ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 2
Anticancer effects of Gardenia jasminoides in HepG2 human hepatoma cells
1Department of Biological and Chemical Engineering, Chongqing University of Education, Chongqing 400067, China
2Chongqing Collaborative Innovation Center of Functional Food, Chongqing University of Education, Chongqing 400067, China
3Chongqing Engineering Technology Research Center for Functional Food, Chongqing University of Education, Chongqing 400067, China
4School of Pharmacy, Shanghai University of Traditional Chinese Medicine, Shanghai 201210, China
5Chongqing Three Gorges Natural Medicine Research Institute, Chongqing 404000, China
#These authors contributed equally to this work
- *Corresponding Author:
- Rui Wang
School of Pharmacy
Shanghai University of Traditional Chinese Medicine
Shanghai 201210, PR China
Yanhong Shi
School of Pharmacy
Shanghai University of Traditional Chinese Medicine
Shanghai 201210, PR China
Accepted on June 20, 2016
Background: Gardenia jasminoides is a natural plant, it has many biological activities. Its ability of cancer cell apoptosis needs to be researched.
Methods: In this study, the important biological activity materials were determined by high efficiency liquid chromatography. The in vitro anticancer effects were measured by MTT and RT-PCR assays in HepG2 human hepatoma cells.
Results: Gardenia jasminoides contained 1.03% genipin gentiobioside, 5.90% gardenoside, 1.26% crocin 1 and 0.17% crocin 2. Gardenia jasminoides had significantly strong HepG2 cells inhibitory effects, and highest effect 80.2% is showed in 400 μg/mL concentration of Gardenia jasminoides which is remarkable. the Gardenia jasminoides also have the ability to increase the mRNA expression of caspase-3, caspase-8, caspase-9, Bax, TRAIL, Fas, Fas/FasL, p53, p21, IκB-α and decrease the Bcl-2, BclxL, XIAP, cIAP-1, cIAP-2, survivin, NF-κB expression in HepG2 cells. The higher concentration of Gardenia jasminoides also showed the higher increasing or decreasing mRNA expression effects in HepG2 cells.
Conclusion: Gardenia jasminoides had a strong anticancer effect through its apoptosis inducing abilities in hepatic cancer; it could be used for the treatment as a medicine or health product in daily human life for good liver health.
Keywords
Gardenia jasminoides, Apoptosis, mRNA, HepG2 human hepatoma cells, Anticancer
Introduction
Gardenia jasminoides is dried mature fruit of Gardenia jasminoides jasminoides Ellis, which belongs to the family Rubiaceae [1]. It has many common beneficial effects for daily life, calm fret, diuresis, cold blood and detoxication. However, it has detumescence analgesic effect for topical use [2]. It includes many kinds of chemicals, such as iridoids, diterpenes (crocins), organic acids, flavonoids, coumarins, naphtha, saponins, lignanoids and polysaccharides. Meanwhile, the study emphasize that Gardenia jasminoides has a number of ingredients with strong physiological activities, mainly iridoids and crocins [3]. Genipin gentiobioside, gardenoside, crocin-1 and crocin-2 are important contents of Gardenia jasminoides, these contents have anticancer effects [3], gardenoside has in vitro anticancer effects in HepG2 liver cancer cells [4], both crocin-1 and crocin-2 could treat liver cancer [5].
As one of the most common malignant tumors, liver cancer is extremely severe and had a great impact on human health, which mostly common in males at the age of 40-50 [6]. As compared to other cancers mortality and morbidity is high, whereas survival rate is short, so it does severe harm to human body. The attack of liver cancer is related to various factors such as virus, chemical compounds, genetics and diet [6,7]. Among these, the main factors that promote liver cancer are alcohol, moldy food, food containing nitrosamines, foods which are lack of trace element selenium [8]. The bioactive elements in Gardenia jasminoides can adjust the harm to human body caused by unhealthy lifestyle, while they also have certain effects on many kinds of cancer cells [2,9]. Studies have shown that geniposide has obvious inhibiting effect on many kinds of cancer, especially pancreatic cancer [10,11]. In addition, modern pharmacological studies have shown that crocin has obvious anti-cancer function, especially inhibiting the cancer cells of cervical cancer, leukemia, bladder cancer, colon cancer, rectal cancer and so on [12-14].
Cancerous tumor form due to the inhibition of apoptosis [15]. It is generally seen that malignant tumor cells are caused by wild growth and excessive proliferation. At the same time, a series of oncogenes and proto-oncogenes in cancer cells are activated and present expressing state. The activation of these genes has close relationship with the development of tumor [16]. Among cancer genes, some belong to the growth factor genes, while others belong to the growth factor receptor genes. The activation and expression of these genes directly stimulate the growth of tumor cells, and these cancer genes and their expressed products are an important regulatory factors of cell apoptosis, as many cancer genes block the apoptosis process of tumor cells and after expression, lead to the increase of tumor cells [17]. Therefore, controlling tumors through controlling cell apoptosis, its mechanism is to rebuild tumor cell transformation system of apoptosis and activate the expression of death gene in cancer cells [18]. The important effective ingredient crocin in Gardenia jasminoides can obviously inhibit the multiplication of human leukemia cell line HL-60 and block cell cycle G0/G1 [19]. The apoptosis-inducing effect may be related to inhibit the expression of Bcl-2 genes and active the expression of Bax genes. Bcl-2 family genes are important cell apoptosis genes [20], and the cancer cell apoptosis-inducing effect of many bioactive substances in Gardenia jasminoides may be the key to the anti-cancer effect of Gardenia jasminoides. At the same time, the associative function of different substances may enhance the effect of Gardenia jasminoides. Therefore, this research study is to investigate the antioxidant effect of Gardenia jasminoides in vitro to clarify anti-cancer mechanism of Gardenia jasminoides by observing its cancer cell apoptosis-inducing effect.
Materials and Methods
Preparations of Gardenia jasminoides extract
Gardenia jasminoides was purchased in a local market (Enshi City, Hubei Province, China) and stored at -80°C prior to being freezedried to produce a powder. A 20fold volume of 70% methanol was added to the powdered Gardenia jasminoides and then extracted by sonic extract (power 250W, frequency 40 kHz). The methanol extract was evaporated using a rotary evaporator (N1100; Eywla, Tokyo, Japan), concentrated and then dissolved in dimethyl sulfoxide (DMSO; Amresco, Solon, OH, USA) to adjust to the stock concentration (20%) [21].
Liquid chromatogram experiment
Genipin gentiobioside, geniposide, crocin 1, crocin 2 standard samples were prepared respectively in 20 mL volumetric flasks. Methanol was added to make reference substance mother liquids with the concentrations of 1.03 mg/mL, 1.07 mg/mL, 0.103 mg/mlL and 1.02 mg/mL, then the four reference substance solutions were mix 5 times dilution and the mixed reference substance solution were got. The liquid chromatogram experiment was done as the follow chromatographicconditions, Waters ACQUITY UPLC BEH C18 chromatographic column (2.1 mm × 50 mm, 1.7 μm); acetonitrile (A) and 0.2 % phosphoric acid solution (B) were used as mobile phase by gradient elution (8%~20 % A in 0~3 min, 20~35 % A in 3~8 min); the detection wavelength were 238 nm in 0~5 min and 440 nm in 5~8 min; the flow rate was 0.3 mL/min; the column temperature was 30oC; the sample volume was 2 μL.
