ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 4
Assessment of causative factors of febrile seizure related to a group of children in Iran
Children's Medical Center, Tehran University of Medical Sciences, Tehran, Iran
- *Corresponding Author:
- Parisa Rahmani
Children's Medical Center
Tehran University of Medical Sciences, Iran
Accepted date: August 10, 2016
Febrile seizure is a common disorder between children with age of 6 month to 6 years and its recurrence is an emotional trauma for parents. Causative factors of febrile seizure have not been completely identified. In this study we determined the correlation between causative factors and probability of febrile seizure occurrence. This study was performed on 344 children factors related to febrile seizure were recorded and the distribution of febrile seizure occurrence was identified with Pearson chi square test. Our results demonstrated that number of febrile seizures increased in evening and autumn. Also, there was a correlation between body temperature and number of febrile seizure. Finally, the history of family with febrile seizure was identified as a suitable causative and prognostic factor.
Keywords
Febrile seizure, Circadian rhythm, Seasonal variations, Family history, Body temperature
Introduction
Febrile seizures are the most common types of seizures in children, and usually occur among children between 6 months and 6 years old and affecting about 2%-5% of all children [1]. Recurrence of febrile seizure is common which about 20%-30% of patients reported to experience more than one febrile seizure [2]. Many attempts have been done to identify the pathogenesis of febrile seizure.
Several studies have attempted to identify risk factors associated with seizure [3]. Febrile seizure is generated from genetic and environmental factors [4]. Presence of a positive family history in first- and second-degree family members is the most important identified risk factor for febrile seizure [5]. It has been demonstrated that one fourth of patients with febrile seizure had a positive family history [6]. Male gender, low birth weight, breast- feeding duration, high body temperature, high blood bilirubin or neonates whose mothers consume alcohol and smoke cigarettes are ascribed to other causative factors of febrile seizure [7]. Also, bacterial and viral infections are considered as significant factors [5].
Many circadian rhythms such as sleep, behaviour, metabolism, and hormone secretion are being regulated with changes in exposure to light [8]. Melatonin is secreted mostly at night and in the dark, and exposure to light reduces its secretion [9]. Some studies have suggested an association between the occurrence of febrile seizures and melatonin [10,11]. According to some studies, melatonin seems to possess some anti-seizure effects [12,13].
In this study, we aimed to determine demographic features and causative factors for febrile seizure in Children’s Medical Center (Tehran, Iran) to prepare an answer for conflicting opinions about causative factors of febrile seizure regarding geographical and seasonal variation. We evaluated this case in Iran which helps the health care provider in better diagnosis and selecting better treatment.
Patients and Methods
Patients
This cross-sectional study was done on 344 children with 6 month to 6 year-old who were diagnosed with febrile seizure, and admitted to Children’s Medical Center (Tehran, Iran) from February 2013 to February 2015.
Inclusion and exclusion criteria
Written informed consent from all parents was obtained for inclusion in the study. All children with a seizure due to reasons such as comorbidity with chronic diseases, electrolyte imbalance, past medical history of seizure or central nervous system infections were excluded from our study.
Performance
Hospital admission time was recorded, and the time the febrile seizure occurred was asked of the parents upon their arrival at the hospital. Patients’ body temperature was measured upon their arrival at the hospital. Patient’s data including age, gender, seizure subtypes such as focal, seizure types (simple or complex), time of seizure occurrence, a duration of more than 15 minutes, and usage of diazepam, month and season of seizure, family history of disease, infectious causes of fever were recorded in specific self-formatted questionnaires. When a febrile seizure was either focal or prolonged (>15 minutes), or if more than one seizure had occurred within a 24 hour period, it was considered complex.
Ethical consideration
The study was carried out in accordance with the Declaration of Helsinki, the ethics committee of the Tehran University of Medical Sciences approved the protocols of the study, and the parents provided written, informed consent.
Statistical analysis
Qualitative data were analysed and reported as frequency (%). Percentage was reported considering missing system data. P-value< 0.05 was considered significant. The Pearson chi-squared test served to detect any possible correlation between different variables and occurrence of febrile seizure. All data were analysed with IBM SPSS Statistics for Windows, version 21.0 (SPSS Inc., Chicago, Illinois, USA).
