ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 11
Astragaloside IV inhibits hypoxia-induced cardiac myocytes death via PKCβ/Egr-1 pathway
Ying Zhang, Hong Li, Qin Zhang, Xiao-Ming Lou and Guo-Qing Chen*
Department of Paediatrics, Peoples Hospital of Zhejiang province, Hangzhou, Zhejiang, PR China
- *Corresponding Author:
- Guo-Qing Chen
Department of Paediatrics
Zhejiang Provincial People’s Hospital, PR China
Accepted on March 20, 2017
Astragaloside IV exerts beneficial effects on hypoxia/reoxygenation-induced cardiomyocyte injury. However, the exact mechanisms needs further disclosed. This study was designed to investigate the role of PKCβ/Egr-1 pathway in the protective effect of Astragaloside IV on hypoxia/reoxygenation injury. Exposed under hypoxia/reoxygenation condition, the primary cultured neonatal rat cardiac myocytes viability was reduced significantly. While the expression of Egr-1, the phosphorylated level of PKCβ and ERK kinase, and the activities of SOD and MDA were up-regulated obviously. On the other hand, the cells with Astragaloside IV preconditioning increased cell survival rate compared with hypoxia/ reoxygenation alone. Moreover, the expression of Egr-1, the phosphorylated level of PKCβ, and ERK was decreased in Astragaloside IV group. Taken together, these results suggest that the cardioprotection of Astragaloside IV may be through the down-regulation of PKCβ/Egr-1 pathway.
Keywords
Astragaloside IV, Hypoxia/reoxygenation injury, PKCβ, Egr-1, Neonatal rat Cardiac myocytes
Introduction
Astragaloside IV (3-O-beta-D-xylopyranosyl-6-O-beta-Dglucopyranosyl- cycloastragenol, AS-IV) is a major active ingredient of the traditional Chinese herb Astragalus membranaceus [1], which has been widely prescribed in traditional Chinese medicine (TCM) for the treatment of cardiovascular disorders [2,3]. Recent studies have shown that Astragaloside IV may play a potential role in protecting the heart from myocardial ischemia. The mechanism of action may involve antioxidative and nitric oxide-inducing properties, reduction of (Ca2+) i and sarcoplasmic reticulum (SR) Ca2+ load, enhanced free radical removal, and decreased lipid peroxidation [4]. Here, we showed that Astragaloside IV could attenuate myocardial ischemia reperfusion injury via PKCβ/ Egr-1 Pathway.
Protein Kinase C (PKC) is a family of serine- and threonine-specific protein kinases that can be activated by calcium and second messenger diacylglycerol. PKC family members phosphorylate a wide variety of protein targets and are known to be involved in diverse cellular signaling pathways and in many different cellular functions [5-7]. Yan et al. reported that activated Protein Kinase C (PKC), especially the β isoform, is a critical upstream regulator of Early Growth Response-1 (Egr-1) in the response to acute vascular stresses, such as hypoxia/hypoxemia and ischemia/reperfusion (I/R) [8-10].
Egr-1 is a nuclear protein and functions as a transcriptional regulator, its target genes are required for differentiation and mitogenesis. Recent researches suggest that ischemia/reperfusion- or hypoxia/reoxygenation-induced myocardial injury might be partly mediated by Egr-1 [11-13].
In the present study, we confirmed that hypoxia/reoxygenation could increase PKCβ and Egr-1 expression, and then induce cardiac myocytes damage in primary cultured neonatal rat cardiac myocytes. Astragaloside IV treatment could inhibit the PKCβ/Egr-1 pathway and protect against hypoxia/ reoxygenation-induced cardiac myocytes death, which contributes to improve our knowledge of mechanism in the cardioprotective function of Astragaloside IV.
