ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 14
Association of extracellular superoxide dismutase Ala40Thr, Arg213Gly, and Leu53Leu polymorphisms with risk of type 2 diabetes mellitus in a Chinese population
Haoyun Li1#, Changmeng Zhang2#, Li He1, Fengjiao Zhang1, Fang Luo1, Yu Mao1, Jiangdong Tang1,Hongmei Liu1, Zhiqiang Kang1 and Qingchu Li1*
1Department of Endocrinology, Zhengzhou Central Hospital, Zhengzhou University, Zhengzhou, PR China
2Department of Orthopaedics, Zhengzhou Central Hospital, Zhengzhou University, Zhengzhou, PR China
#The two authors contribute equally to this study.
- *Corresponding Author:
- Qingchu Li
Department of Endocrinology
Zhengzhou Central Hospital
Zhengzhou University, Zhengzhou ,PR China
Accepted on June 19, 2017
Objective: We performed a study to investigate the association of EC-SOD Ala40Thr (rs2536512), Arg213Gly (rs8192291) and Leu53Leu (rs1799895) polymorphisms and haplotypes with the risk of T2DM, and their correlation with environmental factors.
Methods: A total of 612 Chinese patients with T2DM and 630 healthy volunteers were enrolled in our study between Jun 2013 and Jun 2015. An iPlex GLOD SNP genotyping analysis of EC-SOD Ala40Thr (rs2536512), Arg213Gly (rs8192291) and Leu53Leu (rs1799895) was run in a 384 well plate format using the Sequenom MassARRAY® System.
Results: The CT (OR=1.46, 95% CI=1.13-1.90) and TT (OR=1.92, 95% CI=1.30-2.84) genotype were associated with a moderate increased risk of T2DM, in comparison to the CC genotype. The CT+TT genotype also displayed an elevated risk of T2DM when compared with the CC genotype (OR=1.44, 95% CI=1.14-1.80). The TT+CT genotype was correlated with risk of T2DM in those with older age (OR=1.59, 95% CI=1.19-2.14), never drinkers (OR=1.82, 95% CI=1.25-2.63), a family history of T2DM (OR=1.44, 95% CI=1.14-1.82) and a higher BMI (OR=1.56, 95% CI=1.13-2.17). The G-C-C and G-C-G haplotypes were associated with a reduced risk of T2DM, while the G-T-C haplotype was correlated with an increased risk.
Conclusion: The EC-SOD Arg213Gly (rs8192291) polymorphism is correlated with an increased risk of T2DM, especially in males, never drinkers, those with a family history of T2DM and overweight subjects. The G-C-C, G-C-G and G-T-C contribute to the pathogenesis of T2DM.
Keywords
Type-2 diabetes mellitus, EC-SOD, Polymorphism, Haplotype, Environmental factors.
Introduction
Type 2 diabetes mellitus (T2DM) is the most common type of endocrine disease in developed and development countries and the prevalence of this disease is increasing worldwide, and majority of patients with T2DM occur in China [1,2]. It is reported that the prevalence of T2DM was 2.5% in 1994, but increased to about 10% in 2010 [3,4]. Diabetes and related complications have brought about a major healthcare burden and give tremendous challenges to patients and healthcare systems [1,2]. The pathogenesis of T2DM is a long term process, and the detailed etiology underlying T2DM remains unclear. Many environmental and lifestyle factors play a part in the development of T2DM, such as impaired glucose tolerance, hypertension, high fat dietary, high cholesterol dietary, obesity or overweight, and long-term lack of physical activity practice [5]. However, not all individuals would develop T2DM even when they exposed to the same environmental and lifestyle risk factors, and only a part of individuals can be explained by such factors, and the exact molecular mechanism of developing T2DM is not fully understood.
