ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 20
Association among measures of physical function, functional disability and self-perceived fatigue in individuals with obesity
Zeliha Başkurt1*, Ferdi Başkurt1, Sabriye Ercan2 and Cem Çetin3
1Department of Physiotherapy and Rehabilitation, Süleyman Demirel Üniversity, Isparta, Turkey
2Department of Sports Medicine, Dr. Ersin Arslan Education and Research Hospital, Gaziantep, Turkey
3Department of Sports Medicine, Suleyman Demirel University, Medicine Faculty, Isparta, Turkey
- *Corresponding Author:
- Zeliha Başkurt
Faculty of Health Sciences
Department of Physiotherapy and Rehabilitation
Süleyman Demirel Üniversity, East Campus, Turkey
Accepted date: November 2, 2017
Objective: The purposes of this study were: 1) to compare the levels of self-perceived fatigue, physical function and self-reported functional disability between individuals with obesity and without obesity, and 2) to examine whether self-perceived fatigue predicted physical function and self-reported functional disability in individuals with obesity.
Materials and Methods: Subjects who had a Body Mass Index (BMI) greater than 30 kg/m2 were classified into the participants with obesity group (n=111) while those with BMI between 18.5 and 24.9 kg/m2 were included into control group (n=138). Fatigue was measured with the Turkish version of Checklist Individual Strength (CIS-T). The Physical Component Summary measure of the SF-12 (PCS-12), a 30 s Chair Stand Test (30 s CST), 11-step stair ascend/descend test (STTotal-11), and 10 m Walk Test (10 m WT) were used to evaluate physical functions of the participants. Functional disability was evaluated with the Functional Disability Questionnaire (FDQ).
Results: There were statistically significant differences between the participants with obesity group and the control group regarding functional disability score, physical function measures, and fatigue scores (p<0.05). According to the CIST-T scale, 56.75% of individuals with obesity were fatigued. FDQ scores, PCS-12 scores, 10 m WT times, STTotal-11 times, and 30 s CST scores were significantly poorer in fatigued participants with obesity compared with the non-fatigue participants with obesity (p<0.05). Fatigue showed a significant correlation with functional disability and physical function parameters in participants with obesity (p<0.05).
Conclusions: It is recommended that fatigue coping skills are included in strategies aimed at returning functional losses due to obesity.
Keywords
Obesity, Fatigue, Functional disability, Physical functions
Introduction
Obesity is abnormal or excessive accumulation of fat in the body in a way that is harmful to health. The World Health Organization defines and classifies obesity based on body mass index (BMI ≥ 30 kg/m2) and waist circumference measurement (above 88 cm for women and above 102 cm for men) [1]. Obesity is a chronic disease with rapidly increasing prevalence in developed and developing countries and causes a great burden on national economy. As well as causing medical problems, obesity triggers various psychosocial problems and is a significant risk factor in terms of morbidity and mortality [2,3].
Obesity has physiological effects on functions of many organs. The most affected systems include the cardiovascular system (blood and oxygen change), the respiratory system (oxygencarbon dioxide change) and the musculoskeletal system (mobility and stress) [4]. When asked why they want to receive obesity treatment, patients report deficiency in daily physical functions due to shortness of breath, pain in weight-bearing joints such as knees, low energy level (fatigue) and/or loss of mobility [4]. Moreover, obesity may increase perceived fatigue and physical function loss without affecting other metabolic and physiological markers or morbidity and adversely affect quality of life [5].
The burden caused by obesity on physical functions such as bodily pain and limitations in physical roles is studied using health-related quality of life scales. Results of these scales indicate lower quality of life associated with loss in physical functions [4]. Subjective symptoms such as fatigue are often reported by individuals with obesity [3]. Fatigue perceived by individuals with obesity reduces quality of life in patients by reducing activity tolerance and adversely affecting performance of daily life activities [6].
Fatigue is defined as a subjective sensation containing emotional, cognitive and behavioral components. It is a common complaint in the community, may be felt anytime, refers to lack of energy and is usually temporary. Fatigue causes a sedentary life style and reduces quality of life. It is also known as one of the reasons behind patients’ inability to continue or comply with their exercise program. Fatigue restricts personal, social, professional, educational and mental functions of the patient and accompanies rich clinical findings [7,8].
