Keywords
|
| Astragaloside; D101 adsorption resin; SGC-7901. |
Introduction
|
| Astragalus membranaceus is the dried root of leguminous plant Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao or Astragalus membranaceus (Fisch.) Bge., which was originally recorded in the Shen Nong's Herbal Classic. As an important qi-benefiting traditional Chinese medicine, it is warm, sweet, and has qi-replenishing, superficies-strengthening, dieresis-inducing, toxin-expelling, sore-astringing, tissue regeneration-promoting and middle warmer-tonifying actions [1-3]. |
| Domestic and foreign scholars have conducted extensive research on Astragalus membranaceus, who have confirmed that the major chemical constituents of Astragalus membranaceus are polysaccharides, triterpenoid saponins and flavonoids. Besides, it also contains monosaccharides, amino acids, linoleic acid, palmitic acid and linolenic acid. Sufficient studies have demonstrated that Astragalus membranaceus also contains protein, riboflavin, folic acid, vitamins, vanillic acid, ferulic acid, isoferulic acid, p-hydroxyphenyl acrylic acid, caffeic acid, chlorogenic acid, daucosterol, lupeol, nhexadecanol and trace elements [4-7]. |
| Modern pharmacological studies have shown that Astragalus membranaceus has anti-tumor, immunoregulatory, anti-viral, anti-aging, anti-oxidant, anti-radiation and anti-stress effects, which has been widely used in areas like medicine and agriculture. Owing to multi-target, multi-link and multi-effect characteristics of Astragalus membranaceus, it plays important roles in suppressing and killing tumor cells, improving symptoms and signs, mitigating adverse chemoradiotherapeutic effects and prolonging patients' survival [8-10]. Meanwhile, Astragalus membranaceus can also improve immunity, has small toxic and side effects, and hardly produces drug resistance, which has gradually become the hot topic and focus in clinical immunity enhancement and tumor activity suppression in recent years [11-13]. This study investigates the resin purification process of astragalosides, and explores its inhibitory effect on human gastric cancer cells, in order to lay the foundation for large-scale industrial production and clinical application of Astragalus membranaceus. |
Materials
|
Instruments and reagents
|
| UV-2401 UV-Vis spectrophotometer (Shimadzu, Japan); CO-150 CO2 incubator (NBS, USA); SW-CJ-2F clean bench (Suzhou Purification Equipment Factory); RPMI 1640 medium (Gibco, USA); MTT (Beijing Solarbio Science & Technology Co., Ltd); DMSO, trypsin, and PI (Sigma, USA). Reagents were all of analytical grade. |
Cells and drugs
|
| Human gastric cancer SGC-7901 cells were purchased from China Medical University; Astragalus membranaceus was purchased from pharmacy; and astragalosides, with a purity of 56.78%, was self-extracted. |
Methods and Results
|
Resin pretreatment
|
| Resin was washed with distilled water, and soaked in 95% ethanol for 24 h. After sufficiently swollen, the resin was loaded onto column by wet method, and flow washed with 95% ethanol till the effluent had similar absorbance with 95% ethanol by UV spectrometry. Then, the resin was washed with distilled water until no ethanol was smelt, and set aside. |
Preparation of Astragalus membranaceus sample solution
|
| 1 kg of crude Astragalus membranaceus powder was weighed, and extracted for 2 h twice with 80% ethanol. After filtration, extracts were combined, and ethanol was removed until no ethanol was smelt. The concentrate was then dissolved in distilled water, filtered, and diluted to 1,000 ml to give Astragalus membranaceus sample solution. |
Determination of total astragaloside content
|
