ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2009) Volume 20, Issue 3
Anti-proliferation Effect of Palm Oil γ-tocotrienol and α-tocopherol on Cervical Carcinoma and Hepatoma Cell Apoptosis
Narimah AH Hasani1*, A. Ghapor MT2, Khalid BAK3, Wan Ngah WZ3
1Faculty of Medicine, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia
2Chemistry Division, Malaysian Palm Oil Board, Jalan Persiaran Ilmu, 43600 Bangi, Selangor, Malaysia
3Department of Biochemistry, Faculty of Medicine, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, Kuala Lumpur- 503 00, Malaysia
- *Corresponding Author:
- Wan Zurinah Wan Ngah
Department of Biochemistry
Faculty of Medicine
UKM Medical Molecular Biology Institute (UMBI)
Universiti Kebangsaan
Kuala Lumpur-503 00, Malaysia
E-mail: zurina@medic.ukm.my
Accepted date: May 18 2009
Gamma-tocotrienol has been reported to exhibit a protective effect on hepatocarcinogenesis through the enhancement of immune response and anti-proliferation effect on breast cancer cell lines such as MDA-MB-435 and MCF7. The anti-proliferation mechanism was suggested to be via an increase in apoptosis. The effects of palm oil γ-tocotrienol and α-tocopherol on the cell proliferation were determined using 5-Bromo-2’-deoxy-uridine (BrdU) detection method. CaSki and Alexander cells were sensitive to γ-tocotrienol and α-tocopherol-anti proliferation effects with 93.5 to 97.8% (P less than 0.01, n is equal to 4) and 59.7 to 69.1% (P less than 0.001, n is equal to four) inhibition, respectively beginning with a concentration of 100μM and above. Treatment with α-tocopherol showed a lesser inhibition in the proliferation activity of CaSki cell by 19.7 to 39.4% (P less than 0.01, n is equal to four) and Alexander cell by 16.9 to 19.6% (P less than 0.001, n is equal to four) beginning with a concentration of 50μM and 200μM, respectively. However, both the compounds had no effect on Chang cell. Gamma-tocotrienol showed an IC50 inhibition of CaSki and Alexander cells growth at a dose of 75μM (P less than 0.05, n is equal to four) and 66μM (P<0.05, n=4), respectively. Alpha-tocopherol showed a lesser growth inhibition of CaSki cell at IC40 value of a concentration of 75μM (P less than 0.05, n is equal to four) and Alexander cell at IC20 value of a concentration of 300μM (P less than 0.05, n is equal to four), respectively. Gamma-tocotrienol induced maximum apoptotic activity of both CaSki and Alexander cells at a concentration 150μM of treatment as compared to α-tocopherol at a concentration 300μM and 500μM, respectively. Evidence of the cellular DNA laddering fragments induced by both the compounds at similar doses as above was detected using electrophoresis. The results suggested that palm oil γ-tocotrienol and α-tocopherol exhibit antiproliferation effect on CaSki and Alexander cells via selective induction of apoptosis.
Keywords
Cancer cell apoptosis, γ-tocotrienol, α-tocopherol
Introduction
Vitamin E refers to a group of naturally occurring compounds called α-, β-,γ-, δ-tocopherols and α-, β-,γ-, δ-tocotrienols, as well as acetate and succinate derivatives of both natural and synthetic α-tocopherol [1].
Palm oil vitamin E consists of a mixture of 78 to 82% of α-, γ-, δ-tocotrienols and 18 to 22% of α-, β-, δ-tocopherols [2]. Each of these molecules shares a great similarity in their molecular structures that consist of a chromanol ring and an isoprenoid tail. Both the tocopherol and tocotrienol isomers (α-, β-,γ-, δ-) slightly differ in the number and position of the methyl groups on the chromanol ring. Tocopherols have saturated tails, whereas tocotrienols have three double bonds at 3’, 7’ and 11’ position in their isoprenoid tails [1]. Scientists believed that these unique differences enable tocotrienols to display efficient anti-cancer activity [3]. Biological activities of these isomers depend on their structures. The chromanol ring carries an active antioxidant group and therefore, both tocotrienols and tocopherols are excellent antioxidant [1]. However, tocotrienols are 40 to 50 times more effective in their antioxidant capabilities [4]. The reason may be due to effective uptake of tocotrienols by cells and better distribution throughout cell membranes as compared to tocopherols [5].
