ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2016) Health Science and Bio Convergence Technology: Edition-I
Antimicrobial activity of lichen Bryoria capillaris and its compound barbatolic acid
1Department of Biology, Faculty of Sciences, Anadolu University, Eskişehir, Turkey
2Department of Chemistry, Faculty of Sciences, Anadolu University, Eskişehir, Turkey
- *Corresponding Author:
- Nalan Yılmaz Sarıözlü
Department of Biology
Faculty of Sciences
Anadolu University
Turkey
Accepted date: November 30, 2016
Antibacterial, antifungal activity and MIC values of the acetone, methanol and chloroform extracts of the lichen Bryoria capillaris (Ach.) and its barbatolic acid constituent have been investigated against Gram-positive and Gram-negative bacteria, yeasts and filamentous fungi using disk-diffusion method. Microplate Alamar Blue Assay was also carried out to determine the antitubercular activity. The extracts of lichen were shown considerable antimicrobial effect to tested pathogenic microorganisms. Also, the isolated compound barbatolic acid significantly affected against the infectious pathogen Mycobacterium tuberculosis H37Rv with a MIC value of 31.25 μg/ml. Present study is the first report on antimicrobial activities of barbatolic acid constituent of lichen B. capillaris.
Keywords
Antimicrobial activity, Lichen extracts, Bryoria capillaris, Barbatolic acid.
Introduction
Lichens are stable symbiotic organisms associations of fungus and algae and/or a cyanobacterium [1]. They grow in many ecosystems such as on and within rocks, on soil, tree bark, shrubs, trunks, and animal carapaces and on man-made undisturbed surfaces like bricks, leather, wood, bone, glass, metal, plastic etc. [2-4]. Lichens have been widely used various purposes for centuries. Lichens and their producing unique secondary compounds are beneficial in medicine such as antibacterial, antiviral, antifungal, antiprotozoal, antioxidant, antitumor, anti-inflammatory, antigenotoxic, analgesic, enzyme inhibitory and antipyretic characteristics [5-11]. Antimicrobial activities against human disease organisms of lichens have been known for many years. There are many investigations in literature about the antimicrobial activities of lichen extracts but there are limited studies related to pure lichen substances. To our best knowledge, there are few recorded data on the antimicrobial effects of B. capillaris extracts and also no report in literature about barbatolic acid. Therefore, the aim of the present study is to determine the antibacterial and antifungal activities of the methanol, acetone and chloroform extracts of the lichen B. capillaris and its barbatolic acid constituent.
Materials and Methods
Collection and identification of lichen samples`
The lichen Bryoria capillaris (Ach.) Brodo and Hawksw was collected from Mihalıççık-Catacik Pinus sylvestris forest in Eskişehir province on August 16, 2015. The lichen sample was dried at room temperature and identified by using standart keys [12,13]. A voucher specimen was stored at the herbarium of Anadolu University (ANES), Eskişehir, Turkey.
Preparation of the lichen extracts
Finely ground dry thalli of the lichen sample (10 g) was extracted in 100 ml of acetone, methanol and chloroform in a Soxchlet extractor. Then, the crude extracts were filtered and then concentrated under reduced pressure in a rotary evaporator. The amounts of the lichen B. capillaris extract residues were 135.0 mg of acetone, 220.0 mg of methanol and 90.0 mg of chloroform. The dry extracts were stored at -18°C until they were used in the tests.
Microorganisms and media
The following bacteria, yeasts, and filamentous fungi were used as test organisms in this study: Bacillus cereus ATCC 10876, Bacillus subtilis NRRL NRS-744, Escherichia coli ATCC 25922, Enterococcus faecalis ATCC 51299, Enterobacter aerogenes NRRL B-3567, Klebsiella pneumoniae ATCC 700603, Listeria monocytogenes ATCC 19111, Micrococcus luteus NRRL B-4375, Mycobacterium tuberculosis H37Rv (ATCC 27294), Pseudomonas aeruginosa ATCC 27853, Proteus vulgaris NRRL B-123, Staphylococcus aureus ATCC 6538, Salmonella typhimurium ATCC 14028, and Yersinia enterocolitica Y53 bacteria; Candida parapsilosis ATCC 22019, Candida albicans ATCC 90028, Candida globrata ATCC 90030, and Candida krusei ATCC 6258 yeasts; and Aspergillus niger ATCC 9807, Aspergillus flavus ATCC 9807, Aspergillus parasiticus NRRL 465, Fusarium moniliforme NRRL 2374, Aspergillus fumigatus NRRL 113, Alternaria brassicola, Sclerotium rolfsii, Fusarium solani and Rhizopus sp., filamentous fungi obtained from our laboratory.
