patients with normal serum cardiac troponin I levels were further screened for their medical history, family history and any allergies, if present. Blood was sampled for serum cardiac troponin I into tubes with anticoagulant immediately before, and at 6- and 12- hours after the procedure. The samples were centrifuged at 3,000 rpm for 10 minutes, serum was separated and freezed at -70°C, batch thawed and analyzed using the paramagnetic-particle, chemiluminescent immunoenzymatic assay (Beckman, Coulter Inc., California). Using this assay, serum cardiac troponin I levels in the sample can be accurately measured within the reportable range of the lower limit of detection and the highest calibrated value (0.03 to 50 ng/ml). The upper limit of the 95% non-parametric range for a presumably healthy population is below the mean minimum detectable concentration of the assay (0.03 ng/ml), and the diagnostic cut-off value (as specified by the manufacturer) for myocardial damage based on receiveroperating characteristic (ROC) plots is ≥0.1ng/ml [14]. Therefore, any value equal to or higher than 0.1 ng/ml indicates evidence of myocardial damage.
Study endpoints and patient follow-up
A follow-up study was done at 30 ± 5 days after the procedure to report any in-cathlab complications and major adverse cardiac events. The study endpoint was taken as the occurrence of any major adverse cardiac events (MACE) at 30- days follow up. MACE is defined as cardiac death or Q wave myocardial infarction (MI/). Q-wave MI was diagnosed using clinical symptoms, new significant (> 1 mm depth) Q waves in at least two contiguous ECG leads, and a creatine kinase-MB concentration 99th centile of our own laboratories’ reference control value. Follow-up involved regular clinical review until the study endpoint was reached.
Statistical analysis
Data analyses were performed with SPSS 17.0 for Windows (Chicago, IL, USA). Continuous variables were summarized as mean (SD). Categorical variables were expressed as frequencies and percentages. We used paired Student’s t - test, to compare the means of pre- and postprocedural serum cardiac troponin I levels. A P value of <0.05 was considered significant.
Results
Patient characteristics
The patient cohort was comprised of 264 patients; 157 males and 107 females, aged ≥ 18 years; who underwent diagnostic cardiac catheterization. Of these patients, 42 (16%) had a positive family history of coronary artery disease and 10 (4%) had a past history of coronary artery disease. The demographic and clinical characteristics of the patients are as shown in Table 1. Overall, 98 (37%) were hypertensive, 37 (14%) had type II diabetes mellitus, 74 (28%) uses tobacco and 32 (12%) uses alcohol. Also, among the enrolled patients, 77 (29%) patients were overweight and 63 (24%) patients were obese [Table 1].
Cardiac troponin I level
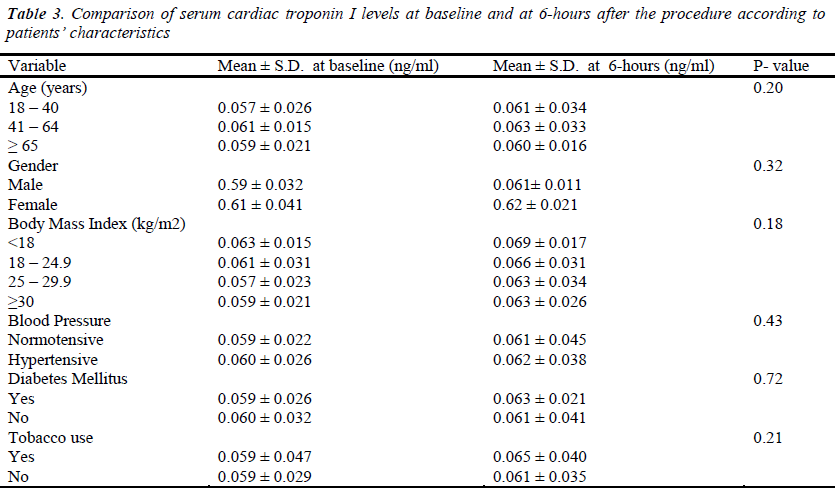
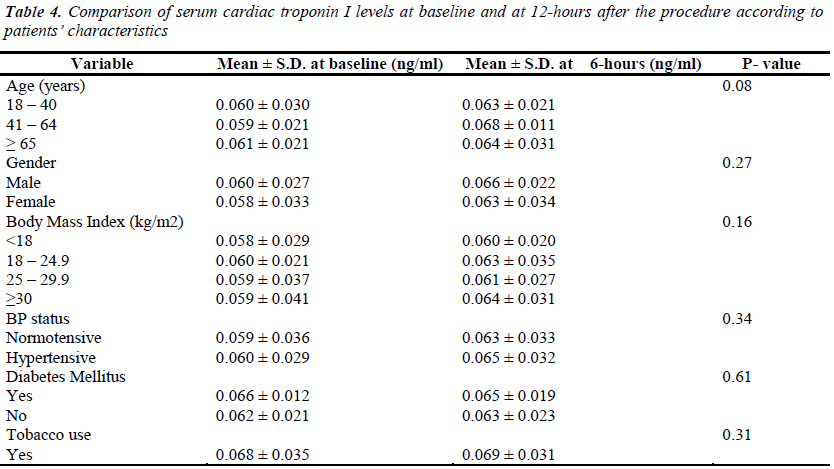