The protective effect of vitamin E in cancer has been postulated through the inhibition of cancer formation by the quenching of free radical and control of tumor growth via the induction of differentiation, cell cycle inhibition at G1-S transition phase and apoptosis [6].
in vitro studies have demonstrated that α-, γ-, δ-tocotrienols effectively inhibit the growth of human cervical carcinoma, HeLa and breast cancer cells (non-estrogen-responsive, MDA-MB-435 and estrogen-responsive, MCF-7) by inducing apoptosis [7]. Another study reported that α-tocopherol inhibits the growth of several human cancer cell lines, including prostate (androgen-resistant, PC-3 and androgen-sensitive, LNCaP) and lung (A549) cells. However, it has no effect on normal prostate epithelial (PrEC) cells [8].
Vitamin E succinate acts as a potent apoptotic inducer for an extensive variety of both epithelial and lymphoid human cancer cells that comprise of more than 90% of all human malignancies [9]. Studies indicated that α-tocopheryl succinate induces apoptosis in human breast cancer cell via the translocation of Bax from the cytosol to the mitochondria and the releases cytochrome c from the mitochondria to the cytosol [10]. Another study has demonstrated that α-tocopheryl succinate also induces apoptosis in prostate cancer cell through the inhibition of Bcl-xL/Bcl-2 function [6].
The mode of action of anti-cancer agents indicates that regardless of the diverse nature of anti-cancer drugs, most of them elicit apoptosis in the target cells [11]. Apoptosis is a natural cell death that complements proliferation [12] to maintain the homeostasis of cells [13]. The ability of tumor cells to detect cellular damage and activate the apoptotic response may determine the ultimate success of cancer chemotherapy treatment [14].
Our study intends to demonstrate that palm oil γ-tocotrienol and α-tocopherol could effectively inhibit the cell proliferation of human cancer cells (CaSki and Alexander cells) via the selective induction of apoptosis.
Material and Methods
Cell culture
CaSki cell was cultured in RPMI, while Alexander (PLC/ PRF/5) and Chang cells were cultured in EMEM with Earle’s balanced salts and supplemented with 10% fetal bovine serum, 20mM Hepes, 20mM sodium bicarbonate, 2mM L-glutamine and 1% penicillin and streptomycin. Cells (American Type Cell Collection, Manassas, VA, USA) were grown on culture flask (Falcon, Becton Dickinson, NJ, USA) as monolayer to approximately 80% of confluence in five per cent CO2, at 37°C. Culture media and the above chemicals were purchased from FLOWLAB, Sydney, Australia.
Vitamin E treatment
Palm oil γ-tocotrienol and α-tocopherol were obtained as 80% concentration (single peak by HPLC) from the Palm Oil Research Institute of Malaysia, Kuala Lumpur. Stock solutions of both the vitamins were dissolved in absolute alcohol at 500μM and then diluted so that the final concentration of alcohol in the culture media was less than 0.1%.
Cell proliferation assay
The 2 x 104 cells were treated separately with different concentrations of γ-tocotrienol and α-tocopherol (0, 10μM, 50μM, 100μM, 150μM, 200μM and 300μM) and incubated in 5% CO2 at 37°C for 48 hours. The effect of both compounds on the cell proliferation was determined using a 5-Bromo-2’-deoxy-uridme (BrdU) labelling and detection method (Bohrienger Mannheim, Mannheim, Germany).
Cell growth assay
2 x 104 of CaSki and Alexander cells were treated separately with γ-tocotrienol at IC50 value (a concentration of 75μM and 66μM, respectively) and α-tocopherol at IC40 and IC20 values (a concentration of 300μM), respectively on different days (0, 2, 4, 6, 8, 10, 12, 14). Cells were stained with 10% trypan blue (Flow General Company, McLean, VA, USA) and counted on different days of treatment as above. Cells without test compound were also cultured as controls.