Results
The demography of studied children has been presented in Table 1. The majority of them are between 1 and 2 years old, and most of them are in generalized tonic-clonic subtype of seizure. The number of children with simple seizure is more than those with complex seizure. The number of febrile seizures increased during the day and in the evening, peaking between 6 pm and 12 mid-night. This number was at lower level between 12 mid-night and 12 mid-day. Body temperature was recorded between 38 and 39˚C for majority of children, and most of them had not used diazepam. The number of febrile seizures was the highest in autumn, coinciding with November, and the lowest during summer, coinciding with September. The number of children without history of family members with febrile seizure was highest, and Upper Respiratory Tract Infections (URI) was the major causative factor of febrile seizure.
| Variable | Frequencya |
|---|---|
| Age | |
| Under 1 year | 53 (15.4) |
| 1 to 2 years | 139 (40.4) |
| 2 to 3 years | 76 (22.1) |
| 3 to 4 years | 24 (7.0) |
| 4 to 5 years | 25 (7.3) |
| Over 5 years | 21 (6.1) |
| Gender | |
| Male | 147 (42.7) |
| Female | 197 (57.3) |
| Type of seizure | |
| Generalized tonic-clonic seizure | 237 (68.9) |
| Tonic | 27 (7.8) |
| Clonic | 15 (4.4) |
| UWG (Myoclonic) | 21 (6.1) |
| Atonic | 9 (2.6) |
| Lateral gaze | 23 (6.7) |
| Staring | 5 (1.5) |
| Apnea | 1 (0.3) |
| Distonia | 1 (0.3) |
| Spasme | 1 (0.3) |
| Fasciculation | 1 (0.3) |
| Focal | 1 (0.3) |
| Type of febrile seizure | |
| Simple | 287 (83.4) |
| Complex | 55 (16.0) |
| Time between seizure and arriving hospital | |
| Less than 1 hour | 18 (5.2) |
| 1 to 6 hours | 288 (83.7) |
| More than 6 hours | 23 (6.7) |
| Time of seizure | |
| 6 am to 12 md | 36 (10.5) |
| 12 md to 6 pm | 117 (34.0) |
| 6 pm to 12 mn | 145 (42.2) |
| 12 mn to 6 am | 42 (12.2) |
| Temperature of seizure | |
| Below 38°C. | 97 (28.2) |
| 38 to 39°C | 167 (48.5) |
| 39 to 40°C | 70 (20.3) |
| Above 40°C | 4 (1.2) |
| Duration of seizure | |
| Less than 15 minutes | 324 (94.2) |
| More than 15 minutes | 17 (4.9) |
| The use of diazepam | |
| Yes | 19 (5.5) |
| No | 323 (93.9) |
| Season of seizure | |
| Spring | 94 (27.3) |
| Summer | 58 (16.9) |
| Autumn | 108 (31.4) |
| Winter | 82 (23.8) |
| Month of seizure | |
| April | 26 (7.6) |
| May | 27 (7.8) |
| June | 30 (8.7) |
| July | 21 (6.1) |
| August | 34 (9.9) |
| September | 14 (4.1) |
| October | 29 (8.4) |
| November | 48 (14.0) |
| December | 30 (8.7) |
| January | 31 (9.0) |
| February | 17 (4.9) |
| March | 34 (9.9) |
| Family history of febrile seizure | |
| Yes | 67 (19.5) |
| No | 275 (79.9) |
| Febrile disease | |
| Fever | 121 (35.2) |
| Cough | 5 (1.5) |
| Upper Respiratory Infection | 147 (42.7) |
| Gastroesophageal | 38 (11.0) |
| Otitis | 10 (2.9) |
| Shigellosis | 3 (0.9) |
| Pharyngitis | 11 (3.2) |
| Fever and rash | 1 (0.3) |
| Fever (Measles, mumps, rubella) | 1 (0.3) |
| Roseola | 1 (0.3) |
| Urinary tract infection | 1 (0.3) |
| Pneumonia | 1 (0.3) |
| Gingivioostomatitis | 1 (0.3) |
| Fifth disease | 1 (0.3) |
| aData are presented as number (%). | |
Table 1: Demography of children with febrile seizure.