Determination of antimicrobial activity of the extracts
The antimicrobial activities of lichen extracts were tested by using the disk-diffusion method and by determining the MIC. Disk-diffusion test was performed according to the guidelines of CLSI [14,15]. The bacterial test strains were incubated for 24 h at 37°C on Mueller-Hinton agar substrate and the dilution according to the 0.5 McFarland standard to approximately 108 CFU/ml. Tested filamentous fungi and yeasts were grown onto Potato Dextrose agar and Sabourad Dextrose agar at 25°C for 3-7 days, respectively. The fungal spore suspensions were prepared using sterile 0.1% Tween 80 and adjusted to about 106 spores/ml. 50 mg of each extract was dissolved in 1 ml same solvents including 60 blank sterile antibiotic disks (7 mm diameter). The final concentration of one disk for each extract was 0.833 mg. The disks were placed on the plates and incubated for 24-48 h at 35-37°C and the fungal plates for 5-7 days at 20-25°C. Antimicrobial activities for total extracts were determined by measuring the inhibition zones around the disk. Pure solvent-treated and dried disks were used as negative control. Then, the MIC assay was performed for only sensitive test bacteria to extracts.
The MIC value determinations of the extracts for sensitive test microorganisms were employed according to the Candan et al. [15]. Ten sets of sixty sterilized disks with 10 mg/ml-19.53 μg/ml amount of each extract were prepared in same solvent. After removed of the solvents, disks containing extracts were placed into the plates. The bacterial plates were incubated for 24-48 h at 35-37°C and the fungal plates were incubated for 5-7 days at 20-25°C. The MICs were determined by the lowest concentration that affected to test microorganisms. Pure solvent-treated and dried disks were used as negative control. Streptomycin was utilized as standard antibacterial agent, whereas ketoconazole was utilized as an antifungal agent. All tests were done twice and checked with the control plates.
Isolation and characterization of barbatolic acid
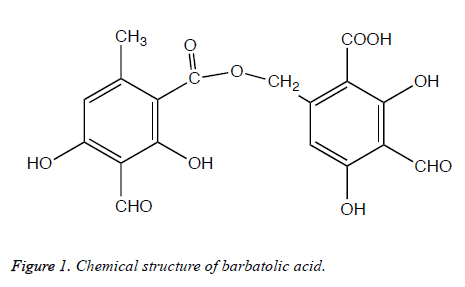
The methanol extract was subjected to TLC plates coated silica gel (60 F254, Fluka). The chromatogram was developed in three solvent systems which are generally used A mix consisting of toluene/dioxane/glacial acetic acid (36:9:1 v/v/v), B mix consisting of hexane/diethyl ether/formic acid (24:8:4 v/v/v, and C mix consisting of toluene/glacial acetic acid (20:3 v/v) [16]. The plates were run in duplicate; one of them was used as reference plate, the other plate was used for bioautography. TLC plates were placed on Nutrient agar petri dishes and poured soft Nutrient agar containing experimental microorganisms (108 CFU/ml). After incubation at 37°C for 24-48 h, antimicrobial activity was detected by spraying of 0.5% TTC (triphenyl tetrazolium chloride, Merck) aqueous solution. The Rf values of the antimicrobial substance was determined by using literature, as well as their melting points and IR spectra, constitutes the basis for their characterization [17-21]. The substance having antimicrobial effect was identified as barbatolic acid (C18H14O10, MA=390.29).
Determination of MIC values of the barbatolic acid
The MIC values of barbatolic acid against to test microorganisms were determined using the microbroth dilution method. Stock solutions of the purified barbatolic acid from methanol extract of lichen were prepared in 20% dimethylsulfoxide (DMSO). Dilution series using sterile distilled water were prepared from 12.8 mg/ml to 0.025 mg/ml and then 100 μl of each dilution was transferred to 96-well microtiter plates. Overnight-grown bacterial and yeast cultures in double-strength Mueller-Hinton broth were standardized to 108 CFU/ml using 0.5 McFarland standard solution. 100 μl of each microorganism suspension was then added into the wells. After incubation at 37°C for 18-24 h, antimicrobial activity was detected by using resazurin. Resazurin represents a nonfluorescent dye of blue color which turns pink and fluorescent in the case of reduction to resazurin with oxidoreductases inside viable cells. Streptomycin was used as standard antibacterial agent, whereas ketoconazole was used as an antifungal agent. MIC was defined as the lowest concentration of compounds that inhibited visible growth, as indicated by the resazurin staining.