Apoptosis assay
(i) Analysis of DNA fragmentation activity
The 2 x 104 cells were treated separately with γ-tocotrienol and α-tocopherol at different concentrations (0, 10μM, 50μM, 100μM, 150μM, 200μM, 300μM and 500μM) and incubated in 5% CO2 at 37°C for 24 hours. The cellular DNA fragmentation induced by both compounds was measured using a Cellular DNA Fragmentation-ELISA method (Bohrienger Mannheim, Mannheim, Germany).
(ii) Detection of DNA laddering via electrophoresis
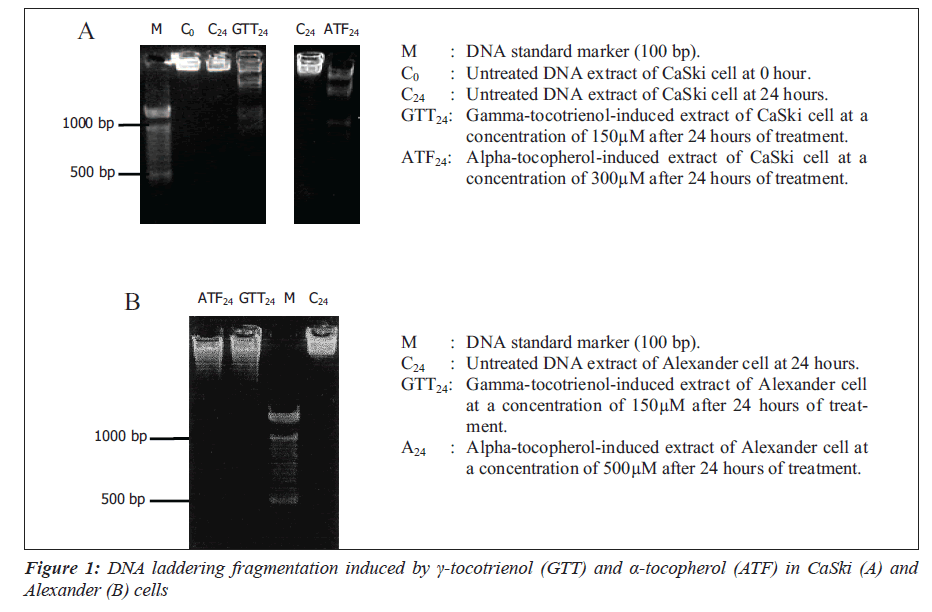
The 106 cells were treated separately with γ-tocotrienol and α-tocopherol at a concentration of 150μM and 300μM, respectively (values obtained from the maximum apoptotic activity assay) for 24 hours. Cells were treated with 10μL of 0.5mg/mL Protenaise K containing 10mM EDTA (pH 8.0), 0.5% SDS and 50mM Tris-HCl (pH 8.0) at 50°C for 1 hour. After 1 hour of incubation in 10μL of RNase A [0.5mg/ mL RNase A, 10mM EDTA (pH 8.0), 50mM Tris-HCl (pH 8.0)] at 50°C for 1 hour, DNA samples and loading buffer [10μL 10mM EDTA (pH 8.0), one per cent (w/v) lowgelling- temperature agarose, 0.25% (w/v) bromophenol blue, 40% (w/v) sucrose] were loaded into two per cent agarose gel stained with 0.5μg/mL ethidium bromide and electrophoresis in 10 x Tris-phosphate buffer [0.9M Trisfosfat, EDTA 0.2M pH (8.0)] at 75 volts for three hours. All the above chemicals were obtained from Sigma Chemical Co, St. Louis, MO, USA.