Superoxide dismutase (SOD) is a critical defence system for antioxidant enzymes, and there are three types of SOD in human body, including a cytosolic form (CuZn SOD or SOD1), a mitochondrial form (Mn SOD or SOD2), and an extracellular form (EC-SOD or SOD3). EC-SOD is mainly observed in the pancreas, skeletal muscles, and blood vessels, and it is the principal cleaner for oxygen free radicals. The enzymatic activity of EC-SOD in uterine, placenta and blood vessels was stronger than that of CuZn SOD and Mn SOD. The EC-SOD is located on chromosome 4p16.3-q21 with a length of 5900bp and 720bp in coding region, including three exons and two introns. The heparin-binding domain of EC-SOD could bind the extracellular surface of endothelial cells and blood vessels, but the lysine residue of EC-SOD is easier to be glycosylation in the high glucose condition, and thus the binding affinity to heparin is reduced [6]. Experimental study showed that the low EC-SOD activity may lead to high alloxan susceptibility of beta-cells, and thus attribute to a high susceptibility to superoxide radicals produced by activated inflammatory leukocytes and in hyperglycaemia [7]. An in vivo study reported the EC-SOD is associated with the altered metabolic state in diabetic skin and increases the reactive oxygen species [8]. Three main genetic polymorphisms (Ala40Thr, Arg213Gly and Leu53Leu) were observed in ECSOD and were located on the 2 and 3 extrons. Mutation of Arg213Gly could increase 8-15 fold human plasma extracellular superoxide dismutase content, and reduce its enzyme activity [9,10]. Currently, only a few studies reported the association between EC-SOD polymorphisms and risk of T2DM, but the results are inconsistent [11,12]. Therefore, we performed a study to investigate the association of EC-SOD Ala40Thr (rs2536512), Arg213Gly (rs8192291) and Leu53Leu (rs1799895) polymorphisms and haplotypes with the risk of T2DM, and their correlation with environmental factors.
Materials and Methods
Subjects
A total of 612 Chinese patients with T2DM were selected from the Department of Endocrinology of Zhengzhou Central Hospital between Jun 2013 and Jun 2015. All enrolled patients were diagnosed by laboratory evaluations according to the criteria specified by the World Health Organization- International Diabetes Federation (WHO-IDF) [13]. The diabetes mellitus was defined as the fasting plasma glucose level (FPG) ≥ 126mg/dl or ≥ 200 mg/dL 2 h after consuming a drink containing 75 g glucose. The exclusion criteria for patients were those with a history of auto-immunologic disease, cancers or end-stage liver or kidney diseases. The main characteristics of T2DM patients were as follows: mean age was 58.40 ± 9.55 y, and the mean T2DM duration was 11.43 ± 6.50 y.
The non-diabetic group comprised 630 healthy volunteers attending the physical examination center at Zhengzhou Central Hospital of Zhengzhou University. All control subjects were not having T2DM according to WHO-IDF criteria. All the controls were confirmed to be without endocrine diseases, severe active inflammatory diseases, receiving any treatments or drugs that could influence the glucose metabolism or weight. The mean age of controls was 59.10 ± 9.20 y.
The proposal of this study obtained the approval from the ethics committee of Zhengzhou Central Hospital.
Demographic and clinical data collection
A standard questionnaire was used to collect information in terms of sex, age, age at T2DM diagnosis, family history of T2DM, history of tobacco smoking and alcohol drinking, physical activity and body mass index (BMI). The tobacco smoking was divided into non-smokers, light smokers, moderate smokers and heavy smokers. Alcohol drinking was categorized into non-drinkers, light drinkers, moderate drinkers and heavy drinkers. Physical activity was divided into seldom, occasionally and often physical activity. BMI was calculated as weight (kg)/height square (meters).
The blood pressure, plasma glucose levels, HbAlc, total plasma cholesterol, HDL cholesterol and triglycerides were collected from medical records. The blood pressure was measured on the left arm in sitting position after 5 min rest, by using a mercury sphygmomanometer. Plasma glucose levels were tested with the glucose oxidase method. The total plasma cholesterol, HDL cholesterol and triglycerides were determined by enzymatic methods.
Genotype analysis
Each subject was asked to provide 3-5 ml peripheral venous blood, and the blood samples were kept in tubes coated with Ethylene Diamine Tetra Acetic Acid (EDTA) at -4°C until further use. DNA was extracted from the peripheral venous blood leucocytes according to the standard procedures, using Tiangen DNA Blood Mini Kit (Tiangen Biotech Co., Ltd. Beijing, China). The primers and probes of Polymerase Chain Reaction (PCR) amplification and single base extension assays were designed using MassARRAY® Assay Design 3.1 Software (Sequenom, Inc. San Diego, USA). An iPlex GLOD SNP genotyping analysis of EC-SOD Ala40Thr (rs2536512), Arg213Gly (rs8192291) and Leu53Leu (rs1799895) was run in a 384 well plate format using the Sequenom MassARRAY® System (Sequenom, Inc. San Diego, USA). The PCR amplification reaction was done in a 5 μL mixture. Then the SAP and iPLEX reactions were performed. Finally, the PCR samples are desalted, dispensed to a SpectroCHIP and analysed with MALDI-TOF MS.