Cell preparation
HepG2 human hepatoma cells were obtained from American Type Culture Collection (ATCC; Manas-sas, VA, USA) and human normal hepatic cell line L-02 were obtained from Cell Research Institute of Chinese Academy of Sciences. The HepG2 and L-02 cells were cultured in RPMI 1640 medium (Thermo Fisher Scientific Inc.; Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Gibco-BRL; Grand Island, NY, USA) at 37°C in a humidified atmosphere with 5% CO2 (incubator model 311 S/N29035; Forma, Waltham, MA, USA). The medium was changed for every two days [22].
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) assay
Growth of HepG2 and L-02 cells by different concentrations of Gardenia jasminoides extract treatment were determined by the MTT assay. Different concentrations of Gardenia jasminoides (50, 100, 200 and 400 μg/mL) and the caspase inhibitor Z-VAD-FMK (10 μg/mL) were added to the RPMI 1640 medium. HepG2 cancer cells were seeded in 96 well plates at the density of 1 × 105 cells/mL in each well for 100 μL, and then the cells were incubated at 37 °C in 5% CO2. After the cancer cells were cultured for 24 h, the culture medium was aspirated and discarded from each well, and then the new culture medium with different concentrations of Gardenia jasminoides was added to each well. The cells were continuing incubated for 48 h and the culture medium was aspirated and discarded in each well again. Then the culture medium contained 5 mg/mL MTT solution (Amresco; Solon, OH, USA) was added in each well and cultured for 4 h. Following removal of the MTT solution culture medium, 100 μL of DMSO was added to each well and mixed for 30 min. Subsequently, the absorbance of each well was measured with an enzyme-linked immunosorbant assay (ELISA) reader (model 680; Bio-Rad; Hercules, CA, USA) at 540 nm [23].
RT-PCR assay
Total RNA from different groups HepG2 cells were isolated using Trizol reagent (Invitrogen; Carlsbad, CA, USA) according to the manufacturer’s recommendations. The HepG2 cells RNA were digested with RNase-free DNase (Roche; Basel, Switzerland) for 15 min at 37°C and purified using the RNeasy kit (Qiagen; Hilden, Germany) according to the manufacturer’s protocol. cDNA in cancer cells were synthesized from 2 μg of total RNA by incubation at 37°C for l h with avian myeloblastosis virus reverse transcriptase (GE Healthcare; Little Chalfont, United Kingdom) with random hexanucleotides according to the manufacturer’s instruction.
Sequences of primers used to specifically amplify the genes of interest are shown in Table 1. Amplification was performed in a thermal cycler (Eppendorf; Hamburg, Germany). The polymerase chain reaction (PCR) products were separated in 1.0% agarose gels and visualized with ethidium bromide staining [23].
| Gene Name | Sequence |
|---|---|
| Caspase-3 | Forward: 5′-CAA ACT TTT TCA GAG GGG ATC G-3′ |
| Reverse: 5′-GCA TAC TGT TTC AGC ATG GCA-3′ | |
| Caspase-8 | Forward: 5′-CCC CAC CCT CAC TTT GCT-3′ |
| Reverse: 5′-GGA GGA CCA GGC TCA CTT A-3′ | |
| Caspase-9 | Forward: 5′-GGC CCT TCC TCG CTT CAT CTC-3′ |
| Reverse: 5′-GGT CCT TGG GCC TTC CTG GTA T-3′ | |
| Bax | Forward: 5′-AAG CTG AGC GAG TGT CTC CGG CG-3′ |
| Reverse: 5′-CAG ATG CCG GTT CAG GTA CTC AGT C-3′ | |
| Bcl-2 | Forward: 5′-CTC GTC GCT ACC GTC GTG ACT TGG-3′ |
| Reverse: 5′-CAG ATG CCG GTT CAG GTA CTC AGT C-3′ | |
| Bcl-xL | Forward: 5′-CCC AGA AAG GAT ACA GCT GG-3′ |
| Reverse: 5′-GCG ATC CGA CTC ACC AAT AC-3′ | |
| XIAP | Forward: 5′-CCG TGC GGT TGC TTT AGT TGT C-3′ |
| Reverse: 5′-ATG GCA GGG TTC CTC GGG TAT-3′ | |
| cIAP-1 | Forward: 5′-TGAGCATGCAGACACATGC-3′ |
| Reverse: 5′-TGACGGATGAACTCCTGTCC-3′ | |
| cIAP-2 | Forward: 5′-AATGGAAGATAGCACGAT-3′ |
| Reverse: 5′-AGAAAGGCTGGAGTAAGA-3′ | |
| Survivin | Forward: 5′-CTT TCT CAA GGC CCA CCG CAT CT-3′ |
| Reverse: 5′-GCA CTT TCT CCG CAG TTT CCT C-3′ | |
| TRAIL | Forward: 5′-GGA ACC CAA GGT GGG TAG AT-3′ |
| Reverse: 5′-TCT CAC CAC ACT GCA ACC TC-3′ | |
| Fas | Forward: 5′-GAA ATG AAA TCC AAA GCT-3′ |
| Reverse: 5′-TAA TTT AGA GGC AAA GTG GC-3′ | |
| FasL | Forward: 5′-GGA TTG GGC CTG GGG ATG TTT CA-3′ |
| Reverse: 5′-TTG TGG CTC AGG GGC AGG TTG TTG-3′ | |
| p53 | Forward: 5′-GCT CTG ACT GTA CCA CCA TCC-3′ |
| Reverse: 5′-CTC TCG GAA CAT CTC GAA GCG-3′ | |
| p21 | Forward: 5′-CTC AGA GGA GGC GCC ATG-3′ |
| Reverse: 5′-GGG CGG ATT AGG GCT TCC-3′ | |
| NF-κB | Forward: 5′-CAC TTA TGG ACA ACT ATG AGG TCT CTG G-3′ |
| Reverse: 5′-CTG TCT TGT GGA CAA CGC AGT GGA ATT TTA GG-3′ | |
| IκB-α | Forward: 5′-GCT GAA GAA GGA GCG GCT ACT-3′ |
| Reverse: 5′-TCG TAC TCC TCG TCT TTC ATG GA-3′ | |
| GAPDH | Forward: 5′-CGG AGT CAA CGG ATT TGG TC-3′ |
| Reverse: 5′-AGC CTT CTC CAT GGT CGT GA-3′ |
Table 1. Sequences of reverse transcription-polymerase chain reaction primers were used in this study.
Statistical analysis
All experiments were determined three times and all the data were presented as mean ± standard deviation (SD). Differences between the mean values for individual groups were assessed with one-way analysis of variance (ANOVA) with Duncan's multiple range test. P<0.05 was considered to indicate a statistically significant difference. SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) was used to conduct the statistical analyses.