The prevalence of fatigue is 10-25% in the general population. This rate reaches up to 50% in the elderly [9]. Functions of the individual start to deteriorate in cases where the complaint of fatigue continues for prolonged periods of time. Studies show that the vast majority of chronic patients experience fatigue, fatigue limits self-care requirements such as working, fulfilling the duties of the house, bathing and dressing, and fatigue affects motivation, concentration, social life and leisure time activities adversely [10].
The link between fatigue observed in obesity and inflammatory mediators has been shown in the current literature [11]. According to studies, increased adipose tissue results in increased C-reactive protein amount in blood and increased systemic inflammatory markers adversely affect the nervous system either directly or indirectly [9]. However, it is still not clear how increased body weight and inflammation affects the fatigue level [11].
As a chronic disease, obesity restricts physical functions such as ascending and descending stairs, walking and incapacitates individuals from performing activities of daily living and selfcare. Combined with the impact of a sedentary life style, it becomes increasingly difficult for patients to perform their basic functions in daily living [12]. Although there are many studies in the literature investigating different results of obesity, there is no study available on effects of fatigue on these results.
The purposes of this study were to compare the levels of selfperceived fatigue between individuals with obesity and individuals without obesity, and to examine whether selfperceived fatigue predicted physical function and self-reported functional disability.
Methods
Ethics approval for this study was obtained from the Ethics Committee of Süleyman Demirel University, Turkey. Informed consent was obtained from all participants in accordance with the Declaration of Helsinki. This is a descriptive observational study carried out in a cross-sectional manner without intervention. Subjects completed a detailed medical history questionnaire to begin the assessment. By the interview, comorbid diseases, general health status, presence of mood disorders, infection or sleep disorders which cause secondary fatigue, endocrine system and hypothalamic disorders, genetic syndromes, the use of drugs such as glucocorticoids, cyproheptadine and phenothiazines which cause secondary development of obesity were questioned. Individuals with secondary obesity and secondary fatigue were excluded from study. A total of 249 volunteers were recruited from the community to participate in the study.
Height and weight measurements were performed using a wallmounted stadiometer (SECA 700, Germany) after exhaling, in bare feet and upright position. Then, body mass index (BMI) (kg/m2) was calculated using the method determined by the World Health Organization. The participants were assigned to two groups based on their BMI values as those with a BMI of 30 kg/m2 and above (participants with obesity group) and those with a BMI in the 18.5-24.9 kg/m2 range (control group) [13]. Table 1 shows the inclusion and exclusion criteria for the present study.
| Inclusion criteria |
| ·To be between the ages of 20-65 |
| · To have a BMI of 30 kg/m2 and above |
| · To have a BMI in the 18.5-24.9 kg/m2 range |
| Exclusion criteria |
| · To have musculoskeletal and neuromuscular problems (neurological, orthopedic, vestibular diseases) affecting walking performance considerably |
| · To have undergone joint surgery or received back pain treatment recently |
| · To have cognitive and psychiatric disorders |
| · To have instable angina or uncontrolled arterial hypertension |
| · To have severe pulmonary hypertension |
| · To have recent history of cardiac arrhythmia or myocardial infarction |
| · To have a clinical condition (malignancy, etc.) which may worsen due to physical effort |
| · To have a BMI below 18.5 or in the 25.1- 29.9 range |
| BMI: Body Mass Index; Kg: Kilogram; M: Meter. |
Table 1. Inclusion and exclusion criteria.
Fatigue
Fatigue was measured with the Turkish version of Checklist Individual Strength (CIS-T), a 20-item self-report instrument. The validity and the reliability of the Turkish version of CIS were established by Ergin and Yldirim [14]. The statements refer to aspects of fatigue experienced during the previous 2 w. The CIS-T covers several aspects of fatigue, such as subjective fatigue (eight items, for example, “I get tired very quickly”); concentration (five items, for example, “Thinking requires effort”); motivation (four items, for example, “I feel no desire to do anything”); physical activity (three items, for example, “I don’t do much during the day”), and a total score. Items are scored on a seven point Likert scale (1=Yes, that is true to 7=No, that is not true). The total score can range from 20 to 140. Higher scores indicate a higher degree of fatigue. A cutoff score (a CIS total score which is a summation of the four aspects higher than 76) was used to distinguish individuals fatigue and non-fatigue [14].