Antitubercular activity of the lichen extracts and pure barbatolic acid
Antimycobacterial activity against of Mycobacterium tuberculosis H37Rv ATCC 27294 (American Type Culture Collection) was tested Microplate Alamar Blue Assay (MABA). M. tuberculosis H37Rv (ATCC 27294) was subcultured in ATCC® Medium 1395: Middlebrook 7H9 broth with ADC enrichment at 37°C for 30 days. Suspensions were standardized using McFarland no. 1 turbidity standard. Rifampicin was utilized as control in this test. Dilution series of lichen extracts and rifampicin were prepared with 5.0 to 0.0097 mg/ml, and barbatolic acid was prepared with 2.0 to 0.0039 mg/ml. The incubation of all black, clear-bottomed, 96- well plates (Corning 3340, USA) was performed at the temperature of 37°C for the period of 7 days. A newly made 1:1 mixture containing Alamar Blue reagent (1 : 10 dilution, Invitrogen, 1025, USA) and 10% Tween 80 was added to one well in a group of the positive controls on the 7th day of the incubation. The further incubation of the plates was performed at the temperature of 37°C for the 24 h period. The reagent mixture was put in each well of the microplate in case the well’s content became pink. A pink color in the well was determined as growth, and a blue color as no growth.
Results and Discussion
The antibacterial and antifungal activities of the lichen extracts against to tested fourteen Gram-negative and Gram-positive bacteria including M. tuberculosis, four yeasts and nine filamentous fungi were presented in Tables 1 and 2, respectively. The antimicrobial activity of the extracts was determined by the disk diffusion method and MIC. The methanol, acetone and chloroform extracts was shown significant antibacterial activity except K. pneumoniae, P. aeruginosa and S. typhimurium. The inhibitions zones of the test bacteria were determined ranged from 12.0 to 21.0 mm. Generally, Gram-negative bacteria were more resistant than Gram-positive bacteria. The acetone extract of B. capillaris was more affected of test bacteria compared to other extracts (Table 1). It was inhibited four tested bacteria at a concentration of 78.12 μg/ml.
| Bryoria capillaris extracts |
Barbatolic acid | Antibiotics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Methanol | Chloroform | Acetone | Streptomycin | Rifampicin | ||||||
| Microorganisms | D | MIC (μg/ml) |
D | MIC (μg/ml) |
D | MIC (μg/ml) |
MIC (μg/ml) |
D | MIC (μg/ml) |
MIC (μg/ml) |
| B. cereus | 12 | 78.12 | 15 | 78.12 | 20 | 78.12 | 400 | 30 | 4.88 | - |
| B. subtilis | 18 | 156.20 | 21 | 156.2 | 16 | 78.12 | 400 | 28 | 4.88 | - |
| E. coli | 12 | 156.20 | 15 | 156.20 | 19 | 156.20 | 200 | 25 | 9.76 | - |
| E. aerogenes | 15 | 312.50 | 17 | 312.50 | 20 | 156.20 | 400 | 26 | 9.76 | - |
| E. faecalis | 20 | 156.20 | 18 | 156.20 | 21 | 156.20 | - | 30 | 9.76 | - |
| L. monocytogenes | 19 | 156.20 | 19 | 312.50 | 19 | 156.20 | 200 | 28 | 39.06 | - |
| M. luteus | 21 | 78.12 | 21 | 312.50 | 20 | 78.12 | 200 | 22 | 19.53 | - |
| P. vulgaris | 16 | 156.20 | 18 | 312.50 | 20 | 156.20 | 400 | 20 | 19.53 | - |
| S. aureus | 17 | 78.12 | 17 | 156.20 | 18 | 78.12 | 200 | 26 | 39.06 | - |
| Y. enterocolitica | 15 | 156.20 | 15 | 156.20 | 14 | 156.20 | 200 | 20 | 39.06 | - |
| S. typhimurium | - | Nt | - | Nt | - | Nt | 400 | 25 | 39.06 | - |
| M. tuberculosisH37Rv | Nt | 156.20 | Nt | 156.20 | Nt | 156.20 | 31.25 | - | - | 25 |
| D: Diameter of inhibition zone (mm); -: No activity; Nt: Not tested | ||||||||||
Table 1. Antibacterial activity of methanol, chloroform and acetone extracts of B. capillaris and MIC values of their and barbatolic acid.