Results
The main constituent of Gardenia jasminoides
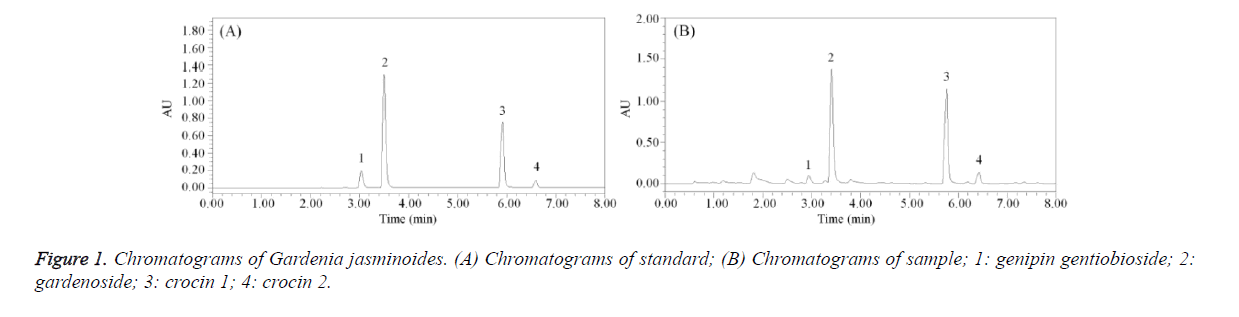
In preliminary experiments, observe extraction solvents (n-hexane, ethanol and methanol), extraction methods (cold leaching, ultrasonic extraction and reflux), extraction time (15, 30, 45, 60 mins) as well as the amount of extraction solvents (50, 75, 100, 125 times). Based on the principles of efficiency, convenience and saving, the extraction method was to use 0.2 g sample powder with 20 mL 70% methanol for 30 mins ultrasonic extraction. Respectively full scan reference substance solutions of all four index components, the result showed that the maximum absorption wavelength of genipin gentiobioside and geniposide were both at 238 nm, while the maximum absorption wavelength of crocin 1 and crocin 2 were both at 440 nm. Peak time of the first two components was from 2 to 5 mins, while peak time of the rest two components was from 5 to 8 mins. As a result, adding wavelength switching events makes the detection of wavelength switch automatically at the 5th minute, achieving detection of the four indicators at the same time.
The standard of genipin gentiobioside, gardenoside, crocin 1 and crocin 2 were dissolved in methanol, the standard solutions were adjusted in different concentrations (genipin gentiobioside: 1.0300, 0.5150, 0.2575, 0.1288, 0.0644 and 0.0322 mg/mL; gardenoside: 1.0700, 0.5350, 0.2675, 0.1338 , 0.0669 and 0.0334 mg/mL; crocin 1: 0.2575, 0.1288, 0.0644, 0.0322, 0.0161 and 0.0080 mg/mL; crocin 2: 0.0638, 0.0319, 0.0159, 0.0080, 0.0040 and 0.0020 mg/mL). Using liquid chromatogram, the standards were determined, the date of standard concentrations was horizontal ordinate (X) and the peak area was vertical ordinate (Y), the standard curves were calculated (Table 2).
| Component | Regression equation | Coefficient (r) | Linear range (mg/mL) |
|---|---|---|---|
| Genipin gentiobioside | Y=5 × 106X+17485 | 0.9999 | 0.0322~1.0300 |
| Gardenoside | Y=1 × 107X+96992 | 0.9999 | 0.0335~1.0700 |
| Crocin 1 | Y=4 × 107X+101764 | 0.9998 | 0.0081~0.2527 |
| Crocin 2 | Y=4 × 107X-5784.4 | 0.9999 | 0.0020~0.0638 |
Table 2. Linearity data obtained for four compounds.
The standard (genipin gentiobioside, gardenoside, crocin 1 and crocin 2) and Gardenia jasminoides sample were determined by liquid chromatogram (Figure 1). After calculating by standard curves, genipin gentiobioside, gardenoside, crocin 1 and crocin 2 contents of Gardenia jasminoides sample were calculated in 1.03%, 5.90%, 1.26% and 0.17% (Table 3).
| Compound | Genipin gentiobioside | Gardenoside | Crocin 1 | Crocin 2 |
|---|---|---|---|---|
| Content (%) | 1.03 | 5.90 | 1.26 | 0.17 |
Table 3. Four compound contents of Gardenia jasminoides.
Growth inhibitory effect of Gardenia jasminoides
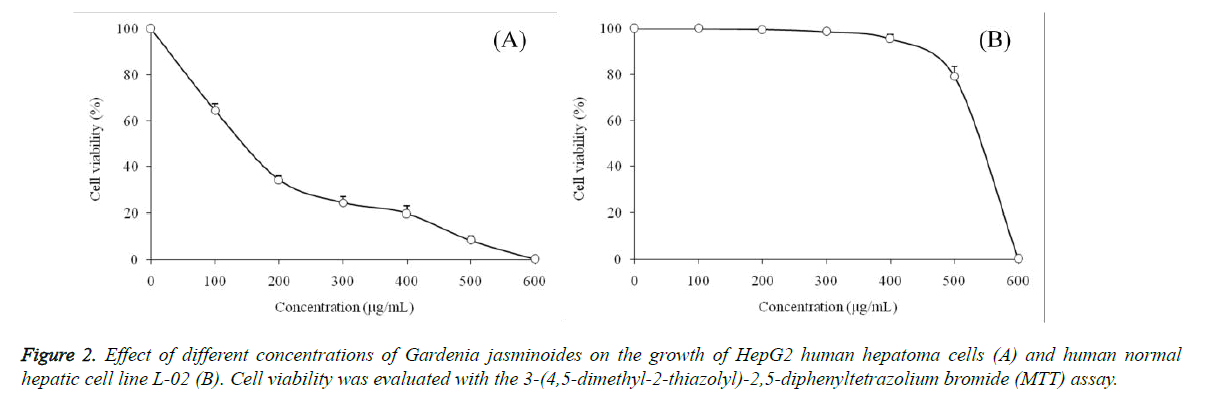
Anticancer effects of Gardenia jasminoides on HepG2 human hepatoma cells were evaluated using an MTT assay (Figure 2).
At the concentrations ranging from 0 to 600 μg/mL of Gardenia jasminoides, HepG2 cells were decreased by the sample in a concentration-dependent manner, at the concentration of 600 μg/mL, the survival rate of the HepG2 cells treated with Gardenia jasminoides reached 0%, but the normal hepatic cells L-02 were not significantly reduced. Consequently, 0-400 μg/mL Gardenia jasminoides were safe concentrations for normal human hepatic cells, and 50, 100, 200 and 400 μg/mL selected for subsequent human cancer cell experiments.
At the concentration of 50, 100, 200 and 400 μg/mL, Gardenia jasminoides extracts showed cancer cells growth inhibitory effects, and these effects were depends on the concentration of Gardenia jasminoides, higher the concentration of Gardenia jasminoides stronger the effect (Table 4). After caspase inhibitor Z-VAD-FMK (10 μg/mL) treatment, the HepG2 cells growth inhibitory of 400 μg/mL Gardenia jasminoides was decreased.
| Treatment | OD540 value | Inhibitory rate (%) | |
|---|---|---|---|
| Control (untreated) | 0.491 ± 0.006a | / | |
| Gardenia jasminoides (µg/mL) | 50 | 0.442 ± 0.010b | 10.0 ± 2.1E |
| 100 | 0.317 ± 0.011d | 35.4 ± 2.8C | |
| 200 | 0.168 ± 0.009e | 65.8 ± 1.7B | |
| 400 | 0.097 ± 0.014f | 80.2 ± 3.2A | |
| Z-VAD-FMK treatment | 0.407 ± 0.012c | 17.1 ± 2.4D | |
| a-f, A-E Mean values with different letters in the same column are significantly different (P<0.05) according to Duncan’s multiple-range test. Z-VAD-FMK treatment: 400 µg/mL Gardenia jasminoides + 10 µg/mL Z-VAD-FMK. | |||
Table 4. Growth inhibition of HepG2 human hepatoma cells by different concentrations of Gardenia jasminoides as evaluated by an MTT assay.
