ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 1
Association of TNF-alpha with type-2 diabetic mechanical hyperalgesia in rats
Department of Pain Medicine, First Affiliated Hospital of Xinjiang Medical University, Urumqi, PR China
- *Corresponding Author:
- Yimei Li
Department of Pain Medicine First
Affiliated Hospital of Xinjiang Medical University PR China
Accepted on June 4, 2016
To study the role of tumour necrosis factors-alpha (TNF-alpha) in mechanical hyperalgesia in type-2- diabetic rat, diabetic mechanical hyperalgesia model was established in female Wister rats by combining high-fat diet for 4 weeks and single intraperitoneal injection of streptozotocin (STZ, 30 mg/kg, serum glucose increased from 12.7 ± 0.44 mmol/L to 28.3 ± 0.59 mmol/L after STZ injection). Based on hyperalgesia symptoms, rats were divided into hyperalgesia group (HFDH) and non-hyperalgesia group (HFDNH). Hyperalgesia rats were further treated with or with TNF-alpha inhibitor (HFDHTNFI). Compared with HFDNH group, hyperalgesia symptom was evident in HFDH rats in that PWT is 26 ± 0 g HFDNH group and 7.371 ± 1.49 g in HFDH respectively. On the other hand, serum level of TNF-alpha was lower in HFDH rats (76.64 ± 3.40 pg/ml) than those in HFDNH rats (109.7 ± 6.23 pg/ml). Sensitization of hyperalgesia and low serum TNF-alpha in HFDH group could be restored by TNF-alpha inhibitor (serum TNF-alpha was 106.1 ± 2.39 pg/ml and PWT was 26 ± 0 g after TNF-alpha application). Thus down-regulation of serum TNF-alpha is involved in progression of type2-diabetic mechanical hyperalgesia.
Keywords
Serum TNF-alpha, Type-2 diabetes, Hyperalgesia.
Introduction
Type-2 diabetes has becoming a major burden to family and society as economy develops. It has been well-documented that neuropathy occurs in early stage of type-2 diabetes [1]. Although there’s controversial whether mechanical hyperalgesia in diabetic patients is inflammatory or not [2], increasing evidences suggest that immune system and proinflammatory factors play an important role in mechanical hyperalgesia [3]. A number of studies have proved that progression of type-2 diabetes is companied and regulated by chronic inflammatory response [4]. TNF-alpha is a critical inflammatory factor whose imbalance between adiponectin is responsible for deficient in insulin-resistance and lipid metabolism [5]. Meanwhile, micro vascular pathology induced by lipid-metabolism dysfunction is considered to be a cause of hyperalgesia in diabetes [6], but the relationship between TNFalpha and type-2 diabetes hyperalgesia is not clear.
In current study, we aimed to investigate correlation between serum TNF-alpha and type-2-diabetic mechanical hyperalgesia. We established type type-2 diabetes and related mechanical hyperalgesia in female rats after high-fat diet feeding. Then we found down-regulation of serum TNF-alpha is responsible for hyperalgesia occurrence. Meanwhile, TNF-alpha receptor inhibition can restore serum TNF-alpha and improve normal hyperalgesia sensation. Our results firstly revealed the role of TNF-alpha in type-2-diabetic mechanical hyperalgesia.
Subjects and Methods
Animals
Wistar female rats (SPF grade; 170-210 g) were purchased from Animal Centre of Xinjiang Medical University and kept in Animal Experimental Centre. All protocols were optimized to minimize animal death and approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University.
Diabetic mechanical hyperalgesia model
Rats were fed with normal or high-fat diet (with 60% normal diet, 10% lard, 20% sucrose, 2% cholesterol and 8% yolk powder). After Four weeks, single dose of streptozotocin (STZ, 30 mg/kg) was administrated via intraperitoneal injection. STZ (Yuanye Bio, China) was dissolved in 0.1 mmol/L Citrate buffer (PH=4.2) and protected from light. Intraperinantal injection was made within 30 minutes after solution preparation. Rats were restricted from food and water for 8 hours before injection and allowed for free food- and wateraccess 3 hours after injection.