Cellular uptake of γ-tocotrienol and α-tocopherol
The 10 x 106 cells were incubated separately with γ-tocotrienol and α-tocopherol at different concentrations (0, 50μM, 100μM, 200μM and 300μM) in 5% CO2 at 37°C for 24 hours. At the end of incubation, the cells were washed thrice with ice-cold phosphate buffer saline (10mM NaF, 0.9% NaCl, pH 7.2) and 50μL of 10mg/mL β-hydroxytoluene was added to prevent oxidation of vitamin E’s isomers. After the final wash with phosphate buffer saline, 100μL ethanol and β-hydroxytoluene were added and the cells were saponificated for 40 seconds. The samples were dissolved into 100μL HPLC grade-hexane containing β-hydroxytoluene, vortex and centrifuged at 300g for three minutes to separate the phases. Aliquots of the top phase were air dried using a vacuum concentrator and again dissolved in 100μL of hexane. The samples were filtered with 0.4μm nitrocellulose membrane and injected into a 150 x 4.6mm normal silica column with a mobile phase of hexane:methanol (99.75:0.25) at a rate of 1.50mL/min. Vitamin E compounds were quantified with a fluorescence detector at 294nm and emission detection at 330nm. All the above chemicals were obtained from Sigma Chemical Co, St. Louis, MO, USA.
Statistical analysis
Paired Student’s t-test was used to compare between control and different levels of each treatment on the measured parameters. Significance was set up at P less than 0.05.
Results
Effects of γ-tocotrienol and α-tocopherol on cell proliferation
To determine the effects of both the vitamin E compounds, on cell proliferation, we examined the incorporation of BrdU into freshly synthesized cellular DNA. Gammatocotrienol, at the lowest dose of 10μM slightly enhanced the proliferation of CaSki cells by 3.8% (P less than 0.01, n is equal to four), however it slightly inhibited the proliferation of Alexander cells by 9.7% (P less than0.001, n is equal to four). At 50μM, γ-tocotrienol enhanced the proliferation of both CaSki and Alexander cells by 22.0 and 9.9% (P less than 0.01, n is equal to four), respectively. Interestingly, at concentrations of 100μM and above, γ-tocotrienol effectively suppressed the proliferation of both cells by 93.5 to 97.8% (P less than 0.01, n is equal to four) and 59.7 to 69.1% (P is less than 0.001, n is equal to four) with IC50 values of 75μM and 66μM, respectively. Alpha-tocopherol reduced the proliferation of CaSki cells by 19.7 to 39.4% (P is less than 0.01, n is equal to four) beginning at a concentration of 50μM, followed by 16.9% to 19.6% (P is less than 0.001, n is equal to four) reduction of Alexander cells beginning at a concentration of 200μM with IC40 value of 75μM and IC20 value of 300μM. On the other hand, both compounds have no effect on the cell proliferation of Chang cells at all concentrations used.
Effects of γ-tocotrienol and α-tocopherol on cell growth
Gamma-tocotrienol reduced the growth of CaSki and Alexander cells by 50.0% (P is less than 0.05, n is equal to four) at IC50 values of a concentration of 75μM and 66μM, respectively. Alpha-tocopherol shows a lesser growth inhibition of CaSki cell at IC40 value of a concentration of 75μM (P is less than 0.05, n is equal to four) and Alexander cell at IC20 value of a concentration of 300μM (P is less than 0.05, n is equal to four), respectively as compared to untreated cultures.
Induction of apoptosis by γ-tocotrienol and α-tocopherol by DNA fragmentation
To further analyze the possible antiproliferation mechanism induced by Vitamin E on CaSki and Alexander cells, we analyzed the apoptotic properties of both compounds by measuring the cellular DNA fragmentation activity. The activity was investigated by determining the BrdUlabeled DNA fragments released into the cytoplasm during apoptosis.
Treatment with γ-tocotrienol at a concentration of 10μM has no effect on CaSki cell, though it enhanced the apoptotic activity of Alexander cell by 2.3-fold (P is less than 0.01, n is equal to four ) as compared to control Figure 1. However at a concentration of 50μM, it has no effect on both cells. Interestingly, at a concentration of 100μM, γ-tocotrienol enhanced the apoptotic activity of both cells by 5.8-fold (P is less tha 0.01, n is equal to four) and 3.9-fold (P is less than 0.01, n is equal to four) with the maximum activity of 6.8-fold (P less than 0.01, n is equal to four) and 5.2-fold (P less than 0.01, n is equal to four) at a concentration of 150μM, respectively. At higher concentrations of 200μM to 500μM, the apoptotic activity induced by γ-tocotrienol was slightly reduced to 5.6-fold to 3.7-fold and 2.7-fold, respectively, but the effect was still significant at P less than 0.01 as compared to untreated cultures. Alpha-tocopherol enhanced the apoptotic activity of both CaSki and Alexander cells by 2.7-fold (P less than 0.01, n is equal to four) and 2.1-fold (P less than 0.01, n is equal to four) beginning at a concentration of 300μM and 500μM, respectively.