Gene expression of caspase family
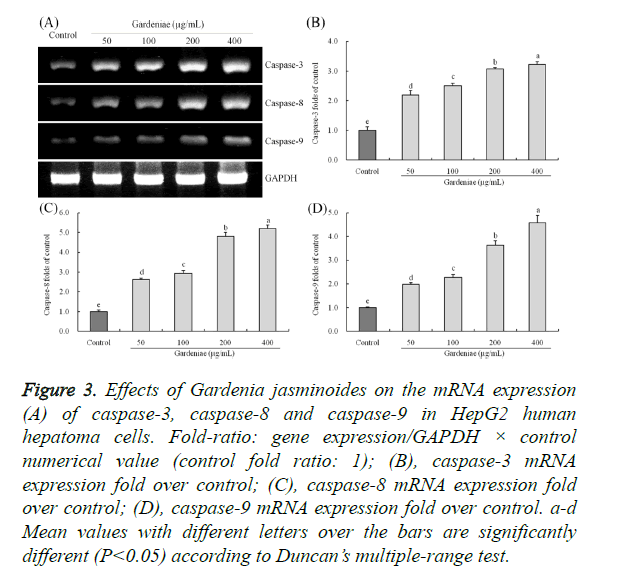
The caspase-3, caspase-8 and caspase-9 mRNA expressions were determined by RT-PCR assay (Figure 3). The untreated HepG2 cancer cells (control) had the lowest caspase-3, caspase-8 and caspase-9 mRNA expression. After treated with different concentrations of Gardenia jasminoides, the caspase-3, caspase-8 and caspase-9 mRNA expressions were raised, these expressions were significantly different (P<0.05) from control cancer cells, and 400 μg/mL has the highest concentration of Gardenia jasminoides treated cells had the strongest caspase-3 (3.23 folds of control), caspase-8 (5.19 folds of control) and caspase-9 (4.57 folds of control) mRNA expressions.
Figure 3. Effects of Gardenia jasminoides on the mRNA expression (A) of caspase-3, caspase-8 and caspase-9 in HepG2 human hepatoma cells. Fold-ratio: gene expression/GAPDH × control numerical value (control fold ratio: 1); (B), caspase-3 mRNA expression fold over control; (C), caspase-8 mRNA expression fold over control; (D), caspase-9 mRNA expression fold over control. a-d Mean values with different letters over the bars are significantly different (P<0.05) according to Duncan’s multiple-range test.
Gene expression of Bcl-2 family
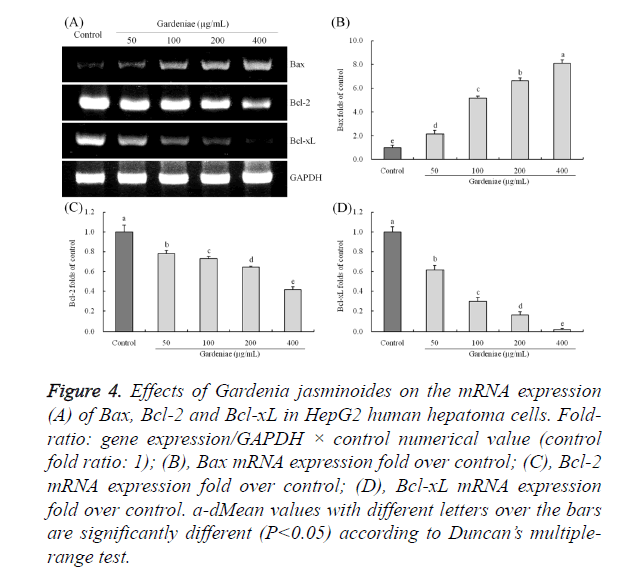
Gardenia jasminoides treated HepG2 cells showed stronger Bax mRNA expressions than control cells, the 50, 100, 200 and 400 μg/mL Gardenia jasminoides treated cells had 2.13, 5.16, 6.62 and 8.09 folds expression than control cells (Figure 4). The Bcl-2 and Bcl-xL expressions of Gardenia jasminoides treated cells were significantly (P<0.05) reduced as compared to the control cells, whereas 400 μg/mL Gardenia jasminoides treated cells has the lowest expressions (0.41 and 0.02 folds of control).
Figure 4. Effects of Gardenia jasminoides on the mRNA expression (A) of Bax, Bcl-2 and Bcl-xL in HepG2 human hepatoma cells. Fold-ratio: gene expression/GAPDH × control numerical value (control fold ratio: 1); (B), Bax mRNA expression fold over control; (C), Bcl-2 mRNA expression fold over control; (D), Bcl-xL mRNA expression fold over control. a-dMean values with different letters over the bars are significantly different (P<0.05) according to Duncan’s multiple-range test.
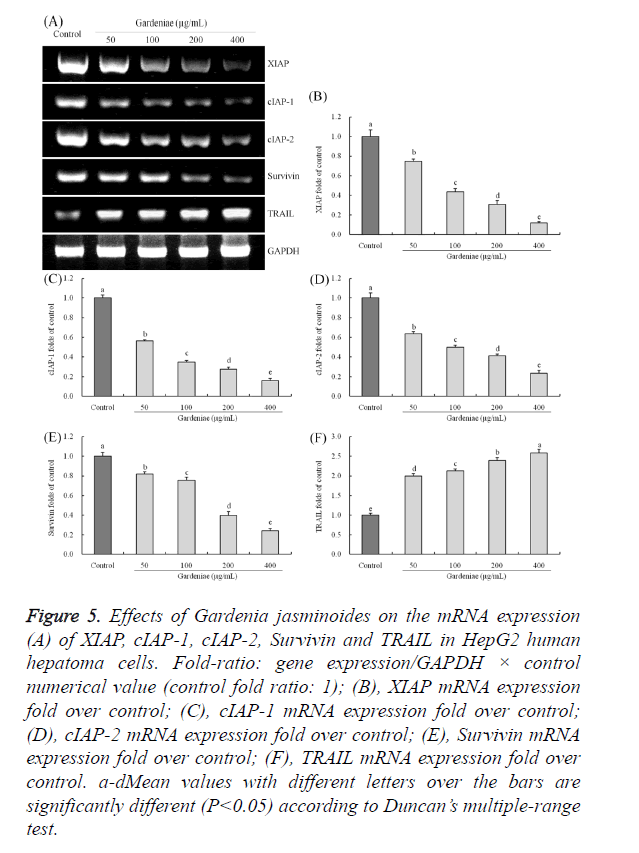
Gene expression of XIAP, cIAP-1, cIAP-2, survivin and TRAIL
The mRNA expression of XIAP, cIAP-1, cIAP-2, survivin were significantly (P<0.05) decreased, and TRAIL gene was (P<0.05) increased significantly by treatment of 50, 100, 200 and 400 μg/mL Gardenia jasminoides in HepG2 cancer cells (Figure 5). After treated with 50, 100, 200 and 400 μg/mL Gardenia jasminoides, the cancer cells showed 0.75, 0.44, 0.31, 0.12 folds XIAP expressions of control, 0.57, 0.35, 0.28, 0.16 folds cIAP-1 expressions of control, 0.64, 0.50, 0.41, 0.23 folds cIAP-2 expressions of control, 0.82, 0.76, 0.40, 0.24 folds survivin expressions of control and 2.00, 2.13, 2.39, 2.60 folds TRAIL expressions of control.