The mean serum cardiac troponin I levels of the patients, at different time intervals, are shown in Table 2. Its variations with patients’ demographic and clinical characteristics are shown in Tables 3 & 4. Before the procedure, the mean serum cardiac troponin I level was 0.059 ± 0.030 ng/mL. The mean serum cardiac troponin I level after 6 hours of catheterization was 0.062 ± 0.041 ng/mL (P = 0.189) and after 12 hours was 0.063 ± 0.026 ng/mL (P = 0.099) indicating that there was a minor statistically insignificant increase in the mean serum cardiac troponin I levels at 6 and 12 hours after diagnostic cardiac catheterization. Mean serum cardiac troponin I levels at 6- and 12- hours post diagnostic catheterization did not differ according to patients demographic or clinical characteristics (P >0.05). Overall, at 6- and 12- hours post catheterization only 2 (1%) asymptomatic patients had serum cardiac troponin I levels of 0.12ng/ml and 0.14ng/ml indicative of some myocardial injury. Furthermore, none of the patients studied reported any major adverse cardiac event during one-month follow-up and there were no in-cathlab complications reported.
Discussion
Diagnostic cardiac catheterization is an invasive procedure that is prone to complications, including death. Our primary aim was to establish the possibility of myocardial injury due to diagnostic cardiac catheterization. We collected complete data prospectively to avoid the bias associated with retrospective studies, particularly where data are collected from subsidiary source records such as theatre logbooks. Over the last two decades, there have been improvements in catheterization technique and technology that have reduced the morbidity and mortality associated with routine diagnostic coronary angiography, which according to published records is between 0.05% and 0.2% [8-12]. Severe and progressive myocardial ischemia starting during or shortly after percutaneous coronary intervention is an important cause of morbidity and mortality requiring emergency surgery [8-10]. Early recognition of ischemia post-catheterization and immediate surgery has been shown to improve survival rate [10].
In this study, there was no significant elevation of serum cardiac troponin I level following diagnostic cardiac catheterization, indicating no significant myocardial injury. Only 1% of the patients who were asymptomatic showed evidence of myocardial injury after diagnostic cardiac catheterization. However, there was no incidence of in-cathlab complications and after one month of follow-up none of the patients in this series showed any major adverse cardiac event. Serum cardiac troponin I elevations in this study was lower compared with a study in children which showed substantial elevations in serum cardiac troponin I following diagnostic cardiac catheterization [13-15]
The potential clinical benefit of this study is in highlighting the need for a cut-off value for the initiation of close monitoring for possible procedure-related complications where myocardial injury is suspected [13- 14]. In such cases, the clinical relevance of high levels of serum cardiac troponin I found in the patients may be difficult to interpret unless a good knowledge of the expected procedure–related elevation after an uneventful procedure is known [13]. Therefore, establishing a reference range of “expected”/normal elevation would allow levels of serum cardiac troponin I to be used as a valid diagnostic tool to detect “unexpected/sub-clinical” myocardial injury following diagnostic/therapeutic cardiac catheterization.