Statistical analysis
Categorical variables are shown by percentages and frequencies (%), and the continued variables were expressed by mean and standard deviation. The differences between study groups in terms of the demographic and lifestyle characteristics as well as EC-SOD Ala40Thr (rs2536512), Arg213Gly (rs8192291) and Leu53Leu (rs1799895) genotype distributions were compared by Chi-square (χ2) test or student t-test. The Hardy-Weinberg Equilibrium (HWE) in T2DM patients and controls EC-SOD Ala40Thr (rs2536512), Arg213Gly (rs8192291) and Leu53Leu (rs1799895) was analysed by Chi-square with one degree of freedom. The association of EC-SOD Ala40Thr (rs2536512), Arg213Gly (rs8192291) and Leu53Leu (rs1799895) with the risk of T2DM was expressed with Odds Ratio (OR) and 95% confident intervals (95% CI), using binary multivariate logistic regression. The linkage disequilibrium and haplotype analyses of EC-SOD Ala40Thr (rs2536512), Arg213Gly (rs8192291) and Leu53Leu (rs1799895) were analysed using SHEsis software (http://analysis.bio-x.cn/myAnalysis.php) [14].
Analyses were performed using IBM SPSS Statistics for Windows, Version 21.0. (IBM Corp, Armonk, NY, USA) and a two-sided P<0.05 was set as a statistical significance.
Results
When compared with controls in terms of demographic, lifestyle and clinical characteristics of investigated subjects,patients with T2DM were more likely to be male, have a habit of heavy smoking and drinking, have a family history of T2DM, and have higher level of systolic and diastolic blood pressure, BMI and HDL-c and a lower level of FPG, triglyceride and LDL-c (Table 1).
| Variables | Patients | Controls | t value or χ2 value | P value | ||
|---|---|---|---|---|---|---|
| N=612 | N=630 | |||||
| N | % | N | % | |||
| Age (y) | 62.27 ± 9.75 | 61.53 ± 10.55 | -1.28 | 0.2 | ||
| Gender | ||||||
| Female | 238 | 38.89 | 284 | 45.08 | ||
| Male | 374 | 61.11 | 346 | 54.92 | 4.88 | 0.03 |
| Duration of T2DM | ||||||
| Systolic blood pressure, mmHg | 137.37 ± 18.67 | 131.35 ± 16.73 | -6 | <0.001 | ||
| Diastolic blood pressure, mmHg | 83.70 ± 11.25 | 77.21 ± 10.44 | -10.56 | <0.001 | ||
| BMI, kg/m2 | 25.16 ± 3.21 | 22.74 ± 3.67 | -12.37 | <0.001 | ||
| <24 | 222 | 36.27 | 403 | 63.97 | ||
| ≥ 24 | 390 | 63.73 | 227 | 36.03 | 95.24 | <0.001 |
| Smoking | ||||||
| Never | 250 | 40.85 | 307 | 48.73 | ||
| Light | 87 | 14.22 | 94 | 14.92 | ||
| Moderate | 101 | 16.5 | 128 | 20.32 | ||
| Heavy | 174 | 28.43 | 101 | 16.03 | 28.41 | <0.001 |
| Drinking | ||||||
| Never | 200 | 32.68 | 267 | 42.38 | ||
| Light | 178 | 29.08 | 155 | 24.6 | ||
| Moderate | 112 | 18.3 | 98 | 15.56 | ||
| Heavy | 122 | 19.93 | 110 | 17.46 | 12.5 | 0.01 |
| Physical activity | ||||||
| less | 177 | 28.92 | 185 | 29.37 | ||
| moderate | 255 | 41.67 | 279 | 44.29 | ||
| Heavy | 180 | 29.41 | 166 | 26.35 | 1.56 | 0.46 |
| Family history of T2DM | ||||||
| No | 546 | 89.22 | 598 | 94.92 | ||
| Yes | 66 | 10.78 | 32 | 5.08 | 13.9 | <0.001 |
| Serum urea, mmol/L | 326.43 ± 105.28 | 330.69 ± 103.88 | -0.72 | 0.47 | ||
| FPG, mmol/L | 4.94 ± 1.03 | 8.55 ± 2.13 | -38.1 | <0.001 | ||
| total cholesterol, mmol/L | 4.59 ± 1.07 | 4.54 ± 1.08 | 0.92 | 0.36 | ||
| Triglyceride, mmol/L | 1.41 ± 0.96 | 1.77 ± 0.93 | -6.78 | <0.001 | ||
| HDL-c, mmol/L | 1.35 ± 0.47 | 1.11 ± 0.40 | 10.05 | <0.001 | ||
| LDL-c, mmol/L | 3.06 ± 0.41 | 3.72 ± 1.41 | -11.21 | <0.001 | ||
| Insulin treatment | ||||||
| No | 190 | 31.05 | ||||
| Yes | 630 | 102.94 | ||||
| Retinopathy | ||||||
| No | 182 | 29.74 | ||||
| Yes | 430 | 70.26 | ||||
| Nephropathy | ||||||
| No | 187 | 30.56 | ||||
| Yes | 425 | 69.44 | ||||
| Cardiovascular disease |
||||||
| No | 173 | 28.27 | ||||
| Yes | 439 | 71.73 | ||||
| Peripheral vascular disease |
||||||
| No | 180 | 29.41 | ||||
| Yes | 432 | 70.59 | ||||
Table 1. Demographic, lifestyle and clinical characteristics of investigated subjects.