| Bryoria capillaris extracts | Barbatolic acid | Antibiotic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Methanol | Chloroform | Acetone | Ketaconazole | ||||||
| Microorganisms | D | MIC (μg/ml) | D | MIC (μg/ml) | D | MIC (μg/ml) | MIC (μg/ml) | D | MIC (μg/ml) |
| Yeasts | |||||||||
| C. albians | 15 | 625 | 13 | 625 | 20 | 625 | - | 36 | 9.76 |
| C. krusei | 12 | 625 | 14 | 625 | 15 | 156.20 | 50 | 35 | 19.53 |
| C. paropilopsis | 15 | 312.50 | 12 | 625 | 20 | 156.20 | - | 40 | 9.76 |
| C. globrata | 15 | 625 | 16 | 625 | 17 | 312.50 | - | 38 | 9.76 |
| Filamentous fungi | |||||||||
| F. solani | 15 | 312.50 | - | Nt | 13 | 156.20 | 200 | 35 | 39.06 |
| F. moniliforme | 15 | 312.50 | - | Nt | 11 | 156.20 | 400 | 38 | 39.06 |
| A. alternata | 12 | 312.50 | - | Nt | 10 | 312.50 | - | 32 | 78.12 |
| A. niger | 12 | 625 | - | Nt | - | Nt | - | 34 | 39.06 |
| A. flavus | 10 | 312.50 | - | Nt | - | Nt | 100 | 28 | 39.06 |
| A. parasiticus | 9 | 625 | - | Nt | 10 | 312.50 | 400 | 35 | 78.12 |
| A. fumigatus | S | 625 | S | 625 | S | 625 | S | 35 | 78.12 |
| S. rolfsii | S | 625 | S | 625 | S | 625 | S | 38 | 19.53 |
| D: Diameter of inhibition zone (mm); S: Inhibition of sporulation; -: No activity; Nt: Not tested | |||||||||
Table 2. Antifungal activities of the extracts of B. capillaris and its substance barbatolic acid.
In the bioautography studies, one substance in the extracts was found to be active in TLC plate. The place of the spot, which demonstrated the inhibition zone was different on every TLC plate depending on the TLC development solvent system. The isolation of this substance was performed with the preparative TLC and identified it as barbatolic acid (Figure 1). The antimycobacterial effects of the lichen extract and barbatolic acid were tested against the infectious pathogen M. tuberculosis H37Rv strain with the Microplate Alamar Blue Assay (MABA). Lichen extracts and barbatolic acid showed significant antituberculosis activity with a MIC value of 156.20 μg/ml and 31.25 μg/ml, respectively (Table 1). The acetone and methanol extracts of the lichen showed antifungal activity on tested filamentous fungi with the range from 9.0 to 15.0 mm. Among the extracts, methanol extract exhibited the largest inhibition zone against F. solani and F. moniliforme with a 15.0 mm.
All the lichen extracts and barbatolic acid inhibited sporulation of A. fumigatus and S. rolfsii. The MIC values of the extracts were found to be between 156.20-625 μg/ml for yeasts and filamentous fungi (Table 2). The barbatolic acid only showed anticandidal activity against C. krusei with a MIC value of 50.00 μg/ml. In literature, there are one or two studies on antibacterial activities extracts of lichen B. capillaris while no report on antimycobacterial and antifungal activity against to filamentous fungi. Also, this study is the first report on antimicrobial activity of its barbatolic acid constituent. We were determined strong antimicrobial activity of against Gram-positive and Gram-negative bacteria, yeasts and filamentous fungi.
Çobanoğlu et al. [22] screened the antimicrobial activities against three gram negative bacteria of acetone and chloroform extracts of taxanomic similar lichen Bryoria fuscescens (Gyeln.) Brodo and Hawksw. They reported acetone extract showed antibacterial activity against Pseudomonas aeruginosa, Escherichia coli and Acinetobacter sp. It is well known that microorganisms have developed resistance to many antibiotics in time and growing resistance especially pose serious threats to human health. Therefore, new alternatives for through antibiotic-resistant microbes are necessary for keeping control pathogens. Natural products from different sources are proposed as a therapeutic alternative to conventional antimicrobial treatment [23,24]. From this point, lichens and their constituents are noteworthy for antibiotic properties. In conclusion, our results clearly indicated that extracts of B. capillaris and its substance barbatolic acid showed considerable antimicrobial effects. On the basis of these results, the lichen B. capillaris and its seconder metabolite barbatolic acid appear to be good and safe natural antimicrobial agent and could be used in the control of various human, animal and plant diseases due to pathogens.