Conclusion
The result of this study extends our understanding of the potential uses of post-diagnostic catheterization elevations in cardiac biomarkers. Unlike what was observed in children, we found that there was no marked substantial myocardial damage from the procedure in adults [13-14]. Although the findings of this preliminary study are limited by small sample size, these results need to be confirmed on a larger scale, and with more diagnostic cases, for more validity, reliability and clinical applicability. Moreover, clinical and demographic predictors of myocardial injury evidenced by elevations in serum cardiac troponin I need to be better characterized.
Acknowledgements
We thank the patients who consented for the study and Chen E. for the editorial assistance.
Funding
This work was supported by 2013 revitalization projects in Anhui Higher Education: 2013zdjy066
References
- Hamm CW, Katus HA. New biochemical markers formyocardial cell injury.CurrOpinCardiol 1995; 10:355-360.
- Gaze DC, Collinson PO. Cardiac troponins as biomarkers of drug- and toxin-induced cardiac toxicity andcardioprotection. Expert Opin Drug MetabToxicol2005; 1: 715-725.
- Saadeddin SM,Habbab MA, Sobki SH, Ferns GA. Biochemical detection of minor myocardial injury after elective, uncomplicated, successful percutaneous coronary intervention in patients with stable angina: clinical outcome. Ann ClinBiochem 2002; 39: 392- 397.
- Cantor WJ, Newby LK, Christenson RH, Tuttle RH, Hasselblad V, Armstrong PW, et al. Cardiac Markers Substudy Investigators. Prognostic significance of elevated troponin I after percutaneous coronary intervention. J Am CollCardiol 2002; 39: 1738-1744.
- Gruberg L, Fuchs S, Waksman R, Pichard AD, Kent KM, Laird JR, et al. Prognostic value of cardiac troponin I elevation after percutaneous coronary intervention in patients with chronic renal insufficiency: a 12-month outcome analysis. Catheter CardiovascInterv 2002; 55: 180-181.
- Califf RM, Abdelmeguid AE, Kuntz RE, Popma JJ, Davidson CJ, Cohen EA, et al. Myonecrosis after revascularization procedures. J Am CollCardiol. 1998; 31: 241-251
- Feldman DN, Kim L, Rene AG, Minutello RM, Bergman G, Wong SC. Prognostic value of cardiac troponin-I or troponin-T following nonemergent percutaneous coronary intervention: A meta analysis. Catheter CardiovascInterv 2011; 77: 1020-1030.
- Nienhuis MB, Ottervanger JP, Bilo HJ, Dikkeschei BD, Zijlstra F. Prognostic value of troponin after elective percutaneous coronary intervention: a meta-analysis. Catheter CardiovascInterv 2008; 71: 318-324.
- Johnson LW, Lozner EC, Johnson S, et al. Coronary arteriography 1984-1987: a report of the Registry of the Society for Cardiac Angiography and Interventions. I. Results and complications. CathetCardiovascDiagn1989; 17: 5-10.
- de Bono D. Complications of diagnostic cardiac catheterisation: results from 34,041 patients in the United Kingdom confidential enquiry into cardiac catheter complications. The Joint Audit Committee of the British Cardiac Society and Royal College of Physicians of London.Br Heart J 1993; 70: 297-300.
- Noto TJ Jr, Johnson LW, Krone R, et al. Cardiac catheterization 1990: a report of the Registry of the Society for Cardiac Angiography and Interventions (SCA&I).CathetCardiovascDiagn 1991; 24: 75-83.
- West R, Ellis G, Brooks N; Joint Audit Committee of the British Cardiac Society and Royal College of Physicians of London. Complications of diagnostic cardiac catheterisation: results from a confidential inquiry into cardiac catheter complications. Heart 2006; 92: 810-814
- Kannankeril PJ, Wax DF, Pahl E. Elevations ofTroponin I after interventional cardiac catheterization. Cardiol Young 2001; 11: 375-378
- Kannankeril PJ, Pahl E, Wax DF. Usefulness of troponin I as a marker of myocardial injury after pediatric cardiac catheterization. Am J Cardiol 2002; 90: 1128-1132.
- Sharma S, Jackson PG, Cardiac troponins. J ClinPathol 2004; 57: 1025-1026.