The CC, CT and TT genotypes of EC-SOD Arg213Gly (rs8192291) displayed significant differences between patients with T2DM and controls (χ2=15.59, P<0.001, Table 2). However, no significant differences were found between the two study groups in terms of EC-SOD Ala40Thr (rs2536512) and Leu53Leu (rs1799895) genotype distributions. In addition, the genotypes of Ala40Thr (rs2536512) and Arg213Gly (rs8192291) were in line with HWE in both patients and controls, while Leu53Leu (rs1799895) genotypes were not.
| EC-SOD | Patients | Controls | χ2 value | P value | χ2 for HWE | P value | χ2 for HWE | P value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| N=612 | % | N=630 | % | |||||||
| N | N | Patients | Controls | |||||||
| Ala40Thr (rs2536512) | ||||||||||
| GG | 438 | 71.57 | 474 | 75.24 | ||||||
| GA | 158 | 25.82 | 143 | 22.7 | ||||||
| AA | 16 | 2.61 | 13 | 2.06 | 2.22 | 0.33 | 0.15 | 0.7 | 0.33 | 0.57 |
| Arg213Gly (rs8192291) | ||||||||||
| CC | 257 | 41.99 | 322 | 51.11 | ||||||
| CT | 259 | 42.32 | 248 | 39.37 | ||||||
| TT | 96 | 15.69 | 60 | 9.52 | 15.59 | <0.001 | 2.06 | 0.12 | 1.46 | 0.23 |
| Leu53Leu (rs1799895) | ||||||||||
| CC | 569 | 92.97 | 571 | 90.63 | ||||||
| CG | 37 | 6.05 | 45 | 7.14 | ||||||
| GG | 6 | 0.98 | 14 | 2.22 | 3.72 | 0.16 | 27.87 | <0.001 | 75.27 | <0.001 |
Table 2. Genotype distributions of EC-SOD between T2DM patients and controls.
By binary multivariate logistic regression, we observed that heavy tobacco smoking (OR=2.00, 95% CI=1.46-2.76), light (OR=1.62, 95% CI=1.19-2.21), moderate (OR=1.65, 95% CI=1.16-2.37) and heavy drinkers (OR=1.75, 95% CI=1.24-2.48), and a family history of T2DM (OR=2.18, 95% CI=1.36-3.49), older age (OR=1.01, 95% CI=1.00-1.02) and higher BMI (OR=1.23, 95% CI=1.19-1.28) were correlated with risk of T2DM (Table 3). We found that the CT (OR=1.46, 95% CI=1.13-1.90) and TT (OR=1.92, 95% CI=1.30-2.84) genotypes were associated with a moderate increased risk of T2DM, in comparison to the CC genotype. The CT+TT genotype also displayed an elevated risk of T2DM compared to the CC genotype, with an OR (95% CI) of 1.44 (1.14-1.80).