| 2.5 mg of astragaloside standard substance was accurately weighed, and diluted to 10 ml. 0.1, 0.2, 0.3, 0.4, 0.5, 0.6 and 0.7 ml of the solutions were drawn, respectively, added precisely with anhydrous ethanol to 0.5 ml, and then with 0.5 ml of 8% vanillin anhydrous ethanol, shaken well, placed in an ice bath, and added with 5 ml of sulfuric acid test solution (volume fraction of 72%). After shaken well, the solutions were kept warm in 60water bath for 20 min, removed, shaken well, and cooled to room temperature. Within 30 min, absorbance was measured at 540 nm by spectrophotometry, standard curve was plotted with absorbance as the ordinate and mass concentration as the abscissa, and the resulting linear regression equation was y=0.028 8x -0.023 1 (r=0.9993). |
Resin type selection
|
| Practicality of different types of resins was investigated by dynamic adsorption method. Approximately 5 g of different types of resins were accurately weighed, and loaded onto column by wet method. 0.858 mg/ml total astragaloside sample solutions were slowly added to different resin columns while controlling volume flow rate at 1.5 ml/min. Fraction was collected once per 10 ml, astragalosides in ethanol elution fraction were measured, and dynamic astragaloside adsorption performances of various model resins were determined. |
| Dynamic adsorption results showed that the astragaloside adsorption capacity and elution rate of D101 macroporous resin reached 27.2 mg/g and 93.3%, respectively, both of which were superior to the other four resin types. Thus, D101 macroporous resin was selected for enrichment and purification of astragalosides, as shown in Figure 1. |
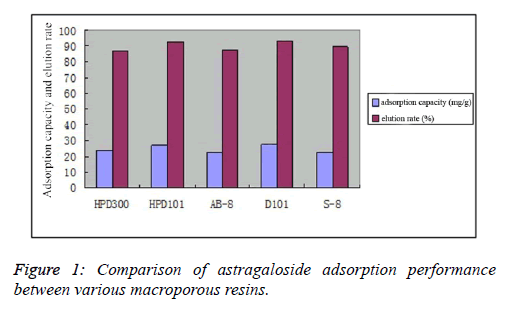 |
Investigation on enrichment and purification processes of D101 macroporous resin
|
| Influence of adsorption rate on adsorption efficiency: 0.858 mg/mL total astragaloside sample solutions were taken, whose outflow rates were controlled at 1, 2, 3 and 4BV/h. The samples were eluted with 5BV of water first, and then desorbed with 5BV of 75% ethanol. Ethanol desorption solutions were collected, respectively, for determination of total astragaloside contents in desorption solutions, followed by calculation of desorption rates, as shown in Figure 2. |
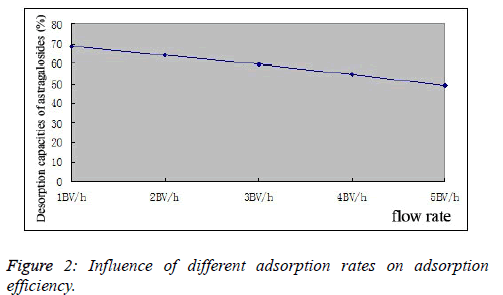 |
| As can be seen from the experimental results, different flow rates have a marked influence on the adsorption results of astragalosides. With the increasing flow rate, astragaloside adsorption efficiency of D101 macroporous resin decreased gradually, which dropped to 48.93% at a 5BV/h flow rate. Based on actual production conditions and comprehensively considering various factors, the flow rate was set as 2BV/h. |
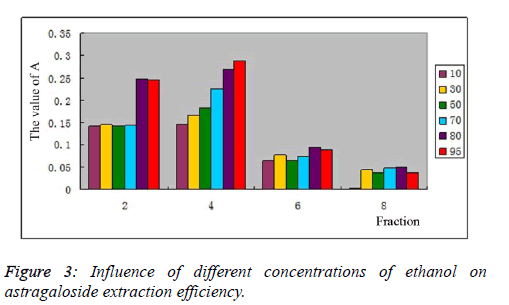 |
| Influence of different volume fractions of ethanol on astragaloside extraction efficiency: 20 g of pretreated D101 macroporous resin was loaded onto column, and then 20 ml of 0.858 mg/ml astragaloside sample solution was injected into the column. 5 BV of 10%, 30%, 50%, 70%, 80% and 95% ethanol were used for elution of astragalosides, respectively, at a 2BV/h flow rate. Total astragaloside content was determined as per the method in 3.3., as shown in Figure 3. With increasing concentration of ethanol, astragaloside desorption efficiency also increased. Total astragaloside content reached its highest in the 4th fraction, and A value of total astragaloside content reached 0.287 when eluted with 95% ethanol. 80% ethanol had enrichment efficiency slightly worse than 95% ethanol, but no statistical difference was noted. After comprehensive consideration, 80% ethanol was selected as the optimal enrichment concentration for total astragaloside content. |
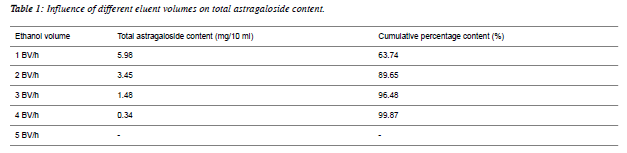
| Determination of optimal elution volume: 10 ml of the above astragaloside solution was accurately weighed, absorbed by D101 macroporous resin column for 30 min, then washed with 4BV of distilled water, and later eluted with 5BV of 80% ethanol at a flow rate of 2BV/h. With 1BV as one fraction, a total of 5 fractions were collected. |
| Data showed that when 3BV of ethanol was used to elute 1.48 mg/10 ml astragalosides, cumulative percentage content was 96.48%, which basically reached the elution end, as shown in Table 1. |