Acknowledgements
The financial support for this study was provided by Anadolu University, Research Projects 1605F401 and 1605F427.
References
- Shukla V, Joshi GP, Rawat MSM. Lichens as a potential natural source of bioactive compounds: a review. Phytochem Rev 2010; 9: 303-314.
- Brightman FH, Seaward MRD. Lichens of man-made substrates: In: Lichen Ecology. Academic Press, London 1977; 253-293.
- Molnár K, Farkas E. Current results on biological activities of lichen secondary metabolites: a review. Z Naturforsch C 2010; 65: 157-173.
- Yousuf S, Choudhary MI, Atta-ur-Rahman. Lichens: Chemistry and biological activities Studies in Natural Products Chemistry 2014; 43: 223-259.
- Lawrey JD. Biological role of lichen substances. Bryologist 1986; 89: 111-122.
- Ingolfsdottir K, Hjalmarsdottir MA, Sigurdsson A, Gudjonsdottir GA, Brynjolfs-dottir A, Steingrimsson O. In vitro susceptibility of Helicobacter pylori to protolichesterinic acid from the lichen Cetraria islandica. Antimicrob Agents Chemother 1997; 41: 215-217.
- Huneck S. The significance of lichens and their metabolites. Naturwissenschaften 1999; 86: 559-570.
- Ingólfsdóttir K. Usnic acid. Phytochemistry 2002; 61: 729-736.
- Haraldsdottir S, Gudlaugsdottir E, Ingolfsdottir K, Ogmundsdottir HM. Anti-proliferative effects of lichen-derived lipoxygenase inhibitors on twelve human cancer cell lines of different tissue origin in vitro. Planta Med 2004; 70: 1098-1100.
- Kosanic M, Rankovic B. Antibacterial and antifungal activity of different lichens extracts and lichen acid. Res J Biotechno. 2011; 6: 23-26.
- Fernandez-Moriano C, Gomez-Serranillos MP, Crespo A. Antioxidant potential of lichen species and their secondary metabolites. A systematic review. Pharm Biol 2016; 54: 1-17.
- Wirth V, Hauck M, Schultz M. Die Flechten Deutschland, Teil 1-2. Ulmer, Stuttgart 2013.
- Smith CW, Aptroot A, Coppins BJ, Fletcher A, Gilbert OL, James PW, Wolseley PA. The lichens of Great Britain and Ireland. 2nd ed, British Lichen Society, London 2009.
- CLSI (Clinical and Laboratory Standards Institute). Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard. CLSI Doc M02-A11. Wayne PA Clinical and Laboratory Standards Institute (11th edn.)2012.
- Candan M, Yilmaz M, Tay T, Kivanç M, Türk H. Antimicrobial activity of extracts of the lichen Xanthoparmelia pokornyi and its gyrophoric and stenosporic acid constituents. Z Naturforsch C 2006; 61: 319-323.
- Culberson CF, Amman K. Standardmethode zur Dünnschichtchromatographie von Flechtensubstanzen. Herzogia 1979; 5: 1-24.
- Huneck S, Yoshimura I. Identification of Lichen Substances, Springer, Berlin 1996; 11-123.
- Culberson CF, Culberson WL, Johnson A. Second Supplement to Chemical and Botanical Guide to Lichen Products. The American Bryological and Lichenological Society, St. Louis, Missouri 1977.
- Orange A, James PW, White FJ. Microchemical Methods for the Identification of Lichens. British Lichen Society, London 2001.
- Schumm F. Dünnschichtchromatogramme auchfür den Amateur möglich. Aktuelle Lichenologische Mitteilungen, NF 9, Essen 2002; pp 8-22.
- Edwards HGM, Newton EM, Wynn-Williams DD. Molecular structural studies of lichen substances II: atranorin, gyrophoric acid, fumarprotocetraric acid, rhizocarpic acid, calycin, pulvinic acid dilactone and usnic acid. J Mol Struct 2003; 651-653: 27-37.
- Çobanoglu G, Sesal C, Gökmen B, Çakar S. Evaluation of the antimicrobial properties of some lichens. South Western Journal of Horticulture, Biology and Environment 2010; 1: 153-158.
- Ali MS, Azhar I, Amtul Z, Ahmad VU, Usmanghani K. Antimicrobial screening of some Caesalpiniaceae. Fitoterapia 1999;70: 299-304.
- Nimri LF, Meqdam MM, Alkofahi A. Antibacterial activity of Jordanian medicinal plants. Pharm Biol 1999; 37: 196-201.