Functional disability
Functional disability was evaluated with the Functional Disability Questionnaire (FDQ), a 10-item self-report instrument. Functional Disability Questionnaire evaluates 10 different daily life activities such as dressing, personal hygiene (bathing, etc.), clipping toenails, urine incontinence, putting on and tying shoes, crouching and standing up, getting up from the couch, picking up something from the floor, carrying shopping bags, picking up something from the bottom drawer. For each question, the participants were asked to choose one of the following: ‘I do not have difficulty', ‘I have a little difficulty', ‘I have difficulty', 'I have a lot of difficulty' or ' It is impossible for me to do’. The total score can range from 10 to 50. Higher scores indicate a higher disability. The questionnaire items were selected from a question pool created by the authors reviewing the relevant literature [12,15]. The data obtained from the questionnaire were imported to SPSS program and the instrument was tested for reliability. The reliability coefficient (Cronbach's alpha) of the instrument was calculated to be 0.88.
Physical function
Physical functions of participants was measured with the physical component summary measure of the SF-12 (PCS-12), a 30 s Chair Stand Test (30 s CST), 11-step stair ascend/ descend (STTotal-11), and 10 m Walk Test (10-m WT).
PCS-12: SF-12 is a 12-item overall quality of life questionnaire assessing functional status, well-being and general health. SF-12 has two sub-dimensions assessing physical and mental status. The physical component consists of sub-questionnaires of physical function, physical role, bodily pain and general health. The physical component part of the questionnaire was utilized in this study. A higher score obtained from the questionnaire indicates a higher quality of life [16].
30 s CST: The chair stand test is a practical method used to assess lower extremity muscle strength. It tests the ability to stand up from a chair of standard height with backrest. In the initial position of the test, the subject is seated on an armless chair, arms folded across the chest and feet on the floor. With the go command, the subject rises into standing position and sits back down. The first rising and sitting movement is accepted as the trial and the test is now introduced to the subject. Then the test is applied and the rising and sitting cycle continues for 30 s. The number of complete rises performed by the subject within 30 s is noted. The number obtained is the score of the subject [17].
STTotal-11: In this test, the subject is asked to ascend and descent an 11-step stair with normal walking pace. The measurement starts at the first step of ascending and ends at the last step of descending. 11-step stair ascend and descend time is measured with a chronometer and the ascend time and the total time are recorded in seconds [18].
10 m WT: For this test, a distance of 10 m is marked on a level floor and the subject is asked to walk 10 m at a comfortable pace. The time starts when the subject’s foot is on the start line and stops when the subject’s foot passes the finish line. Two measurements are made and the average walking time is recorded in seconds [19].
Statistical analysis
The data were analysed statistically using the SPSS program for Windows, version 20.0. Results for continuous variables were given as mean ± standard deviation and categorical variables were given as number and frequencies. A Chi-square test was used to compare the qualitative variables. For the comparison between the two groups the Student t-test for independent was used. Correlations between CIS-T scores, physical function and self-reported functional disability parameters in participants with obesity were calculated with Pearson correlation analysis. The statistical significance was considered at 0.05.
Results
A total of 249 individuals, 111 with obesity and 138 without obesity, participated in the study. For the participants with obesity (57.7% female, 42.3% male), the mean ± standard deviation age was 41.31 ± 10.65 y, the mean height was 166.68 ± 9.3 cm, the mean body weight was 92.76 ± 11.25 kg, the mean BMI was 33.32 ± 2.94 kg/m2; whereas for the participants in control group (51.5% female, 48.5% male), these parameters were 39.38 ± 9.2 y, 171.27 ± 9.18 cm, 67.05 ± 9.91 kg, and 22.74 ± 1.77 kg/m2 respectively. A statistically significant difference was found between the groups in terms of height, body weight, BMI and work status (p<0.05) (Table 2).