Figure 5. Effects of Gardenia jasminoides on the mRNA expression (A) of XIAP, cIAP-1, cIAP-2, Survivin and TRAIL in HepG2 human hepatoma cells. Fold-ratio: gene expression/GAPDH × control numerical value (control fold ratio: 1); (B), XIAP mRNA expression fold over control; (C), cIAP-1 mRNA expression fold over control; (D), cIAP-2 mRNA expression fold over control; (E), Survivin mRNA expression fold over control; (F), TRAIL mRNA expression fold over control. a-dMean values with different letters over the bars are significantly different (P<0.05) according to Duncan’s multiple-range test.
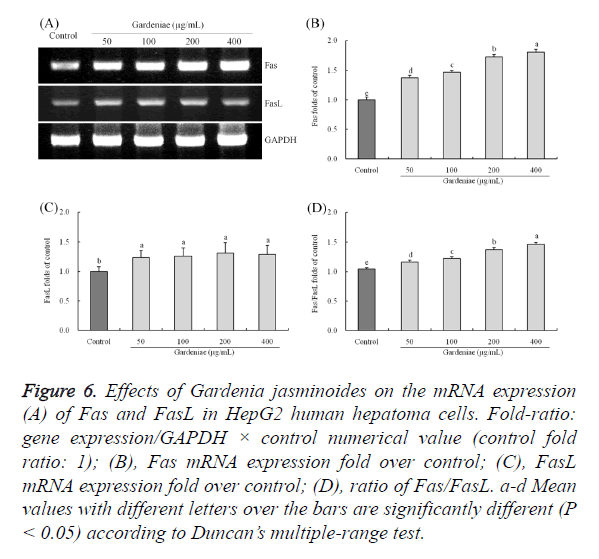
Gene expression of Fas and FasL
The Fas expressions in Gardenia jasminoides treated HepG2 cells were stronger than control cancer cells (Figure 6), the expressions of 50, 100, 200, 400 μg/mL Gardenia jasminoides treated cells were 1.37, 1.47, 1.73, 1.80 folds than the control cells. The FasL expressions in Gardenia jasminoides treated group cells were not have a meaningful difference (P<0.05), but the FasL expressions in Gardenia jasminoides treated cells were (P<0.05) raised remarkably as compared to the control cells. Fas/FasL ratio which (P<0.05) increased after treated with Gardenia jasminoides in HepG2 cells, and the ratio of 400 μg/mL concentration treatment were significantly high in 1.46.
Figure 6. Effects of Gardenia jasminoides on the mRNA expression (A) of Fas and FasL in HepG2 human hepatoma cells. Fold-ratio: gene expression/GAPDH × control numerical value (control fold ratio: 1); (B), Fas mRNA expression fold over control; (C), FasL mRNA expression fold over control; (D), ratio of Fas/FasL. a-d Mean values with different letters over the bars are significantly different (P < 0.05) according to Duncan’s multiple-range test.
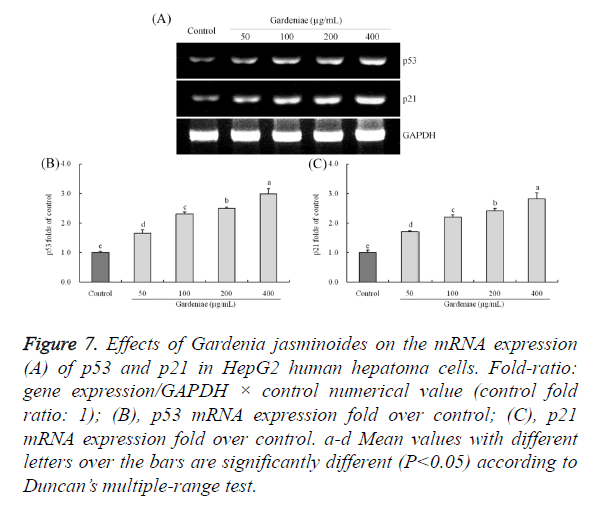
Gene expression of p53 and p21
The p53 and p21 mRNA expressions of HepG2 cells were (P<0.05) increased remarkably after different concentrations of Gardenia jasminoides treatment (Figure 7). The p53 and p21 expressions of 400 μg/mL (8.09 and 2.81 folds of control) treatment were higher than 50 μg/mL (2.13 and 1.71 folds of control), 100 μg/mL (5.16 and 2.21 folds of control) and 200 μg/mL (6.62 and 2.40 folds of control) treatment.
Figure 7. Effects of Gardenia jasminoides on the mRNA expression (A) of p53 and p21 in HepG2 human hepatoma cells. Fold-ratio: gene expression/GAPDH × control numerical value (control fold ratio: 1); (B), p53 mRNA expression fold over control; (C), p21 mRNA expression fold over control. a-d Mean values with different letters over the bars are significantly different (P<0.05) according to Duncan’s multiple-range test.
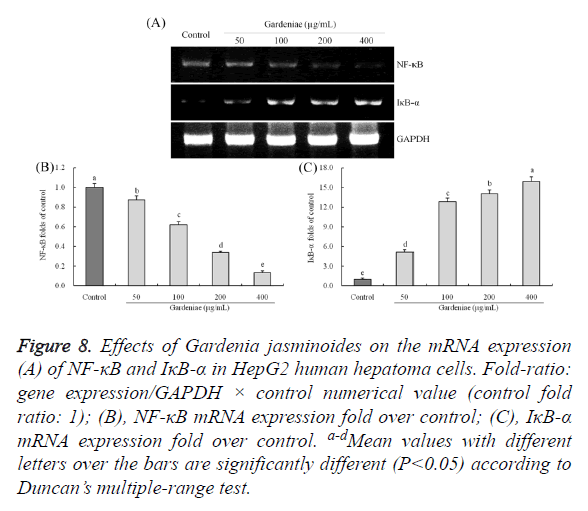
Gene expression of NF-κB and IκB-α
The m-RNA expression variation tendency of NF-κB and IκB- α in Gardenia jasminoides treated HepG2 cells were opposite (Figure 8). After treated with Gardenia jasminoides with cancer cells, NF-κB expressions were (P<0.05) reduced greatly and IκB-α expressions were (P<0.05) rose. The 50, 100, 200 and 400 μg/mL concentration treatment could make the NF-κB (0.87, 0.62, 0.34 and 0.13 folds of control) and IκB-α (5.13, 12.85, 14.03 and 15.90 folds of control) expressions deviate from control cells.
Figure 8. Effects of Gardenia jasminoides on the mRNA expression (A) of NF-κB and IκB-α in HepG2 human hepatoma cells. Fold-ratio: gene expression/GAPDH × control numerical value (control fold ratio: 1); (B), NF-κB mRNA expression fold over control; (C), IκB-α mRNA expression fold over control. a-d Mean values with different letters over the bars are significantly different (P<0.05) according to Duncan’s multiple-range test.