| Variables | β | S.E | Wals | OR | 95% CI | P value |
|---|---|---|---|---|---|---|
| Tobacco smoking | ||||||
| Never | 20.16 | |||||
| Light | 0.25 | 0.19 | 1.82 | 1.29 | 0.89-1.85 | 0.177 |
| Moderate | 0 | 0.17 | 0 | 1 | 0.72-1.41 | 0.982 |
| Heavy | 0.7 | 0.16 | 18.16 | 2 | 1.46-2.76 | <0.001 |
| Alcohol drinking | ||||||
| Never | 1 | Reference | ||||
| Light | 0.48 | 0.16 | 9.21 | 1.62 | 1.19-2.21 | 0.002 |
| Moderate | 0.5 | 0.18 | 7.58 | 1.65 | 1.16-2.37 | 0.006 |
| Heavy | 0.56 | 0.18 | 9.95 | 1.75 | 1.24-2.48 | 0.002 |
| Family history of T2DM | ||||||
| No | 1 | Reference | ||||
| Yes | 0.78 | 0.24 | 10.52 | 2.18 | 1.36-3.49 | 0.001 |
| Age | 0.01 | 0.01 | 1.73 | 1.01 | 1.00-1.02 | 0.188 |
| BMI, kg/m2 | 0.21 | 0.02 | 120 | 1.23 | 1.19-1.28 | <0.001 |
| EC-SOD Ala40Thr (rs2536512) | ||||||
| GG | 1 | Reference | ||||
| GA | 0.2 | 0.15 | 1.77 | 1.22 | 0.91-1.62 | 0.183 |
| AA | 0.08 | 0.41 | 0.04 | 1.09 | 0.49-2.41 | 0.839 |
| GA+AA | 0.19 | 0.13 | 2.11 | 1.21 | 0.94-1.57 | 0.147 |
| EC-SOD Arg213Gly (rs8192291) | ||||||
| CC | 1 | Reference | ||||
| CT | 0.38 | 0.13 | 8.12 | 1.46 | 1.13-1.90 | 0.004 |
| TT | 0.65 | 0.2 | 10.58 | 1.92 | 1.30-2.84 | 0.001 |
| CT+TT | 0.36 | 0.12 | 9.61 | 1.44 | 1.14-1.80 | 0.002 |
| EC-SOD Leu53Leu (rs1799895) | ||||||
| CC | 1 | Reference | ||||
| CG | -0.32 | 0.25 | 1.64 | 0.72 | 0.44-1.19 | 0.2 |
| GG | -0.61 | 0.55 | 1.24 | 0.55 | 0.19-1.59 | 0.265 |
| GC+GG | -0.33 | 0.21 | 2.36 | 0.72 | 0.47-1.10 | 0.124 |
Table 3. Association of environmental factors and EC-SOD polymorphisms with the risk of T2DM.
Additionally, we conducted stratified analysis by age, gender, smoking, alcohol drinking, physical activity, family history of T2DM and BMI, and we observed that TT+CT genotype was correlated with risk of T2DM among those with older age (OR=1.59, 95% CI=1.19-2.14), never drinkers (OR=1.82, 95% CI=1.25-2.63), a family history of T2DM (OR=1.44, 95% CI=1.14-1.82) and a higher BMI (OR=1.56, 95% CI=1.13-2.17) (Table 4). However, we did not find any interaction between EC-SOD Arg213Gly (rs8192291) polymorphism and clinical characteristics of patients with T2DM (Table 5).