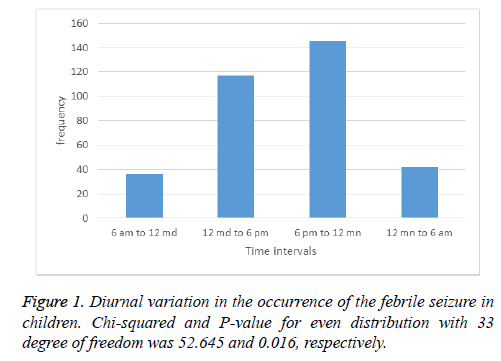
The correlation of different variables with febrile seizure was analysed using Pearson chi square test. Our results showed that occurrence of febrile seizure among children were at lowest in morning, from 6 am to 12 mid-day as shown in Figure 1. This number increased from 12 mid-day to 6 pm, and reached the highest level in evening from 6 pm to 12 midnight. During the night, from 12 midnight to 6 pm the occurrence of febrile seizure back to the lower level.
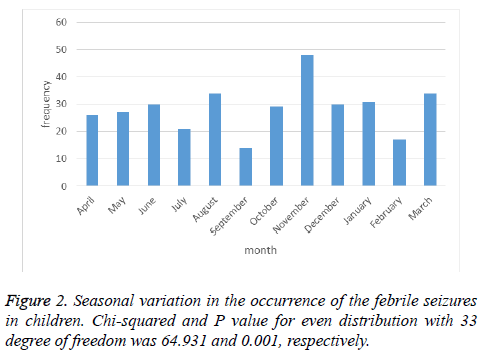
Seasonal variations, also, showed significant difference in occurrence of febrile seizure. The number of febrile seizures was highest in autumn, and November was the month with higher level of febrile seizure. On the other hand, this number decreased in summer, and reached to lower level in September as shown in Figure 2.
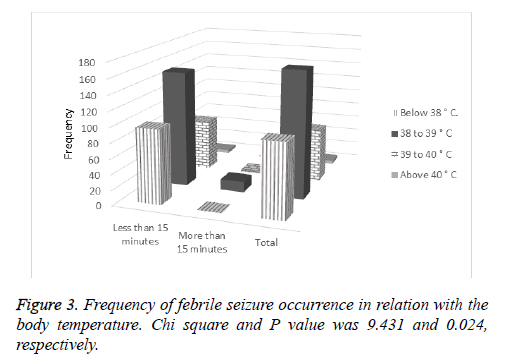
Moreover, the results of this study showed correlation between body temperature and duration of seizure as shown in Figure 3. For seizures either more or less than 15 minutes, the number of children with body temperature between 38 and 39˚C was at higher level. Body temperatures below 38˚C and between 39 and 40˚C were at next levels, and finally, the number of children with body temperatures above 40˚C was at lower level.
Finally, we analysed the distribution and relation between family history of febrile seizure and type of seizure which showed dependence of family history to occurrence of febrile seizure. The number of seizure occurrence among children with no family members suffered by febrile seizure, either for simple or complex type, is more than those with family history of febrile seizure as shown in Table 2.
| Family history of febrile seizure | Type of febrile seizure | Chi square test | ||||
|---|---|---|---|---|---|---|
| simple | complex | Total | Pearson chi square | Df a | P value | |
| yes | 50 | 17 | 67 | 5.33 | 1 | 0.021 |
| no | 237 | 38 | 275 | |||
| Total | 287 | 55 | 342 | |||
| a Degrees of freedom. | ||||||
Table 2: Distribution of febrile seizure in relation with family history of febrile seizure and type of febrile seizure.