| Participants with obesity group (n=111) | Control group (n=138) | p | |
|---|---|---|---|
| Age (y) (mean ± SD) | 41.31 ± 10.65 | 39.38 ± 9.20 | 0.126 |
| Height (cm) (mean± SD) | 166.68 ± 9.30 | 171.27 ±9.18 | 0.001* |
| Weight (kg) (mean ± SD) | 92.76 ± 11.25 | 67.05 ± 9.91 | 0.001* |
| BMI (kg/m2) (mean ± SD) | 33.32± 2.94 | 22.74 ± 1.77 | 0.001* |
| Gender (n/%) | |||
| Female | 64/57.7 | 71/51.5 | 0.163 |
| Male | 47/42.3 | 67/48.5 | |
| Work status (n /%) | |||
| House wife | 40/36.03 | 32/23.18 | |
| Retired | 12/10.81 | 17/12.31 | 0.006* |
| Actively Working | 51/45.94 | 78/56.52 | |
| Student | 8/7.20 | 11/7.97 | |
| Tobacco use (n/%) | |||
| Yes | 43/38.73 | 61/44.20 | 0.267 |
| No | 68/61.26 | 77/55.79 | |
| Medications (n/%) | |||
| Not using | 89/80.18 | 121/87.68 | 0.066 |
| At least one regular | 22/19.81 | 17/12.31 | |
| cm: Centimeter, kg: Kilogram, m: Meter, SD: Standard Deviation, BMI: Body Mass Index, *p<0.05 |
Table 2. Characteristics of the participants with obesity and without obesity.
When the comorbid diseases of the participants were evaluated, 22% had dislipidemia, 24% had controlled hypertension, 14% had diabetes mellitus, and 19% had arthritis in the participants with obesity group. In the control group, 13% had dislipidemia, 11% had controlled hypertension, 4% had diabetes mellitus and 9% had arthritis.
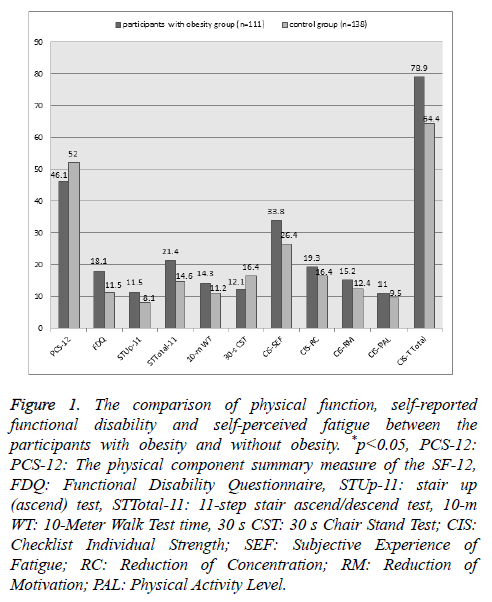
A statistically significant difference was found between the participants with obesity and the participants in control group in terms of PCS-12 (p: 0.006), 30 s CST (p: 0.048), STUp-11 and STTotal-11 (p: 0.001), 10 m WT (p: 0.008) and Functional Disability Score (p: 0.001) and CIST-T (p: 0.001) (Figure 1).
Figure 1: The comparison of physical function, self-reported functional disability and self-perceived fatigue between the participants with obesity and without obesity. *p<0.05, PCS-12: PCS-12: The physical component summary measure of the SF-12, FDQ: Functional Disability Questionnaire, STUp-11: stair up (ascend) test, STTotal-11: 11-step stair ascend/descend test, 10-m WT: 10-Meter Walk Test time, 30 s CST: 30 s Chair Stand Test; CIS: Checklist Individual Strength; SEF: Subjective Experience of Fatigue; RC: Reduction of Concentration; RM: Reduction of Motivation; PAL: Physical Activity Level.
According to the CIST-T scale, 56.75% of individuals with obesity were fatigued. FDQ scores, 10 m WT times, STTotal-11 times were significantly higher in fatigued participants with obesity compared with the non-fatigue participants with obesity (p<0.05). PCS-12 and 30 s CST scores were significantly lower in fatigued participants with obesity compared with the non-fatigue participants with obesity (p<0.05) (Table 3). Fatigue showed a significant correlation with functional disability and physical function parameters in participants with obesity (p<0.05).
| Fatigue the participants with obesity (n=63) | Non-fatigue the participants with obesity (n=48) | p | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| PCS-12 | 43.39 ± 9.67 | 49.23 ± 7.23 | 0.004* |
| 30 s CST | 11.43 ± 4.93 | 12.87 ± 5.07 | 0.030* |
| STUp-11 | 12.14±6.73 | 10.17±4.63 | 0.006* |
| STTotal-11 | 22.52 ± 10.70 | 18.86 ± 8.55 | 0.004* |
| 10 m walk test time | 16.14 ± 6.77 | 12.05 ± 5.01 | 0.005* |
| Functional disability score | 19.40 ± 6.71 | 16.16 ± 5.51 | 0.001* |
Table 3. The comparison of physical functions and self-reported functional disability between fatigue and non-fatigue the participants with obesity.