Serum glucose was monitored before, 48 hours after, and weekly 5th ~ 12th weeks after STZ injection. Animals were grouped into diabetes group if serum glucose level exceeds 16.7 mmol/l at the 8th week (D). From 4th to 14th week, 50% paw withdrawal threshold (PWT; δ=0.184) was monitored weekly. In 10th week, if PWT is lower than 10, the diabetic rats were grouped in diabetic hyperalgesia group (HFDH). Furthermore, HFDH rats were treated with TNF-alpha receptor inhibitor (HFDHTNFI). If PWT is equal to or higher than 26, the rats were grouped into diabetic no hyperalgesia group (HFDNH).
TNF-alpha receptor inhibitor was delivered via intrathecal injection as described [7]. During first three days at the 12th week after STZ injection, Etanercept (TNF-alpha receptor inhibitor, 0.5 mg/0.5 ml) was injected by puncture at the lumber 5/6 intervertebral space every day. PWT was monitored 2 hours after the last injection.
Parameters monitored.
Serum glucose: Blood was collected through tail vein from the 4th to the 12th week after STZ injection. Glucose level (mmol/l) was monitored with Blood glucose meter (Glucoleader, Taiwan).
TNF-alpha: Concentration (pg/ml) of serum TNF-alpha at the 12th week was detected with an ELISA kit from Yuanye Bio (Shanghai).
PWT test: Von Frey fibre (RWD Life Science) was used to test the PWT. We used 1.4 g or 26.0 g as lower or upper limits for 8 fibres, respectively. 6.0 g served as the first stimulus. Eight fibres (1.4 g, 2.0 g, 4.0 g, 6.0 g, 8.0 g, 10.0 g, 15.0 g and 26.0 g) were used. The upper limit fibre (26.0 g) was selected based on 10% of body weight of rats (260 g) at 4 weeks after high-fat diet feeding as described [8]. An up-down protocol were adopted to test 50% PWT. 50% PWT = (10 Xf+kδ)/ 10,000 where δ=0.184, Xf=log value of hardness of the last fibre in the stimulus series; the value of K is from the threshold series table. If animal did not show hyperalgesia response to 26.0 g stimuli, it would be labelled as no hyperalgesia (NH) and its threshold is denoted as 26.
PWT was tested from 10:00-18:00 (Beijing time). Rats were placed in a transparent plastic box placed in a metal rack and allowed to accommodate for 30 minutes. Tests were started after animal stopping any carding activity. Fibre approached to the middle point of rat thenar perpendicularly and was kept for slight bending for 6-8 seconds. Positive reaction was accepted if rat exhibited rapid paw withdrawing or foot licking behaviour. The second stimulus was delivered 60 seconds later [8].
Statistics
Graph Pad Prism software was used for statistics. Significance was determined by t-test. One-way ANOVA followed by post hoc test was performed to test the difference among multiple groups. Bars represent mean ± SE. All P values <0.05 were considered statistically significant (*P<0.05; **P<0.01; ***P<0.001).
Results
Establishment of diabetes model
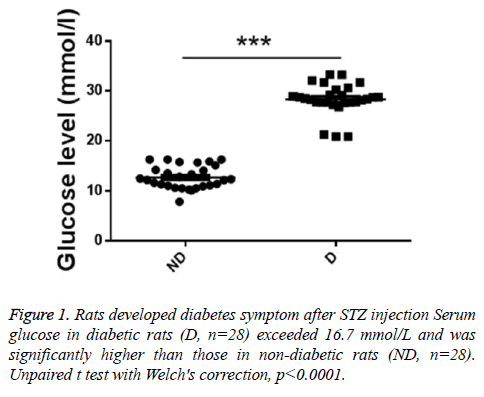
To investigate whether the diabetes model was successfully established, serum glucose levels were detected. As showed in Figure 1, serum glucose in diabetic rats (D, n=28) exceeded 16.7 mmol/L and was significantly higher than those in nondiabetic rats (ND, n=28).