Discussion
The present study aimed to determine the factors dependent with febrile seizures in children. In a study in Finland, children experienced their first febrile seizure most often in the evening and less frequently at night [14]. In a study performed in Italy, the first febrile seizures peaked in the evening between 6 PM and 11:59 PM [11]. Also, another study in Japan showed that febrile seizures occurred five times more often in the evening, peaking in incidence at 4 PM and reaching its lowest incidence at 4 AM in the morning, [15]. Our findings was consistent with others studies and implied to more occurrence of febrile seizure in evening, especially from 6 pm to 12 midnight, and low occurrence in morning. Melatonin concentrations are higher at night than during the day, no matter in healthy children, children with epilepsy, and children with febrile seizures, with the highest concentration occurring at 4 AM [16]. Moreover, the studied children in Finland experienced febrile seizures most often in the winter and less frequently during the summer [14]. Febrile seizures often occur in winter, peaking from November to January, and back to the lowest in summer [11]. In the present study, the number of febrile seizure occurrence was at higher level in autumn, peaking in November, and this measurement back to the lowest in summer, especially in September. In winter, Melatonin concentrations are at the highest level, in summer is at the lowest, and its measure is intermediate in spring and fall [17]. The duration and amplitude of the melatonin peak is in negative correlation with the amount of daylight [18]. During a sleep period, the body temperature decreases further due to endogenous circadian rhythm of body temperature [19]. Decline in oxygen consumption, following with dilatation of the peripheral vessels, and risen sweating (which elevates thermal emission), reduced sympathetic-nerve activity during sleep are known as reasons of decrease in body temperature [20]. Also, Fever has been considered as a provocative factor for a first presentation of seizure in patients with epileptic potential [21]. Thus, the lower body temperature could be an attribution of less number of febrile seizures during night and seasons with longer nights. The results of our study identified that duration of seizure depends on body temperature. Studies have identified high body temperature during infections as the strongest explanatory variable for the occurrence of febrile seizures [22,23]. Also, febrile seizure occur only to a limited extent between age 6 months and preschool age, during which period the circadian rhythm of body temperature seems to be robust [24,25]. Although researchers have also discussed the role of normal body temperature variation as a pathogenesis of febrile seizures [11,26] children at risk for febrile seizures (i.e., between 6 months and 6 years of age) have more stable body temperatures over a 24 hour period than do adults [15,25]. Moreover, body temperature has shown no difference between the low- and high frequency times of febrile seizures in a given day [15] or during the year [14]. According to the results of present study, seizures with duration of more than 15 min occurred at body temperature between 38 and 39˚C. It has been reported that patients with febrile seizure were reported to exhibit significantly higher temperatures than age matched control subjects [20,23]. Immunologic factors are in correlation with generation of febrile seizure, and polymorphisms in the interleukin gene may be used as markers of susceptibility to febrile convulsions [27,28]. Hypothesis that febrile convulsions occur when temperature reaches a certain level in children who have a genetic tendency to develop higher temperatures during infections [15]. Results of present study indicated to the correlation between family history of seizure and its occurrence (P=0.021). Febrile seizure occurrence depends on both genetic and environmental elements, so in some cases in has a familial tendency [29]. Studies have demonstrated that febrile seizure occurs with higher probability of incidence in first- and second-degree relatives of children with febrile seizure [30]. Twenty-five to 40% of patients showed a positive family history for febrile seizure [30] which this proportion is observable in our results, especially for children with complex febrile seizure. It seems that recurrence of seizure, as an indication of complex type, is more common among children with family history of seizure. Some genes have been identified which are linked with febrile seizure and their mutations have been observed in patients with febrile seizure [31,32]. As a result, family history is a remarkable factor to prognosticate febrile seizure.
In conclusion, the occurrence of febrile seizure depends on diurnal and seasonal variation. Also, the body temperature and history of family with febrile seizure are significant factors for diagnosis.
References
- Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League against Epilepsy. Epilepsia 1989; 30: 389-399.
- Nelson KB, Ellenberg JH. Prognosis in children with febrile seizures. Pediatr 1978; 61: 720-727.
- Vaswani RK, Dharaskar PG, Kulkarni S, Ghosh K. Iron deficiency as a risk factor for first febrile seizure. Indian Pediatr 2010; 47: 437-439.
- Sadleir LG, Scheffer IE. Febrile seizures. BMJ 2007; 334: 307-311.
- Mohebbi MR, Holden KR, Butler IJ. FIRST: a practical approach to the causes and management of febrile seizures. J Child Neurol 2008; 23: 1484-1488.
- Omran MS, Khalilian E, Mehdipour E, Juibary AG. Febrile seizures in North Iranian children: Epidemiology and clinical feature. J Pediatr Neurol 2008; 6: 39-42.
- Vahidnia F, Eskenazi B, Jewell N. Maternal smoking, alcohol drinking, and febrile convulsion. Seizure 2008; 17: 320-326.
- Hastings M. The brain, circadian rhythms, and clock genes. BMJ 1998; 317: 1704-1707.
- Haldar C, Ahmad R. Photoimmunomodulation and melatonin. J Photochem Photobiol 2010; 98: 107-117.