 |
Cell cultivation
|
| Gastric cancer SGC-7901 cells were cultured statically in a 37, 5% CO2 incubator with RPMI1640 medium containing 10% FBS, penicillin (100 ml/L) and 100 ku/L penicillin and streptomycin (1 mg/ml). |
| Well growing exponential phase cells were used in the experiment. The monolayer cultured SGC7901 cells were digested with 0.25% trypsin, prepared into a 1×105/ml cell suspension, seeded in a 96-well plate at 1×104 cells per well, and treated by adding astragalosides (concentrations of 5, 10 and 20 µmol/L). 72 h later, 20 µl of MTT solution (5 mg/ml) was added to each well, and the incubation was continued for an additional 4 h. After discarding the supernatant, each well was added with 150 µl of DMSO, shaken on horizontal shaker for 10 min, and then a value was measured at 490 nm with ELISA reader. The experiment was repeated three times, and the growth inhibition (CI) rate was calculated. |
| CI= (A value of negative control group - A value of treatment group)/A value of negative control group × 100%. |
| The results showed that different concentrations of astragalosides had marked anti-proliferative effects on gastric cancer SGC7901 cells, which were enhanced with increasing astragaloside dose and prolonging treatment time, showing dose- and time-dependence. The differences were significant between varying astragaloside dose groups and untreated group (P<0.05), as shown in Figure 4. |
 |
Discussion
|
| With the increase of social pressure and the changes in living environment and lifestyle, tumor is increasingly becoming a common disease seriously harming human health. According to relevant statistics, there are 1.6 million new cases of cancer, 1.3 million deaths and more than 2 million active cancer patients in China annually, which has brought heavy disasters and losses to families and society. Gastric cancer, as a malignancy with high incidence, high metastasis and high mortality, has a tremendous impact on people's lives. |
| Currently, radiotherapy and chemotherapy are considerably effective for cancer treatment, but they have large side effects, which seriously affect the patients' quality of life. In comparison, Chinese medicine has its unique advantage in improving the quality of life of patients. Characterized by low toxicity and high efficiency, Chinese medicine differs from the awkward situation of Western medicine where single drug corresponds to single disease. In the treatment process of diseases, Chinese medicine can reasonably combine traditional Chinese drugs through syndrome differentiation from a holistic approach to achieve better outcomes. Combined with radiotherapy and chemotherapy, traditional Chinese medicine can achieve toxicity attenuating and efficacy enhancing effects, not only inhibiting tumors, but also improving immunity. Active exploration of effective Chinese medical treatment regimens has important clinical significance to improving the quality of life for patients with advanced gastric cancer and prolonging their survival. |
| Cao Liping et al.'s [14] study showed that many tumor cells can increase the synthesis of prostaglandins, especially PGE2, both in vitro and in vivo. Excessively increased PGE2 has the ability to promote tumor cell proliferation, migration and tumor angiogenesis and inhibit tumor cell apoptosis and host immunity, which is thus closely associated with tumorigenesis, development and metastasis. Chinese herbal drug Astragalus membranaceus can effectively inhibit PGE2, whose inhibitory effect is stronger than the chemical drug celecoxib. Besides, Astragalus membranaceus can also prevent the occurrence of tumor immune escape, and thereby prevent the tumorigenesis through inhibiting COX-2 expression and reducing PGE2- induced inflammation and immune responses. |
Conclusion
|