Discussion
Gardenia jasminoides has many physiological activities, and the antioxidant activities could help Gardenia jasminoides treat cancer [24,25]. Oxygen free radicals are one of the main causes of cell damage, aging and some diseases, Gardenia jasminoides contains many flavanoids, these flavanoids make a strong antioxidant activity, this effect might help Gardenia jasminoides has anticancer effects [25,26]. Gardenia jasminoides only had inhibitory effects in HepG2 cancer cells, but had no effects in normal hepatic cells L-02 at 0-400 μg/mL, its anticancer effects were determined at these concentrations in this study. Caspase-3 is a kind of proteolytic enzyme mediating apoptosis that makes it a key effect enzyme in caspase, which is an important effector molecule to perform apoptosis. It is widely expressed in normal human tissues and a variety of tumor tissues [27]. Studies have shown that the activation of Caspase-3 protease is closely related to the apoptosis of liver cancer cells. The activation of Caspase-3 exists in two different ways, including caspase-8 or caspase-10 dependent way and caspase-9 dependent way [28,29]. Caspase-3 is the core protease to trigger cell apoptosis protease cascade reaction, which plays a very important role. Caspase-3 is activates the liver cancer cell apoptosis induced by a variety of factors, Therefore, inhibiting the activity of caspase-3 can prevent apoptosis of liver cancer cell [30]. Z-VAD-FMK is a caspase inhibitor, Z-VAD-FMK treatment could determine whether the anticancer effects of drug from its caspase change ability [31]. In this study, after Z-VAD-FMK treatment, the anticancer effects of Gardenia jasminoides were reduce; these results showed that the anticancer of Gardenia jasminoides might through its caspase decreasing effects. Bcl-2 family including gene products Bcl-2 and Bcl-xL can prevent apoptosis, while gene products such as Bax fight against the above-mentioned genes usually promote cell apoptosis. Bcl-2 family plays an important regulating role in the activation process of caspase-3 [32]. Anti-apoptosis Bcl-2 family member Bcl-xL inhibits the oligomeric function of Apaf-1 molecules, making them dysfunctional. Mainly existing on the outer membrane of mitochondrion, anti-apoptotic Bcl-2 family inhibits the activation of pro caspase-9 by inhibiting the release of cytochrome C from mitochondrion, thus inhibiting the activation of caspase-9 which Apaf-1 depends on [33]. Pro-apoptosis Bcl-2 family members Bak and Bik can inhibit contact between Apaf-1 and Bcl-xL, thus promoting the activation of caspase which Apaf-1 depend on. Bid belongs to Bcl-2 family, which causes apoptosis. Studies have found that caspase-8 can crack Bid, and cracked Bid can react with Bcl-xL which exists on the surface of mitochondrion to induce mitochondrion to release cytochrome C, which activates caspase-9. Caspase-8 and caspase-9 are connected by Bid. This kind of crosstalk can amplify the process of caspase activation [34].
As one TNF family member, APRIL can promote the proliferation and apoptosis of tumor cells by interfering with apoptosis signaling pathway or regulating expression of related anti-apoptotic genes in autocrine or paracrine way. APRIL shows high expression in many kinds of malignant tumor tissues, while only a small amount expresses in normal tissues [35]. As an important member in IAPs family, XIAP has special BIR structure, so it can combine with caspase and inhibit its activity, which promotes the occurrence and development of tumors. XIAP shows high expression in many tumor tissues and cells [36]. Survivin is a kind of strong apoptosis-inhibiting factor, and many studies have shown that high expression of survivin inhibits apoptosis induced by Fas, Bax, caspases, tumor necrosis factors, as well as anti-cancer drugs [37]. At the same time, many studies have also shown that survivin may be regarded as an indicator for diagnosing liver cancer and predicting metastasis, recurrence and prognosis of liver cancer, so it is a suitable target for tumor biological treatment [38-40].
As a kind of transcription factor, NF-κB fights against apoptosis mainly by regulating downstream anti-apoptotic genes. NF-κB can increase apoptosis functional elements, such as Bcl-2 family and IAP family, etc [41]. Bcl-2 is a recognized anti-apoptotic protein, as many tumor cells show high expression of Bcl-2 and play an anti-apoptotic role through Bcl-2. Besides, recent study finds that Bcl-2 is one of the downstream genes of NF-κB [42]. As endogenous cells, IAPs are apoptosis-inhibiting proteins, including cIAP1, cIAP2, XIAP and survivin, etc. cIAP1 and cIAP2 can not only directly inhibit the activation of caspases 3, caspases 7 and caspases 9 to inhibit cell apoptosis, but also induce the activation of NF- κB [43]. On one hand, gene coding sequence of cIAP1 and CIAP2 has κB site, so the activation of NF-κB can increase the expression of cIAP1 and cIAP2. On the other hand, a large number of expressed cIAP1 and cIAP2 can degrade IκB by combining with TRAF, leading to the activation of NF-κB. This forms the positive feedback loop mechanism, which causes high expression of NF-κB, cIAP1 and cIAP2 in order to prevent the apoptosis of tumor cells [44]. FLIP gene coding sequence has κB site, so the high expression of activated NF- κB protein in tumor cells can increase the transcriptional expression of FLIP, which prevents tumor cells from controlling by Fas-mediated apoptosis signals [45]. After cross-linking with FasL or anti-Fas antibody, Fas can conduct death signals into cells and induce cell apoptosis. Fas/FasL is also one of CTL mechanisms. FasL is natural ligand of Fas and exists on the surface of CTL. By combination between FasL and CTL cells, the expression of Fas cells can lead to cell apoptosis. As a result, Fas/FasL is a kind of immune defense system, which is directly related to cancer cell apoptosis [46].
P53 protein is a key ingredient checkpoint in cell cycle G1. The checkpoint of Gl can examine whether DNA is damaged before synthesis. If there is damage, it needs to be repaired at first. If repair fails, gene transcription which induces apoptosis will be activated, such as activation of p21 gene [47]. Through cyclin-dependent kinase (CDK), cell cycle stops at G1 and begins apoptosis. Studies have shown that p53 gene mutation often happens in HCC. Besides, p53 mutation is closely related to histological grading of liver cancer as well as attack and metastasis of liver cancer. If the expression of P21 gene, an important anti-cancer gene, is abnormal, the regulation of cell proliferation and differentiation will be influenced, leading to malignant tumor [48,49]. By depending on p53 way, p21 is involved in cell cycle inhibition. Studies have shown that within the cells, when the expression of p21 is low, the combination between a molecule of p21 and cyclin-PCNA-CDK is essential during cell cycle. When the expression of p21 is high, the combination between more molecules of p21 and the above compounds inhibits cell cycle [50,51].
Genipin gentiobioside, geniposide, crocin 1, crocin 2 are all substances with strong biological activity. Genipin gentiobioside can treat heart failure [52], while geniposide, crocin 1, crocin 2 are confirmed to have anti-cancer effects, such as on colon cancer and bladder cancer [12,53]. It has been proved that gardenoside has certain effect on HepG2 liver cancer cells cultured in vitro. The anti-cancer effect of Gardenia jasminoides also comes from these four kinds of ingredients. The four kinds of ingredients can induce cancer cell apoptosis through its influence on the expression of Bcl-2 and other kind of genes, which may be the main mechanism of how Gardenia jasminoides achieve its anti-cancer effect. Gardenia jasminoides contains genipin gentiobioside, geniposide, crocin 1and crocin 2, these chemical compositions has the clear anticancer effects [54], geniposide injection fits three compartment model in rat, its detention time in body was short [54], pharmacokinetics of crocin 1and crocin 2 were also determined in vitro, their anticancer effects were confirmed by their cancer cells cytotoxic effect [55]. In this study, geniposide content was highest, the key effect of Gardenia jasminoides might from geniposide.