Discussion
The results of this study showed that individuals with obesity had lower physical functions and higher functional disability levels and self-perceived fatigue levels compared to participants in the control group. Besides, significant correlations were found between total self-perceived fatigue levels, all physical function measurements and functional disability levels of participants with obesity.
Obesity is associated with low physical function and limitations in overall life activities [20]. It is known that physical well-being is disrupted due to obesity and physical disability rate is over 66% in individuals with obesity [21]. This fact indicates that obesity is an independent factor related to physical function [4]. In a study conducted by Lean et al. between 1993-1995 to assess health status and daily functions of 14000 randomly selected Dutch individuals, it was observed that almost all parameters tested in the study were affected by BMI. It was found in the study that low quality of life associated with decreased respiratory system functions, back pain and decreased physical function even worsened with increasing severity of obesity and women were more likely to be affected [22]. Friedmann et al. obtained a similar result as well [23]. Larsson and Mattsson explored cases of functional disability such as indoors walking distance, walking pace and stair ascend/descend difficulty and found that individuals with obesity had difficulty playing sports, walking outdoors, climbing stairs, performing moderate housework, lifting an object and reaching a high place [12]. The functional disability questionnaire used in this study was consisting of dressing, personal hygiene, cutting toenails, urinary stress incontinence, wearing shoes, rising from squatting rising from sofa, picking things up from floor, carrying grocery bags and taking things out from bottom cupboards activities.
Disease-specific instruments were developed to determine the burden caused by obesity on daily life and these instruments facilitated making a comparison between individuals with obesity and normal weight. For example, Le et al. developed statements such as “I do not move around very much”, “When I climb the stairs, I have to rest to catch my breath after several steps”, “I walk as little as possible”, “I have trouble squatting and kneeling”, and “I have trouble getting on and off buses, trains, subway, etc.” to assess overall quality of life associated with the disease in case of obesity [24]. Mannucci et al. included questions such as “Does sweating affect your daily activities?” or “Does your weight constitute an obstacle for your sexual activity?” in their questionnaires and observed that symptoms worsened as obesity level increased [25]. In our study, two self-report questionnaires were used to assess physical functions. The first of these questionnaires is SF-12’s Physical Component (PCS-12). The second is the functional disability questionnaire formed as a result of literature review and tested for reliability. Difficulty levels of individuals with obesity in activities of daily living were investigated with this questionnaire. In our study, functional disability and PCS-12 scores of participants with obesity were found to be low, which supports findings in the literature.
Fatigue is one of the symptoms accompanying obesity. It is necessary to assess fatigue and plan activities appropriate for the individual to prevent the symptom of fatigue from adversely affecting the individual. Thus, it is possible to cope with this symptom effectively and reduce losses in physical functions [26]. Health professionals must be able to identify fatigue in obesity to plan appropriate treatment strategies, distinguish fatigue from depression or lack of motivation, assess fatigue level and manage the condition. However, adequate instruments to identify fatigue, distinguish it from similar concepts such as depression and assess verbal expressions of patients are not available [10]. Despite the fact that fatigue is a frequently studied condition in chronic diseases, the number of studies on the symptom of fatigue in individuals with obesity and fatigue’s effects on this population is very limited in the literature. Among the limited number of diagnostic instruments, the Checklist Individual Strength (CIS) questionnaire, which presents an all-round assessment of chronic fatigue and was culturally adapted for the Turkish society, was used in our study. The participants in our study were examined for fatigue using this instrument and it was observed that the individuals with obesity had a fatigue level of 56.75% and fatigue reached a high level in obesity as in other chronic diseases. Also our study showed that fatigue level have a strong correlation with functional disability level (except for CIS-Physical Activity Level) and PCS-12 scores (except for CIS-Reduction of Concentration) in individuals with obesity.