Serum level of TNF-alpha in rats with hyperalgesia
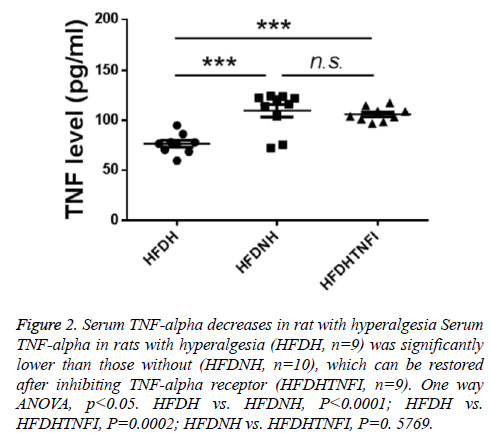
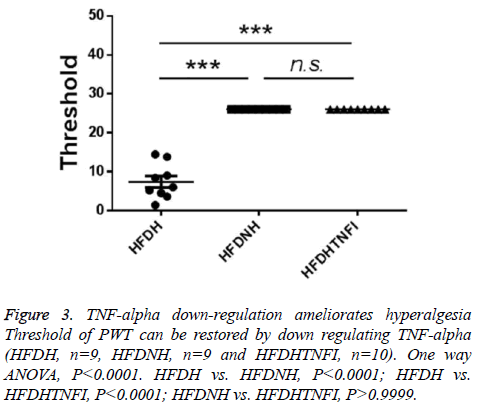
Based on whether exhibited hyperalgesia, diabetic rats were divided into two groups including diabetic no hyperalgesia group (HFDNH) and diabetic hyperalgesia group (HFDH). As showed in Figure 3, left two columns, rats in HFDH group are more sensitive to fibre stimulation than those in HFDNH group. TNF-alpha is one of central cytokines, which were involved in hyperalgesia pathogenesis [7-14], so we tested the serum level of TNF-alpha in those two types of rats. We found that, serum TNF-alpha was significantly lower in HFDH group (n=9) than those in HFDNH rats (n=10, left and mid columns in Figure 2), which indicated that down-regulation of serum TNF-alpha may be responsible for hyperalgesia generation in diabetic rats.
Figure 2: Serum TNF-alpha decreases in rat with hyperalgesia Serum TNF-alpha in rats with hyperalgesia (HFDH, n=9) was significantly lower than those without (HFDNH, n=10), which can be restored after inhibiting TNF-alpha receptor (HFDHTNFI, n=9). One way ANOVA, p<0.05. HFDH vs. HFDNH, P<0.0001; HFDH vs. HFDHTNFI, P=0.0002; HFDNH vs. HFDHTNFI, P=0. 5769.
The effect of TNF-alpha inhibitor on hyperalgesia in HFDH rats
To further identify the role of TNF-alpha in hyperalgesia symptom in diabetic rats, we tested the effect of blocking TNFalpha on hyperalgesia. We found that TNF-alpha receptor inhibitor treatment restored the serum level of TNF-alpha which was down-regulated in in HFDH group (Figure 2, right two columns). Furthermore, inhibition of TNF-alpha ameliorates hyperalgesia symptom in HFDH rats (Figure 3, right two columns).
Discussion
In the current study, we succeeded in establishing type-2- diabetic mechanical hyperalgesia rat model by combining a high-fat diet and STZ administration. Diabetic rats showed higher serum glucose (higher than 16. 7 mmol/L). Meanwhile, diabetic hyperalgesia rats exhibited mechanical allodynia (threshold lower than 10). All of these phenotypes are consistent with the features of neuropathy and hyperalgesia in human type-2 diabetes [9].