In summary, we used high performance liquid chromatography to analyze the profound chemical composition contents and also used various in vitro experimental methods, including MTT, RT-PCR assays, to evaluate the HepG2 cancer cells apoptosis inducing effects of Gardenia jasminoides. Gardenia jasminoides was rich in genipin gentiobioside, gardenoside, crocin 1 and crocin 2. These rich bioactivity chemical substances made Gardenia jasminoides having strong cancer cells inhibitory effects. And Gardenia jasminoides also could raise the mRNA expression of caspase-3, caspase-8, caspase-9, Bax, TRAIL, Fas, Fas/FasL, p53, p21, IκB-α and reduce the Bcl-2, Bcl-xL, XIAP, cIAP-1, cIAP-2, survivin, NF-κB mRNA expression in HepG2 cells. Gardenia jasminoides might be used as liver cancer treatment traditional Chinese medicine or functional food for liver protection.
Acknowledgments
This research project was supported by Science and Technology Innovation Action Plan of Shanghai (14495800400), Basic Research Project of Chongqing Frontier and Application (cstc2014jcyjA1466) and Project of Chongqing Social Science and Technology Innovation (cstc2015shmszx120087), China.
References
- Zhu XY, Mang YL, Shen FQ, Xie J, Su WK. Homogenate extraction of gardenia yellow pigment from Gardenia Jasminoides Ellis fruit using response surface methodology. J Food Sci Technol 2014; 51: 1575-1581.
- Lin WH, Kuo HH, Ho LH, Tseng ML, Siao AC, Hung CT, Jeng KC, Hou CW. Gardenia jasminoides extracts and gallic acid inhibit lipopolysaccharide-induced inflammation by suppression of JNK2/1 signaling pathways in BV-2 cells. Iran J Basic Med Sci 2015; 18: 555-562.
- Uddin R, Saha MR, Subhan N, Hossain H, Jahan IA, Akter R, Alam A. HPLC-analysis of polyphenolic compounds in Gardenia jasminoides and determination of antioxidant activity by using free radical scavenging assays. Adv Pharm Bull 2014; 4: 273-281.
- Zhou J, Li SP, Fng KX, Cao PT, Shi J, Yang WB. Impact of Gardenoside on insulin receptor and nuclear factor kappa B of insulin resistant in HepG2 cells. Chin J Clin Pharmacol 2015; 31: 362-365.
- Liu Y, Gu TM, Zhou H. Pharmacological studies of crocins. J Chengdu Univ (Nat Sci Edit) 2008; 27: 16-19.
- Vogtmann E, Li HL, Shu XO, Chow WH, Ji BT, Cai H, Gao J, Zhang W, Gao YT, Zheng W, Xiang YB. Dietary glycemic load, glycemic index, and carbohydrates on the risk of primary liver cancer among Chinese women and men. Ann Oncol 2013; 24: 238–244.
- Yang Y, Wu QJ, Xie L, Chow WH, Rothman N, Li HL, Gao YT, Zheng W, Shu XO, Xiang YB. Prospective cohort studies of association between family history of liver cancer and risk of liver cancer. Int J Cancer 2015; 135: 1605–1614.
- Zhang W, Shu XO, Li HL, Yang G, Cai H, Ji BT, Gao J, Gao YY, Zheng W, Xiang YB. Vitamin intake and liver cancer risk: a report from two cohort studies in China. J Natl Cancer Inst 2012; 104: 1174–1182.
- Park J, Seok JK, Suh HJ, Boo YC. Gardenia jasminoides Extract attenuates the UVB-induced expressions of cytokines in keratinocytes and indirectly inhibits matrix metalloproteinase-1 expression in human dermal fibroblasts. Evid Based Complement Alternat Med 2014; 2014: 429246.
- Wang ZC, Yang XL, Zhang K, Xiao P. Research on pharmacological activities of Gardenoside. J Henan Univ Sci Tech (Med Sci) 2012; 30: 159–160.
- Huang XY, Zhao Y, Mi JX, Yang Y, Ding Y, Zhang CC, Zhang T, Cai ZZ. Effect of genipin and the clathrate on inhibiting growth of human PANC-1 in BALB/C-nu Mouse. Acta Univ Tradit Med Sinensis Pharm Shanghai 2015; 29: 43–46.
- Aung HH, Wang CZ, Ni M, Fishbein A, Mehendale SR, Xie JT, Shoyama AY, Yuan CS. Crocin from Crocus Sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp Oncol 2007; 29: 175–180.
- Samarghandian S, Borji A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacognosy Res 2014; 6: 99–107.
- Bhandari PR. Crocus sativus L. (saffron) for cancer chemoprevention: A mini review. J Tradit Complement Med 2015; 5: 81–87.
- Su ZY, Yang ZZ, Xu YQ, Chen YB, Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer 2015; 14: 48.
- Ranzani M, Annunziato S, Adams DJ, Montini E. Cancer gene discovery: exploiting insertional mutagenesis. Mol Cancer Res 2013; 11: 1141–1158.
- Witsch E, Sela M, Yarden Y. Roles for growth factors in cancer progression. Physiology (Bethesda) 2010; 25: 85–101.
- Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int 2014; 2014: 150845.
- Ren Q, Sun LH, Liu XM, Liu CY, Quan LH, Xie C, Liao YH. Active constituents from Gardenia jasminoides against acute lymphoblastic leukemia. J Guangdong Pharm Coll 2009; 25: 141-143.
- Kvansakul1 M, Hinds MG. Structural biology of the Bcl-2 family and its mimicry by viral proteins. Cell Death Dis 2013; 4: e909.
- Zhao X. Hawk tea (Litsea coreana Levl. var. lanuginose) attenuates CCl4 ‑induced hepatic damage in Sprague‑Dawley rats. Exp Ther Med 2013; 5: 555-560.
- Zhao X, Kim SY, Park KY. Bamboo salt has in vitro anticancer activity in HCT-116 cells and exerts anti-metastatic effects in vivo. J Med Food 2013; 16: 9-19.
- Pang L, Zhao X, Liu WW, Deng J, Tan XT, Qiu LH. Anticancer effect of ursodeoxycholic acid in human oral squamous carcinoma HSC-3 cells through the caspases. Nutrients 2015; 7: 3200-3218.
- Wu XT, Wu MJ, Wang QP, Cai YY. Study on ultrasonic extraction and optimization of gardenia flavonoids by uniform design method and its antioxidant activity. J Chinese Inst Food Sci Technol 2012; 12: 66-71.
- Singh AK, Pandey P, Tewari M, Pandey HP, Gambhir IS, Shukla HS. Free radicals hasten head and neck cancer risk: A study of total oxidant, total antioxidant, DNA damage, and histological grade. J Postgrad Med 2016; 62: 96-101.