The relationship between BMI and self-reported functional limitations has been frequently investigated in the literature. Physical function limitations determined with scales or questionnaires may be determined with functional tests as well [23]. However, the number of studies investigating physical function limitations with functional tests in individuals with obesity is very limited. It was found in the limited number of studies in the literature that individuals with obesity had an oxygen expenditure of 56% during a comfortable walk outdoors and their metabolic rate was 10% higher than normal [12,27]. In another study assessing functional performance in individuals with obesity, it was found that individuals with obesity had a lower normalized shoulder muscle strength and endurance [2]. A similar result was obtained for lower extremity muscle functions as well [28]. Decrease in lower and upper muscle functions obstructs daily functions of individuals with obesity and increases functional losses [2,28]. Sharkey et al. assessed physical performances of older participants with obesity for 1 year with timed walking, static and dynamic balance, and chair rise. The researchers found that severe obesity (BMI>35.0 kg/m2) was an independent risk factor for losses in lower extremity functions [29]. In obesity, compensations in the locomotor system such as changes in temporospatial walking characteristics and plantar foot pressure limit movement and functionality as well [30]. Besides, the fact that obesity is frequently accompanied with diseases affecting the musculoskeletal system such as osteoarthritis in particular increases losses in lower extremity functions even further [31]. Other musculoskeletal system diseases are commonly seen in individuals with obesity as well. Okifuji et al. noted that obesity was associated with higher fibromyalgia pain severity and sensitivity, poorer sleep quality, declined walking duration and distance due to pain, and reduced physical strength-endurance and flexibility [32]. As is seen, not only obesity, but also compensations and diseases related to obesity worsen the condition of the patient. In the present study, physical function performances of individuals with obesity was assessed and it was shown that individuals with obesity had significantly lower performance compared to the control group in walking, ascending and descending stairs, and rising from chair and low performance was associated with fatigue.
The most important strength of this study is that fatigue was assessed in detail in the population with obesity and physical performance was assessed in detail using valid methods in the same population. However, the present study has certain limitations. These include the cross-sectional design of the study, some self-report assessments included in the study and relatively lower BMI values of the obesity population compared to other samples in the literature.
In conclusion, impairments in multiple domains of physical function, low functional disability level and high fatigue level were associated with obesity. Furthermore, poor physical performance and disability score were related to higher fatigue level in participants with obesity. Individuals with obesity have a low quality of life with respect to physical dimension, have limited lower extremity functions and these limitations affect their functionality in daily living. Also, the present study has shown for the first time that obesity associated fatigue adversely affects the functional capacity of the individual. In the light of this information, it is recommended that fatigue coping skills are included in strategies aimed at returning functional losses due to obesity.
References
- Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care 2008; 11: 693-700.
- Cavuoto LA, Nussbaum MA. Differences in functional performance of the shoulder musculature with obesity and aging. Int J Ind Ergon 2013; 43: 393-9.
- Froehlich-Grobe K, Lollar D. Obesity and disability: time to act. Am J Prev Med 2011; 41: 541-545.
- Kushner RF, Foster GD. Obesity and quality of life. Nutrition 2000; 16: 947-952.
- Seidell JC. The impact of obesity on health status: some implications for health care costs. Int J Obes Relat Metab Disord 1995; 19 Suppl 6: S13-16.
- Katz DA, McHorney CA, Atkinson RL. Impact of obesity on health-related quality of life in patients with chronic illness. J Gen Intern Med 2000; 15: 789-796.
- Working Group of the Royal Australasian College of Physicians. Chronic fatigue syndrome. Clinical practice guidelines. Med J Aust 2002; 176: 17-55.
- Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry 2003; 160: 221-236.
- Valentine RJ, McAuley E, Vieira VJ, Baynard T, Hu L, Evans EM, Woods JA. Sex differences in the relationship between obesity, C-reactive protein, physical activity, depression, sleep quality and fatigue in older adults. Brain Behav Immun 2009; 23: 643-648.
- Sutcu Cicek H, Akbayrak N. KOAH Olan Bireylerde Yorgunluk ve Basetme Stratejileri. Fatigue on patients with COPD and coping strategies. Iç Hastaliklari Dergisi 2009; 16: 135-138.