In neuron-injury model, activations of microglia and astrocytes are important mechanisms underlying initiation and maintenance of mechanical hyperalgesia. Activation of microglia induces hypertrophy and proliferation of glial cells [10]. The proliferated microglia and astrocytes can secreted large amount of chemotactic factors, such as MCP-1, TNFalpha and so on which can penetrate through abnormal brainblood barrier [11] and bind to corresponding receptors on surface of glial cells and activate P38MAK signalling. Then secretions of those chemotactic factors can be further increased by transcriptional activation via NF-KB or ATF2, leading to sensitization of injured neurons in the dorsal horn via both preand post-synaptic mechanisms, thus initiate and maintain mechanical hyperalgesia [12]. It is well known that TNF-alpha is one of the cytokines involved in neuropathic hyperalgesia [13]. However, the role of TNF-alpha in diabetic mechanical hyperalgesia is still unknown. Our result showed that serum TNF-alpha is significantly lower in HFDH rats than those in HFDNH group (P<0.001). Previous study has shown that type-2-diabetic mechanical hyperalgesia can be ameliorated in TNF-alpha-knockout mice [14]. Accordingly, we observed that the treatment of TNF-alpha inhibitor not only restore the down-regulated serum level of TNF-alpha, but also and more importantly, ameliorated hyperalgesia in diabetic rats. Taken together, all of these results suggested TNF-alpha may involve in progression of type-2 diabetic neuropathic hyperalgesia. To a more general sense that patients do not exhibit the severe symptoms after diagnosed as diabetic, prediabetes for example, deregulated serum TNF-alpha may lead to enhanced activation of central TNF-alpha receptor, thus involved in hyperalgesia in prediabetic patients [15].
In summary, we found that rat developed type type-2 diabetes and related mechanical hyperalgesia after high-fat diet feeding. Down-regulation of serum TNF-alpha is responsible for hyperalgesia generation. Meanwhile, TNF-alpha inhibition can restore serum TNF-alpha and improve normal hyperalgesia sensation. Our finding suggests that TNF-alpha signalling is a potential target for preventing and treating type-2-diabetic neuropathic hyperalgesia.
Acknowledgements
This study was supported by Research award fund of the First Affiliated Hospital of Xinjiang Medical University (no: 2012YFY17, 2012).
References
- Obrosova IG, Ilnytska O, Lyzogubov VV. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of "healthy" diet and aldose reductase inhibition.Diab 2007;56:2598-2608.
- Leung L,Cahill CM. TNF-aand neuropathic hyperalgesia-a review. J Neuroinfl2010; 7: 1-11.
- Calvo M, Dawes JM,Bennett DLH. The role of the immune system in the generation of neuropathic hyperalgesia Lancet Neurol 2012;11:629-642.
- Calle MC,Fernandez ML. Inflammation and type 2 diabetes.Diab Metabol 2012; 38: 183-191
- Maury E,Brichard SM. Adipokine deregulation, adipose tissue inflammation and metabolic syndrome Mol Cell Endocrinol 2010; 314: 1-16.
- Nguyen DV, Shaw LC,Grant MB. Inflammation in the pathogenesis of microvascular complications in diabetes Frontier Endocrinol 2012; 3: 1-7.
- Hylden JK,Wilcox GL. Intrathecal morphine in mice:a new technique Europ J pharmacol 1980;67: 313-316.
- Chaplan SR, Bach FW,Pogrel JW. Quantitative assessment of tactile allodynia in the rat pawsJ Neurosci Meth 1994; 53: 55-63.
- Obrosova IG. Diabetic hyperalgesiaful and insensate neuropathy: pathogenesis and potential treatments. J American Soc Exper NeuroTherap2009; 6:638-647.
- Gao YJ,Ji RR. Targeting astrocyte signaling for chronic hyperalgesiaNeurotherap 2010; 7:482-493.
- Lim TKY, Shi XQ,Martin HC. Blood-nerve barrier dysfunction contributes to the generation of neuropathic hyperalgesia and allows targeting of injured nerves for hyperalgesia reliefHyperalgesia.Pain 2014; 155: 954-967.
- Ji RR,Suter MR. P38MAPK,microglial signaling, and neuropathic hyperalgesiaMol Hyperalg 2007;3:331-339.
- GallowayC,Chattopadhyay M. Increases in inflammatory mediators in DRG implicate in the pathogenesis of hyperalgesiaful neuropathy in type 2 diabetesCytokine 2013;63: 1-5.
- Yamakawa I, Kojima H, Terashima T. Inactivation of TNF-a ameliorates diabetic neuropathy in mice Am J Physiol Endocrinol Metab 2011; 301: 844-852.
- Ciccone MM, Scicchitano P, Cameli M.Endothelial Function in Pre-diabetes, Diabetes and Diabetic Cardiomyopathy.J Diabetes Metab 2014; 5: 364-373.