| Variables | Patients | Controls | OR | 95% CI | P value | ||
|---|---|---|---|---|---|---|---|
| CC | TT+CT | CC | TT+CT | TT+CT vs. CC | |||
| Age | |||||||
| <60 | 101 | 138 | 133 | 143 | 1.27 | 0.90-1.80 | 0.18 |
| ≥ 60 | 156 | 217 | 189 | 165 | 1.59 | 1.19-2.14 | 0.002 |
| Gender | |||||||
| Female | 98 | 140 | 142 | 142 | 1.43 | 1.01-2.02 | 0.04 |
| Male | 159 | 215 | 180 | 166 | 1.46 | 1.09-1.97 | 0.01 |
| Tobacco smoking | |||||||
| Never | 108 | 142 | 157 | 150 | 1.38 | 0.98-1.93 | 0.06 |
| Light | 37 | 50 | 52 | 42 | 1.68 | 0.93-3.01 | 0.09 |
| Moderate | 41 | 60 | 65 | 63 | 1.51 | 0.89-2.56 | 0.13 |
| Heavy | 71 | 103 | 48 | 53 | 1.31 | 0.80-2.15 | 0.28 |
| Alcohol drinking | |||||||
| Never | 77 | 123 | 142 | 125 | 1.82 | 1.25-2.63 | 0.002 |
| Light | 70 | 108 | 71 | 84 | 1.3 | 0.84-2.02 | 0.23 |
| Moderate | 52 | 60 | 53 | 45 | 1.36 | 0.79-2.34 | 0.27 |
| Heavy | 58 | 64 | 56 | 54 | 1.14 | 0.68-1.92 | 0.61 |
| Family history of T2DM | |||||||
| No | 230 | 316 | 306 | 292 | 1.42 | 0.62-3.38 | 0.39 |
| Yes | 27 | 39 | 16 | 16 | 1.44 | 1.14-1.82 | 0.002 |
| BMI, kg/m2 | |||||||
| <24 | 89 | 133 | 199 | 204 | 1.37 | 0.97-1.98 | 0.06 |
| ≥ 24 | 168 | 222 | 123 | 104 | 1.56 | 1.13-2.17 | 0.008 |
Table 4. Stratified analyses between EC-SOD Arg213Gly (rs8192291) polymorphism and T2DM risk by age, gender, smoking, alcohol drinking, physical activity, family history of T2DM and BMI.
| Variables | Patients | OR | 95% CI | P value | |
|---|---|---|---|---|---|
| CC | TT+CT | TT+CT vs. CC | |||
| Insulin treatment | |||||
| No | 88 | 102 | |||
| Yes | 169 | 253 | 1.29 | 0.91-1.82 | 0.15 |
| Retinopathy | |||||
| No | 78 | 104 | |||
| Yes | 179 | 251 | 1.05 | 0.74-1.49 | 0.78 |
| Nephropathy | |||||
| No | 77 | 110 | |||
| Yes | 180 | 245 | 0.95 | 0.67-1.35 | 0.79 |
| Cardiovascular disease |
|||||
| No | 80 | 93 | |||
| Yes | 177 | 262 | 1.27 | 0.89-1.82 | 0.18 |
| Peripheral vascular disease |
|||||
| No | 76 | 104 | |||
| Yes | 181 | 251 | 1.01 | 0.71-1.44 | 0.94 |
Table 5. Association between EC-SOD Arg213Gly (rs8192291) polymorphism and clinical characteristics in T2DM.
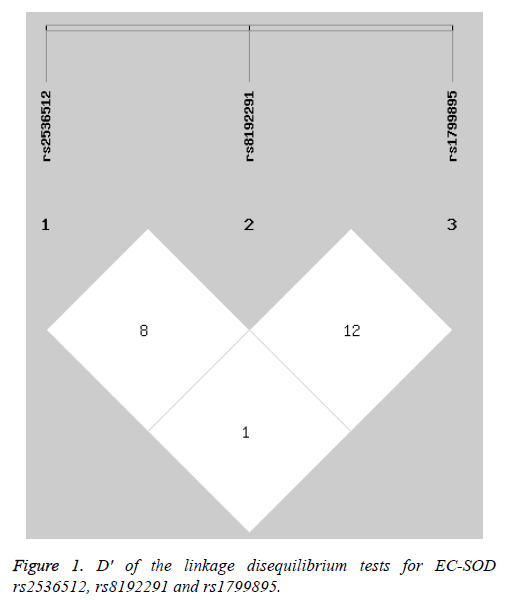
The haplotype analysis revealed no linkage disequilibrium among EC-SOD rs2536512, rs8192291 and rs1799895 (Figure 1). Five common haplotypes (frequency>0.03 in both patients with T2DM and controls) were observed, and the G-C-C and G-C-G haplotypes were associated with a reduced risk of T2DM, while the G-T-C haplotype was correlated with an increased risk (Table 6).
| Patients with T2DM | % | Controls | % | P value | OR (95% CI) | |
|---|---|---|---|---|---|---|
| N=1224 | N=1260 | |||||
| A-C-C | 96 | 7.84 | 113 | 8.97 | 0.15 | 1.23 (0.93-1.64) |
| A-T-C | 62 | 5.07 | 69 | 5.48 | 0.4 | 1.16 (0.82-1.66) |
| G-C-C | 741 | 60.54 | 630 | 50 | <0.001 | 0.73 (0.63-0.86) |
| G-C-G | 47 | 3.84 | 26 | 2.06 | 0.02 | 0.56 (0.35-0.92) |
| G-T-C | 289 | 23.61 | 363 | 28.81 | <0.001 | 1.42 (1.18-1.70) |
Table 6. Haplotype analysis of EC-SOD rs2536512-rs8192291- rs1799895. Total Chi-square=24.69, P value<0.001.