- Sanchez-Barcelo EJ, Mediavilla MD, Reiter RJ. Clinical uses of melatonin in pediatrics. Int J Pediatr 2011; 2011: 892624.
- Manfredini R, Vergine G, Boari B, Faggioli R, Borgna-Pignatti C. Circadian and seasonal variation of first febrile seizures. J Pediatr 2004; 145: 838-839.
- Goldberg-Stern H, Oren H, Peled N, Garty BZ. Effect of melatonin on seizure frequency in intractable epilepsy: a pilot study. J Child Neurol 2012; 27: 1524-1528.
- Banach M, Gurdziel E, Jadrych M, Borowicz KK. Melatonin in experimental seizures and epilepsy. Pharmacol Rep 2011; 63: 1-11.
- Mikkonen K, Uhari M, Pokka T, Rantala H. Diurnal and seasonal occurrence of febrile seizures. Pediatr Neurol 2015; 52: 424-427.
- Ogihara M, Shirakawa S, Miyajima T, Takekuma K, Hoshika A. Diurnal variation in febrile convulsions. Pediatr Neurol 2010; 42: 409-412.
- Ardura J, Andres J, Garmendia JR, Ardura F. Melatonin in epilepsy and febrile seizures. J Child Neurol 2010; 25: 888-891.
- Davis S, Kaune WT, Mirick DK, Chen C, Stevens RG. Residential magnetic fields, light-at-night, and nocturnal urinary 6-sulfatoxymelatonin concentration in women. Am J Epidemiol 2001; 154: 591-600.
- de Almeida EA, Di Mascio P, Harumi T, Spence DW, Moscovitch A. Measurement of melatonin in body fluids: standards, protocols and procedures. Childs Nerv Syst 2011; 27: 879-891.
- Czeisler CA, Buxton OM, Khalsa SBS. The human circadian timing system and sleep-wake regulation. Principles practice of sleep medicine. Philadelphia Elsevier Saunders (4th edn.) 2005; 375-394.
- Gander PH, Connell LJ, Graeber RC. Masking of the circadian rhythms of heart rate and core temperature by the rest-activity cycle in man. J Biol Rhythms 1986; 1: 119-135.
- Lee SH, Byeon JH, Kim GH, Eun BL, Eun SH. Epilepsy in children with a history of febrile seizures. Korean J Pediatr 2016; 59: 74-79.
- Rantala H, Uhari M, Hietala J. Factors triggering the first febrile seizure. Acta Paediatr 1995; 84: 407-410.
- Berg AT, Shinnar S, Shapiro ED, Salomon ME, Crain EF. Risk factors for a first febrile seizure: a matched case-control study. Epilepsia 1995; 36: 334-341.
- Herman JH. Chronobiology of sleep in children. Principles and practice of sleep medicine. Philadelphia Elsevier Saunders (4th edn.) 2005; 85-99.
- Garcia J, Rosen G, Mahowald M. Circadian rhythms and circadian rhythm disorders in children and adolescents. Semin Pediatr Neurol 2001; 8: 229-240.
- Tsuboi T, Okada S. Seasonal variation of febrile convulsion in Japan. Acta Neurol Scand 1984; 69: 285-292.
- Matsuo M, Sasaki K, Ichimaru T, Nakazato S, Hamasaki Y. Increased IL-1beta production from dsRNA-stimulated leukocytes in febrile seizures. Pediatr Neurol 2006; 35: 102-106.
- Heida JG, Pittman QJ. Causal links between brain cytokines and experimental febrile convulsions in the rat. Epilepsia 2005; 46: 1906-1913.
- Berg AT, Shinnar S, Levy SR, Testa FM. Childhood-onset epilepsy with and without preceding febrile seizures. Neurology 1999; 53: 1742-1748.
- Greenberg DA, Holmes GL. The genetics of febrile seizures. Febrile seizures San Diego Acad Press 2002; 249-261.
- Herini ES, Gunadi, Harahap IS, Yusoff S, Morikawa S, Patria SY. Generalized epilepsy with febrile seizures plus (GEFS+) spectrum: clinical manifestations and SCN1A mutations in Indonesian patients. Epilepsy Res 2010; 90: 132-139.
- Patel AD, Vidaurre J. Complex febrile seizures: a practical guide to evaluation and treatment. J Child Neurol 2013; 28: 762-767.