- Valentine RJ, Woods JA, McAuley E, Dantzer R, Evans EM. The associations of adiposity, physical activity and inflammation with fatigue in older adults. Brain Behav Immun 2011; 25: 1482-1490.
- Larsson UE, Mattsson E. Perceived disability and observed functional limitations in obese women. Int J Obes Relat Metab Disord 2001; 25: 1705-1712.
- Xu X, Mirka GA, Hsiang SM. The effects of obesity on lifting performance. Appl Ergon 2008; 39: 93-98.
- Ergin G, Yildirim Y. A validity and reliability study of the Turkish Checklist Individual Strength (CIS) questionnaire in musculoskeletal physical therapy patients. Physiother Theory Pract 2012; 28: 624-32.
- Larsson UE. Influence of weight loss on pain, perceived disability and observed functional limitations in obese women. Int J Obes Relat Metab Disord 2004; 28: 269-277.
- Wee CC, Davis RB, Hamel MB. Comparing the SF-12 and SF-36 health status questionnaires in patients with and without obesity. Health Qual Life Outcomes 2008; 6: 11.
- Gardner AW, Montgomery PS. The relationship between history of falling and physical function in subjects with peripheral arterial disease. Vasc Med 2001; 6: 223-227.
- Almeida GJ, Schroeder CA, Gil AB, Fitzgerald GK, Piva SR. Interrater reliability and validity of the stair ascend/descend test in subjects with total knee arthroplasty. Arch Phys Med Rehabil 2010; 91: 932-938.
- Metli NB, Kurtaran A, Akyüz M. Impaired balance and fall risk in heumatoid arthritis patients. Turk J Phys Med Rehab 2015; 61: 344-351.
- Seidell JC. Societal and personal costs of obesity. Exp Clin Endocrinol Diabetes 1998; 106: 7-9.
- Rimmer JH, Wang E, Yamaki K, Davis B. Documenting disparities in obesity and disability. Focus: NCDDR 2010; 24: 1-16.
- Lean ME1, Han TS, Seidell JC. Impairment of health and quality of life using new US federal guidelines for the identification of obesity. Arch Intern Med 1999; 159: 837-843.
- Friedmann JM, Elasy T, Jensen GL. The relationship between body mass index and self-reported functional limitation among older adults: a gender difference. J Am Geriatr Soc 2001; 49: 398-403.
- Le Pen C, Levy E, Loos F, Banzet MN, Basdevant A. Specific scale compared with generic scale: a double measurement of the quality of life in a French community sample of obese subjects. J Epidemiol Comm Health 1998; 52: 445-450.
- Mannucci E, Ricca V, Barciulli E, Di Bernardo M, Travaglini R, Cabras PL, Rotella CM. Quality of life and the overweight; the obesity related well-being (Orwell 97) Questionnaire. Addict Behav 1999; 24: 345-357.
- Yurtsever S. The fatigue in chronic illnesses and nursing care. CNJ 2000; 4: 16-20.
- Browning RC, McGowan CP, Kram R. Obesity does not increase external mechanical work per kilogram body mass during walking. J Biomech 2009; 42: 2273-2278.
- Maffuletti NA, Jubeau M, Bizzini UMM, Agosti F, Lafortuna ADCL, Sartorio A. Differences in quadriceps muscle strength and fatigue between lean and obese subjects. Eur J Appl Physiol 2007; 101: 51-59.
- Sharkey JR, Ory MG, Branch LG. Severe elder obesity and 1-year diminished lower extremity physical performance in homebound older adults. JAGS 2006; 54: 1407-1413.
- Wearing SC, Hennig EM, Byrne NM, Steele JR, Hills AP. The biomechanics of restricted movement in adult obesity. Obes Rev 2006; 7: 13-24.
- Ells LJ, Lang R, Shield JP, Wilkinson JR, Lidstone JS, Coulton S, Summerbell CD. Obesity and disability-a short review. Obes Rev 2006; 7: 341-345.
- Okifuji A, Donaldson GW, Barck L, Fine PG. Relationship between fibromyalgia and obesity in pain, function, mood, and sleep. J Pain 2010; 11: 1329-1337.