Discussion
In the present study, we conducted a case-control study to investigate the association of EC-SOD Ala40Thr (rs2536512), Arg213Gly (rs8192291) and Leu53Leu (rs1799895) polymorphisms with the development of cerebral infarction. We observed that the CT and TT genotypes of EC-SOD Arg213Gly (rs8192291) were correlated with an increased risk of T2DM, and the G-C-C, G-C-G and G-T-C haplotypes were correlated with an elevated risk of this disease.
In normal condition, the affinity of EC-SOD towards heparin could combine the extracellular surface of endothelial cells and blood vessels, but amino acid residues were glycosylated and the affinity was reduced in the condition of high glucose concentration, and finally the enzyme activity was reduced. An experimental study indicated that the low EC-SOD activity may contribute to the high alloxan susceptibility of β cells, and may also cause a high susceptibility to superoxide radical produced by activated inflammatory leukocytes and in hyperglycaemia [7]. Previous study reported that the EC-SOD Arg213Gly genetic polymorphism could reduce the affinity of EC-SOD and be related to renal failure of T2DM patients, which showed that this genetic mutation was associated with risk of ischemic cardiovascular and cerebrovascular diseases [15,16]. Park et al. reported that the serum EC-SOD concentrations were a sensitive biochemical marker of insulin resistance in patients with T2DM and hypertension [17]. Tahkashi et al. firstly reported the association between ECSOD genetic polymorphisms and development of diabetes nephropathy [18]. The genetic mutation of EC-SOD Arg213Gly could elevate 8-15 folds levels of EC-SOD in serum, and decrease the affinity towards heparin and endothelial cells, while the EC-SOD Ala40Thr and Leu53Leu (rs1799895) genetic polymorphisms could not alter the capacity of affinity to heparin and enzymatic activity [18].
Several previous studies reported inconsistent results for the association between EC-SOD genetic polymorphism and risk of diabetes [10-12,19,20]. Ukkola et al. firstly reported the association between EC-SOD genetic polymorphisms and development of T2DM, and found that EC-SOD genetic polymorphism was not related to the macroangiopathy in T2DM patients [20]. Stroke et al. performed a study in Russian population, and reported an association of EC-SOD Arg213Gly genetic polymorphism with the pathogenesis of diabetic polyneuropathy [10]. Tamai et al. carried out a study in a Japanese population, and indicated that EC-SOD Ala40Thr polymorphism was related to the development of T2DM, while the EC-SOD Arg213Gly was not [12]. A recent study in a Chinese population revealed that the AA genotype of EC-SOD Ala40Thr showed a 2.19-fold higher risk of developing T2DM than the GG+GA genotype [11]. However, we found that the CT and TT genotypes of EC-SOD Arg213Gly showed a 1.46-1.92 folds risk of developing T2DM, and the discrepancies in these studies may be attributed to differences in populations, sample size, study design and random by chance.
Our study revealed that EC-SOD Arg213Gly polymorphism had interaction with family history of T2DM, which showed that there might be other genetic factors interacted with this gene to increase the risk of T2DM. Marklund et al. reported that the plasma levels of EC-SOD increased with age and could be modulated by lifestyle factors, such as obesity, and the ECSOD contributed to the cardiovascular disease risk [21]. Our results showed that EC-SOD Arg213Gly mutation had an interaction with BMI and age, which was in line with previous results. Moreover, we firstly reported a relationship between three haplotypes and risk of T2DM, which suggested that the haplotypes of EC-SOD could be molecular risk factors for this disease.
Two strengthens should be mentioned in this study. First, this study included 612 patients with T2DM and 630 healthy subjects, which is a relative large sample size study and could provide enough statistical power to find differences between studies. Second, we firstly reported the association of EC-SOD haplotypes with risk of T2DM, which could provide scientific information for further studies to investigate the association between them. Moreover, only one limitation should be considered. The lifestyles of included subjects were selfreported and recalled. Therefore, recalling bias and information bias are inevitable.