The apoptotic effect of γ-tocotrienol and α-tocopherol was confirmed by the DNA fragmentation patterns extracted from CaSki (A) and Alexander (B) cells.
Further evidence of apoptosis was obtained by the formation of DNA laddering fragments detected by electrophoresis.
Cell uptake
To ensure that both γ-tocotrienol and α-tocopherol were incorporated into all three cells, each concentration of treatment was analyzed using high performance liquid chromatography.
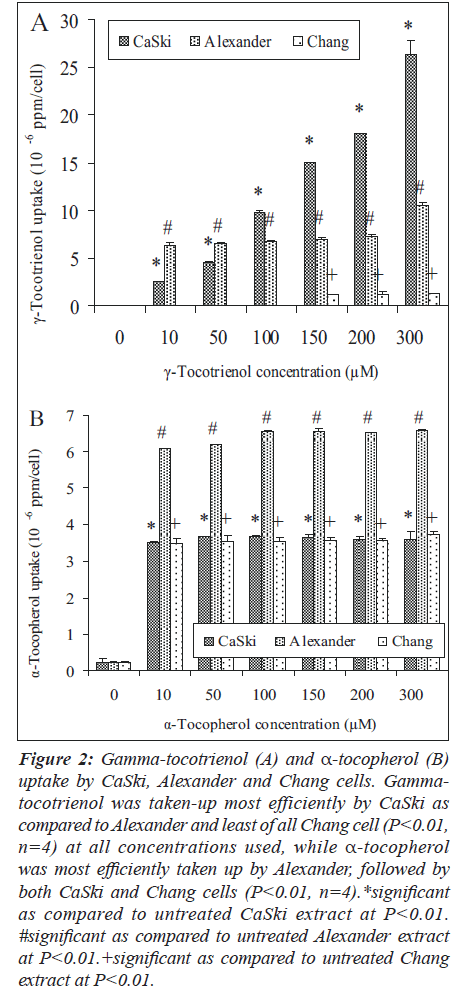
Gamma-tocotrienol was taken-up by CaSki and Alexander cells at all concentration used (Figure 2A). However, the extent of cellular uptake varied between the two cells. The increment uptake of γ-tocotrienol by CaSki cell correlated with all the concentrations used (P less than 0.01, n is equal to four ), while Alexander cell shows a plateau uptake (P less than 0.01, n is equal to four). In contrast, smaller amount of -tocotrienol (P less than 0.01, n is equal to four) was only detected at concentrations from 150μM to 300μM of treatments. Gamma-tocotrienol was taken-up most efficiently by CaSki as compared to Alexander and least of all Chang cell with a ratio of 12.4:5.8:1 (at a concentration of 150μM) and 19.7:7.8:1 (at a concentration of 300μM).
Figure 2: Gamma-tocotrienol (A) and α-tocopherol (B) uptake by CaSki, Alexander and Chang cells. Gammatocotrienol was taken-up most efficiently by CaSki as compared to Alexander and least of all Chang cell (P<0.01, n=4) at all concentrations used, while α-tocopherol was most efficiently taken up by Alexander, followed by both CaSki and Chang cells (P<0.01, n=4).*significant as compared to untreated CaSki extract at P<0.01. #significant as compared to untreated Alexander extract at P<0.01.+significant as compared to untreated Chang extract at P<0.01.
On the other hand, all three cells show plateau increment of α-tocopherol uptake (Figure 2B) at all concentrations used (P less than 0.010.05, n is equal to four). However, α-tocopherol was most efficiently taken up by Alexander, followed by both CaSki and Chang cells (P less than 0.010.01, n is equal to four) with a ratio of 1.7:1:1.