| This study investigates the process conditions for extraction, purification and enrichment of astragalosides with different macroporous resin types. The results show that D101 macroporous resin has astragaloside adsorption capacity and elution rate of 27.2 mg/g and 93.3%, respectively, both of which are superior to the other four resin types. Astragaloside adsorption results at different flow rates reveal that with the increase in flow rate, astragaloside adsorption efficiency of D101 macroporous resin decreases gradually, which drops to 48.93% at a 5BV/h flow rate. Based on actual production conditions and comprehensively considering various factors, the flow rate is set as 2BV/h. In the investigation of extraction solvent concentration, with the increasing concentration of ethanol, astragaloside desorption efficiency also increases. A value of total astragaloside content reaches maximum when eluted with 95% ethanol. 80% ethanol has an enrichment efficiency slightly worse than 95% ethanol, but no statistical difference is noted. After comprehensive consideration, 80% ethanol is selected as the optimal enrichment concentration for total astragaloside content. |
| MTT results demonstrate that different concentrations of astragalosides have marked anti-proliferative effects on gastric cancer SGC-7901 cells, which strengthen with increasing dose and medication time, showing dose- and time-dependence. |
Conflict of Interests
|
| The authors declare that they have no conflict of interests. |
|
|
References
- Sang GY, Wei SX, Liu CJ. Study progress on anti-tumormechanisms and clinical applications of astragalus. Lishizhen Medicine and MateriaMedica Research 2008; 19: 3032-3034.
- Li LQ, Zhang J, Zhai XH. Efficacy of QiBaicollapsepickling treatment for chemotherapeutic phlebitis. Chinese Journal of Misdiagnostics 2008; 8: 6641-6644.
- Hu B, Shen KP. Antitumor effect and mechanism of astragalus. Journal of Chinese Medicinal Materia1s 2008; 28: 83-87.
- Choi EM, Kim AJ, Kim YO, Hwang JK. Immunomodulating activity of arabinogalactan and fucoidan in vitro. J Med Food 2005; 8: 446-453.
- Lto Y. New contimuous extraction method with a coil planet centrifuge. J. Chromatogr. A 1981; 207: 161-170.
- Kong Z, Rinehart KL, Milberg RM. Application of high-speed counter-current chromatography/electrospray ionization mass spectrometry in natural product chemistry. J. Liq. Chrom. and Rel. Technol. 1998; 21: 65-82.
- Yang FQ, Zhang TY, Zhang R, Yoichiro I. Application of analytical and preparative high-speed counter-current chromatography for separation of alkaloids from chinensisFranch. J. Chromatogr. A. 1998; 829: 137-141.
- Zhang F, Gao P, Peng JH. Effect of Astragalus polysaccharide and astragaloside on macrophage phagocytosing mycobacterium tuberculosis. Medical Journal of National Defending Forces in Northwest China 2005; 26: 434-436.
- Zhang CX. Influence of Huangqi injection on bone marrow in patients with mid and advanced stage nonsmall-cell lung cancer. Journal of Hainan Medical University 2007; 13: 437-438.
- Gu JC, Yu WB, Wang Y. Effects of astragalus polysaccharides on tumor growth and the expression of HSP70 in transplanted breast cancer for MA891 in TA2 mice abstract. Chinese Journal of Cancer Prevention and Treatment 2006; 13: 1534-1537.
- Zhang ZX, Qi F, Zhou DJ, Liang XY, Zhu LW, Wang PZ. Effect of 5-flurouracil in combination with astragalusmembranaceus on amino acidmetabolism in mice model of gastric carcinoma. Chinese Journal of Gastrointestinal Surgery 2006; 9: 445-447.
- Xiao ZM, Zhao LH, Qiu J. Influence of astragalus aqueous extract on human liver cancer cells and immune cells in mice bearing tumor. Journal of Shandong University of Traditional Chinese Medicine 2004; 28: 136-139.
- Lin GB, Zhong ZG, Zhang WY, Anti-tumor mechanism of active components from extract of ctinidiarufa root. China Journal of Chinese MateriaMedica 2008; 33: 2011-2014.
- Cao LP, Shen H, Liu L, Liu Y. Effect of astragaloside and ß-elemene on expression of COX-2 and PGE2 in gastric cancer cell line SGC7901. Modern Journal of Integrated Traditional Chinese and Western Medicine 2010; 19: 798-780.
|