Conclusions
Our study suggests that the EC-SOD Arg213Gly (rs8192291) polymorphism is correlated with an increased risk of T2DM, especially in males, never drinkers, those with a family history of T2DM and overweight subjects. In addition, the G-C-C, GC- G and G-T-C haplotypes contribute to the pathogenesis of T2DM. Further studies with larger sample size, better design are greatly needed to confirm our findings.
Acknowledgement
We thank the great help from staffs in Zhengzhou Central Hospital, and they help us to collect the blood samples for our analysis.
Conflict of Interest
The authors declare no conflict of interest in preparing this article.
References
- Meyers JL, Parasuraman S, Bell KF. The high-cost, type 2 diabetes mellitus patient: an analysis of managed care administrative data. Arch Public Health 2014; 72: 6.
- Wilf-MR, Bolotin A, Gordon N. The association between improved quality diabetes indicators, health outcomes and costs: towards constructing a "business case" for quality of diabetes care-a time series study. BMC Endocr Disord 2014; 14: 92.
- Pan XR, Yang WY, Li GW. Prevalence of diabetes and its risk factors in China. Diab Care 1997; 20: 1664-1669.
- Yang W, Lu J, Weng J. Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362: 1090-1101.
- Pearson ER. Dissecting the etiology of type-2 diabetes in the pima Indian population. Diabetes 2015; 64: 3993-3995.
- Adachi T, Kodera T, Ohta H. The heparin binding site of human extracellular-superoxide dismutase. Arch Biochem Biophys 1992; 297: 155-161.
- Sentman ML, Jonsson LM, Marklund SL. Enhanced alloxan-induced beta-cell damage and delayed recovery from hyperglycaemia in mice lacking extracellular-superoxide dismutase. Free Radic Biol Med 1999; 27: 790-796.
- Kim CH. Expression of extracellular superoxide dismutase protein in diabetes. Arch Plast Surg 2013; 40: 517-521.
- Sandstrom J, Nilsson P, Karlsson K. 10-fold increase in human plasma extracellular superoxide dismutase content caused by a mutation in heparin-binding domain. J Biol Chem 1994; 269: 19163-19166.
- Strokov IA, Bursa TR, Drepa OI. Predisposing genetic factors for diabetic polyneuropathy in patients with type-1 diabetes: a population-based case-control study. Acta Diabetol 2003; 40: 375-379.
- Yang YM, Xie XR, Jin AL. Genetic polymorphisms in extracellular superoxide dismutase Leu53Leu, Arg213Gly, Ala40Thr and susceptibility to type 2 diabetes mellitus. Genet Mol Res 2016; 15.
- Tamai M, Furuta H, Kawashima H. Extracellular superoxide dismutase gene polymorphism is associated with insulin resistance and the susceptibility to type 2 diabetes. Diabetes Res Clin Pract 2006; 71: 140-145.
- Diagnosis and classification of diabetes mellitus. Diabetes Care 2013; 36: 67-74.
- Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction and genetic association at polymorphism loci. Cell Res 2005: 97-98.
- Rashidi A, Nakhjavani M, Esteghamati A. Association between oxidant/antioxidant markers and proteinuria in type 2 diabetes: results in 142 patients. J Nephrol 2009; 22: 733-738.
- Fukuda M, Nakamura T, Kataoka K. Potentiation by candesartan of protective effects of pioglitazone against type 2 diabetic cardiovascular and renal complications in obese mice. J Hypertens 2010; 28: 340-352.
- Park H, Hasegawa G, Obayashi H. Relationship between insulin resistance and inflammatory markers and anti-inflammatory effect of losartan in patients with type 2 diabetes and hypertension. Clin Chim Acta 2006; 374: 129-134.
- Yamada H, Yamada Y, Adachi T. Protective role of extracellular superoxide dismutase in haemodialysis patients. Nephron 2000; 84: 218-223.
- Samoila OC, Carter AM, Futers ST. Polymorphic variants of extracellular superoxide dismutase gene in a Romanian population with atheroma. Biochem Genet 2008; 46: 634-643.
- Ukkola O, P.H. E, Savolainen MJ. Lack of association between polymorphisms of catalase, copper-zinc superoxide dismutase (SOD), extracellular SOD and endothelial nitric oxide synthase genes and macroangiopathy in patients with type 2 diabetes mellitus. J Intern Med 2001; 249: 451-459.
- Marklund SL, Nilsson P, Israelsson K. Two variants of extracellular-superoxide dismutase: relationship to cardiovascular risk factors in an unselected middle-aged population. J Intern Med 1997; 242: 5-14.