Discussion
Treatment of cancer cells with high dose of 13-cisretinoic acid, α-tocopheryl succinate, or β-carotene clearly alters the expression of specific genes, levels of proteins, and translocation of certain proteins from one cellular compartment to another, causing differentiation, proliferation inhibition, and apoptosis, depending on the type and form of antioxidant, treatment schedule, and type of tumor cell [15]. Alterations in gene expressions and protein levels are directly related to proliferation inhibition and apoptosis [16].
Our findings demonstrated that treatment with concentrations from 100μM to 300μM of γ-tocotrienol efficiently inhibits the proliferation of CaSki and Alexander cells by 93.5 to 97.8% and 59.7 to 69.1%, respectively as compared to α-tocopherol at a lesser magnitude of 19.7 to 39.4% beginning at a concentration of 50μM and 16.9% to 19.6% reduction of the later cell at concentrations of 200μM and above. Gamma-tocotrienol at IC50 values (a concentration of 75μM and 66μM, respectively) reduced the growth of both cells by 50% as compared to α-tocopherol at IC40 and IC20 values (a concentration of 300μM) caused a 40 and 20% reduction, respectively.
Other in vitro studies reported that α-, β-, γ-tocotrienol and α-tocopherol efficiently function as potent inhibitors of DNA synthesis in human breast cancer cells (non-estrogenresponsive, MDA-MB-435 and estrogen-responsive, MCF 7). The effectiveness of growth inhibition by tocotrienols is independent of estrogen sensitivity. Therefore, tocotrienols have great potential for possible natural aid in the prevention and management of breast cancer. Treatment with a concentration of 180μg/mL and 2μg/mL of palm oil γ-tocotrienol reduced the proliferation of MDA-MB-435 and MCF-7 by 50%, respectively as compared to palm oil α-tocopherol at a concentration of 125μg/mL in the later cells [17]. Other study reported that treatment with a concentration of α-tocopherol at concentrations of 25μM and 50μM, inhibits the growth of several human cancer cell lines, including prostate (androgen-resistant, PC-3 and androgen-sensitive, LNCaP) and lung (A549) cells. In contrast, at similar concentrations α-tocopherol has no effect on normal prostate epithelial (PrEC) cells [8].
To further investigate the antiproliferation mechanism induced by γ-tocotrienol and α-tocopherol, we analyzed the apoptotic properties of both the compounds on CaSki and Alexander cells by measuring the apoptotic activity of the cellular DNA fragmentation. Gamma-tocotrienol at a concentration of 150μM on CaSki and Alexander cells enhanced the maximum DNA fragmentation activity by 6.8-fold and 5.2-fold, while α-tocopherol exhibit lesser magnitude of 2.0-fold at higher concentrations of 300μM and 500μM, respectively. Gamma-tocotrienol is more potent as apoptotic inducer as compared to α-tocopherol. Our finding is in accordance with other data reported that γ-tocotrienol, RRR-α-tocopheryl succinate possessed the ability to induce cancer cells to undergo apoptosis without affecting normal cells. However, RRR-α-tocopherol does not induce cancer cell to undergo apoptosis [18]. Cellular DNA laddering fragments of CaSki and Alexander cells induced with a concentration of 150μM of γ-tocotrienol were evident using electrophoresis. Treatment with -tocopherol at concentrations of 300μM and 500μM also produced similar patterns. Previous study using the cellular DNA laddering detection indicated that α-tocopherol or combination with α-tocopherol, induced apoptosis in androgen-sensitive (LNCaP) but not the androgen-resistant (PC-3) human prostate cancer cells through the induction of cytochrome c release, activation of caspase-9 and -3, cleavage of poly-ADP-ribose polymerase and involvement of caspaseindependent pathways [8]. Another study reported that α-tocopheryl succinate induces apoptosis of human breast cancer cells via translocation of Bax from the cytosol to the mitochondria and releasing cytochrome c from the mitochondria to the cytosol [10].