- Blair CK, Kelly AS, Steinberger J, Eberly LE, Napurski C, Robien K, Neglia JP, Mulrooney DA, Ross JA. Feasibility and preliminary efficacy of the effects of flavanoid-rich purple grape juice on the vascular health of childhood cancer survivors: a randomized, controlled crossover trial. Pediatr Blood Cancer 2014; 61: 2290-2296.
- Walters J, Pop C, Scott FL, Drag M, Swartz P, Mattos C, Salvesen GS, Clark AC. A constitutively active and uninhibitable caspase-3 zymogen efficiently induces apoptosis. Biochem J 2009; 424: 335-345.
- Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ. Ordering the cytochrome c–initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9–dependent manner. J Cell Biol 1999; 144: 281-292.
- Raulf N, El-Attar R, Kulms D, Lecis D, Delia D, Walczak H, Papenfuss K, Odell E, Tavassoli M. Differential response of head and neck cancer cell lines to TRAIL or Smac mimetics is associated with the cellular levels and activity of caspase-8 and caspase-10. Br J Cancer 2014; 111: 1955-1964.
- Ma JH, Zou CB, Guo LD, Seneviratne DS, Tan XP, Kwon YK, An JY, Bowser R, DeFrances MC, Zarnegar R. A novel death defying domain in met entraps the active site of caspase-3 and blocks apoptosis in hepatocytes. Hepatology 2014; 59: 2010-2021.
- Xia L, Bao Y, Yuan YZ, Zhang XJ. The effect of all-trans retinoic acid and caspase inhibitor Z-VAD-FMK on apoptosis of pancreatic cancer cells. Chin J Pancreatol 2003; 3: 198-202.
- Eichhorn JM, Alford SE, Sakurikar N, Chambers TC. Molecular analysis of functional redundancy among anti-apoptotic Bcl-2 proteins and its role in cancer cell survival. Exp Cell Res 2014; 322: 415-424.
- Li T, Zeng LL, Gao W, Cui MZ, Fu XQ, Xu XM. PSAP induces a unique Apaf-1 and Smac-dependent mitochondrial apoptotic pathway independent of Bcl-2 family proteins. Biochim Biophys Acta 2013; 1832: 453-474.
- Guo XX, Guo Q, Li Y, Lee SK, Wei XN, Jin YH. Ginsenoside Rh2 induces human hepatoma cell apoptosis via Bax/Bak triggered cytochrome c release and caspase-9/caspase-8 activation. Int J Mol Sci 2012; 13: 15523-15535.
- Pelekanou V, Kampa M, Kafousi M, Darivianaki K, Sanidas E, Tsiftsis DD, Stathopoulos EN, Tsapis A, Castanas E. Expression of TNF-superfamily members BAFF and APRIL in breast cancer: Immunohistochemical study in 52 invasive ductal breast carcinomas. BMC Cancer 2008; 8: 76.
- Flanagan L, Sebastià J, Tuffy LP, Spring A, Lichawska A, Devocelle M, Prehn JHM, Rehm M. XIAP impairs Smac release from the mitochondria during apoptosis. Cell Death Dis 2010; 1: e49.
- Jaiswal PK, Goel A, Mittal RD. Survivin: A molecular biomarker in cancer. Indian J Med Res 2015; 141: 389-397.
- Bai HB, Gayyed MF, Lam-Himlin DM, Klein AP, Nayar SK, Xu Y, Khan M, Argani P, Pan DJ, Anders RA. Expression of yes associated protein, YAP, modulates survivin expression in primary liver malignancies. Hum Pathol 2012; 43: 1376-1385.
- Zhao XX, Ogunwobi OO, Liu C. Survivin inhibition is critical for Bcl-2 inhibitor-induced apoptosis in hepatocellular carcinoma cells. PLoS One 2011; 6: e21980.
- Dai DJ, Lu CD, Lai RY, Guo JM, Meng H, Chen WS, Gu J. Survivin antisense compound inhibits proliferation and promotes apoptosis in liver cancer cells. World J Gastroenterol 2005; 11: 193-199.
- Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 2013; 12: 86.
- Braun F, Trécesson SDC, Bertin-Ciftci J, Juin P. Protect and serve: Bcl-2 proteins as guardians and rulers of cancer cell survival. Cell Cycle 2013; 12: 2937-2947.
- Makhov P, Golovine K, Uzzo RG, Rothman J, Crispen PL, Shaw T, Scoll BJ, Kolenko VM. Zinc chelation induces rapid depletion of the X-linked inhibitor of apoptosis (XIAP) and sensitizes prostate cancer cells to TRAIL-mediated apoptosis. Cell Death Differ 2008; 15: 1745-1751.
- Mahoney DJ, Cheung HH, Lejmi Mrad R, Plenchette S, Simard C, Enwere E, Arora V, Mak TW, Lacasse EC, Waring J, Korneluk G. Both cIAP1 and cIAP2 regulate TNFα-mediated NF-κB activation. Proc Natl Acad Sci USA 2008; 19: 11778-11783.
- Yang HJ, Wang MA, Wang L, Cheng BF, Lin XY, Feng ZW. NF-κB regulates caspase-4 expression and sensitizes neuroblastoma cells to Fas-Induced apoptosis. PLoS One 2015; 10: e0117953.
- Siena L, Pace E, Ferraro M, Di Sano C, Melis M, Profita M, Spatafora M, Gjomarkaj M. Gemcitabine sensitizes lung cancer cells to Fas/FasL system-mediated killing. Immunology 2014; 141: 242-255.
- Hyun SY, Jang YJ. p53 activates G1 checkpoint following DNA damage by doxorubicin during transient mitotic arrest. Oncotarget 2015; 10: 4804-4815.
- Giovannini C, Minguzzi M, Baglioni M, Fornari F, Giannone F, Ravaioli M, Cescon M, Chieco P, Bolondi L, Gramantieri L. Suppression of p53 by Notch3 is mediated by Cyclin G1 and sustained by MDM2 and miR-221 axis in hepatocellular carcinoma. Oncotarget 2014; 5: 10607-10620.
- Zhang MF, Zhang ZY, Fu J, Yang YF, Yun JP. Correlation between expression of p53, p21/WAF1, and MDM2 proteins and their prognostic significance in primary hepatocellular carcinoma. J Transl Med 2009; 7: 110.
- Funk JO, Waga S, Harry JB, Espling E, Stillman B, Galloway DA. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Gene Dev 1997; 11: 2090-2100.
- Waga S, Stillman B. Cyclin-dependent kinase inhibitor p21 modulates the DNA primer-template recognition complex. Mol Cell Biol 1998; 18: 4177-4187.
- Chen L, Luo ZK, Peng GP, Li XH, Liu L, Shen XB, Wang ZY. The cardiac systolic and diastolic effects of genipin-1-β-D-gentiobioside in the experimental heart failure. Pharm Clin Chinese Mat Med 2013; 29: 39-41.
- Zhao P, Luo CL, Wu XH, Hu HB, Lv CF, Ji HY. Proliferation apoptotic influence of crocin on human bladder cancer T24 cell line. China J Chinese Mat Med 2008; 33: 1869-1873.
- Li D, Yan S. Advance of jasminoidin’s pharmacokinetic research. Tianjin Pharm 2012; 24: 51-54.
- Mei LL, Hu X. Study on the pharmacology and pharmacokinetic of crocin in Gardenia Jasminoides Ellis. Chin J Clin Pharmacol Ther 2013; 18: 837-840.