The greater apoptotic effect on the CaSki and Alexander cells by γ-tocotrienol may be due to its shorter unsaturated isoprenoid tail that allows easier mobility, more uniform distribution in cell membranes and greater recycling activity as compared to α-tocopherol [4]. This is in accordance with our findings of cellular uptake of both the compounds by both the cell types using HPLC method. Gamma-tocotrienol was taken-up most efficiently by CaSki cell with a ratio of 4:1 (at a concentration of 150μM) and 7:1 (at a concentration of 300μM) as compared to α-tocopherol, followed by Alexander cell with a ratio of 2:1 at a concentration of 300μM. The differential magnitude in the incorporation of γ-tocotrienol by CaSki and Alexander cells may also be due to the presence of specific receptor(s) that specifically binds to the compound.
In summary, our results showed that palm oil vitamin E, especially γ-tocotrienol, effectively induces apoptosis of the cells of the cervical carcinoma and hepatoma (CaSki and Alexander cells). The apoptosis inducing ability of γ-tocotrienol makes this compound a promising candidate for further characterization of its antitumor profile in vivo as a possible natural therapeutic agent in cancer management.
Acknowledgments
This study was funded by IRPA research grant 06-02-02-0013 from the Ministry of Science, Technology and Innovation, Malaysia. Authors wish to appreciate valuable suggestions of Prof. Dr. Amar Chatterjee during the preparation of this manuscript.
References
- Kamal-Eldin A, Appelqvist LA. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996;31:671-701.
- Sambanthamurthi R, Sundram K, Tan YA. Chemistry and biochemistry of palm oil. Progress in Lipid Research 2000;39:507-58.
- He L, Mo H, Hadisusilo S, Qureshi AA, Elson CE. Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J Nutr 1997;127:668-74.
- Packer L, Weber S, Rimbach G. Molecular aspects of alpha tocotrienol antioxidant action and cell signaling. J. Nutr 2001;131:369S-73S.
- Sen CK, Khanna S, Roy S, Packer, L. Molecular basis of vitamin E action. J Biol Chem 2000;275:13049-55.
- Shiau CW, Huang JW, Wang DS, Weng JR, Yang CC, Lin CH, Li CL, Chen CS. α-Tocopheryl succinate induces apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 function. J Biol Chem 2006;281:11819-25.
- Nesaretnam K, Ambra R, Selvaduray KR, Radhakrishnan A, Canali R, Virgili F. Tocotrienol-rich fraction from palm oil and gene expression in human breast cancer cells. Annals of the New York Academy of Sciences 2004;1031:143-57.
- Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN. γ-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci U S A 2004;101:17825-30.
- Kline K, Yu W, Sanders BG. Vitamin E: mechanisms of action as tumor cell growth inhibitors. J Nutr 2001;131:161S-3S.
- Yu W, Sanders BG, Kline K. RRR--tocopheryl succinateinduced apoptosis of human breast cancer cells involves Bax translocation to mitochondria. Cancer Research 2003;63:2483-91.
- Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 1980;284:555-6.
- Kerr FR, Winterford CM, Harmon BV. Apoptosis: It significance in cancer and cancer therapy. Cancer 1994;73:2013-26.
- Raff CM. Social controls on cell survival and cell death. Nature 1992;356:397-400.
- Jiang MC, Yang-Yen HF, Lin JK, Yen JJ. Differential regulation of p53, c-Myc, Bcl-2 and Bax protein expression during apoptosis induced by widely divergent stimuli in human hepatoblastoma cells. Oncogene 1996;13:609-16.
- Prasad KN, Kumar B, Yan XD, Hanson AJ, Cole WC. Alphatocopheryl succinate, the most effective form of vitamin E for adjuvant cancer treatment: a review. J Am Coll Nutr 2003;22:108-17.
- Prasad KN. Multiple Dietary Antioxidants Enhance the Efficacy of Standard and Experimental Cancer Therapies and Decrease Their Toxicity Integrative Cancer Therapies 2004;3:310-22.
- Guthrie N, Gapor A, Chambers AF, Carroll KK. Inhibition of proliferation of estrogen receptor negative MDAMB- 435 and -positive MCF-7 human breast cancer cells by palm oil tocotrienols and tamoxifen, alone and in combination. J Nutr 1997;127:544S-8S.
- Kline K, Yu W, Sanders BG. Vitamin E and breast cancer. J Nutr 2004;134:3458S-62S.