Materials and Methods
Animals and reagents
Sprague-Dawley rat at 6 weeks of age were purchased from the Model Animal Research Center of Zhejiang University and housed in the laboratory animal center of Zhejiang University at 22°C with a 12 h light/dark cycle. All animals used in the study were housed and cared for in accordance with the Chinese Pharmacological Society Guidelines for Animal Use. The work was approved by the Committee on the Ethics of Animal Experiments of the Zhejiang University. All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Astragaloside IV and LY-333531 were purchased from Sigma- Aldrich (Shanghai, China).
Rat cardiomyocyte culture
Neonatal cardiomyocytes from the ventricles of 1-3-day-old Sprague-Dawley rat hearts were dissociated by an adaptation of the method of Simpson and Savion. Briefly [14,15], the ventricles were digested with 0.125% Trypsin at 37°C. Dissociated cells were preplated for 2 h in whole DMEM medium to selectively remove nonmuscle cells. Myocytes were then plated in 6-well dishes (1 × 106 cells/well) in a 5% CO2 incubator and maintained in DMEM solution containing 20% fetal bovine serum/0.1 mM Brdu for an additional 72 h. Cell were cultured in 1% Oxygen for 2 h and then moved to regular incubator to modify Hypoxia/reoxygenation condition.
Cell survival assay
Cell survival was evaluated by CCK-8 assay kit (Beyotime Biotechnology, Nantong, China) according to the manufacturer’s instructions.
Immunoblotting
Immunoblotting was conducted with standard procedures, using antibodies against PKCβ, Egr-1, ERK, HIF-1α and actin (Santa Cruz Biotechnology, Santa Cruz, CA).
Real-time quantitative PCR
Total RNA was extracted with Trizol according to the manufacturer’s instructions and was transcribed using Prime ScriptTM RT reagent Kit (TaKaRa, Dalian, China). The cDNA template was amplified by real-time PCR using SYBR-PremixExTaqTM Kit (TaKaRa, Dalian, China). The primer sequences were as follows: 5’- AGGACTTGATlTrGCATGGTATTGCA -3’ (forward), 5’- ATGCAGGGCAGGGTTCTGAG -3’ (reverse) for Egr-1, 5’- GCACCGTCAAGGCTGAGAAC-3’ (forward), 5’- GCCTTCTCCATGGTGGTGAA-3’ (reverse) for GAPDH. Thermal cycling was programmed as follows: 95 for 30 sec followed by 40 cycles of 95°C for 5 s, 60°C for 20 s and 72°C for 15 s, and then 72°C for 10 min. Gene expression was assessed by delta Ct method and mRNA levels of Egr-1 were normalized to those of GAPDH internal standard.
Result
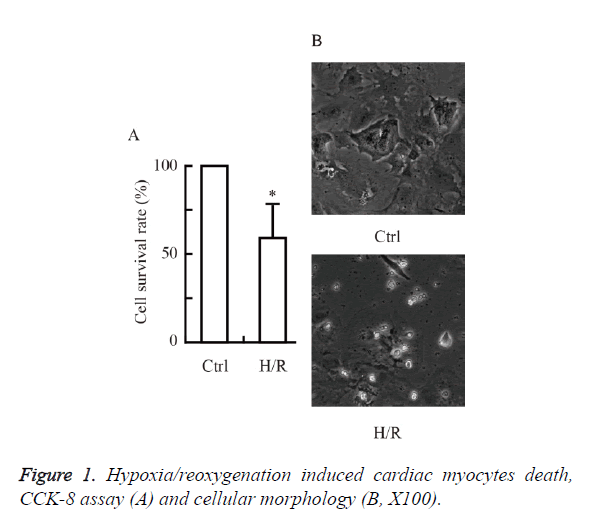
Hypoxia/reoxygenation induced cardiac myocytes death
We first investigated whether hypoxia/reoxygenation induced cardiac myocytes death in primary cultured neonatal rat cardiac myocytes. As shown in Figure 1, the cell viability was reduced significantly by hypoxia/reoxygenation injury. The hypoxia/ reoxygenation group (~60 %) had a significant decrease in cell survival rate versus control (P<0.05 vs. Ctrl).
Hypoxia/reoxygenation activated PKCβ/Egr-1 pathway
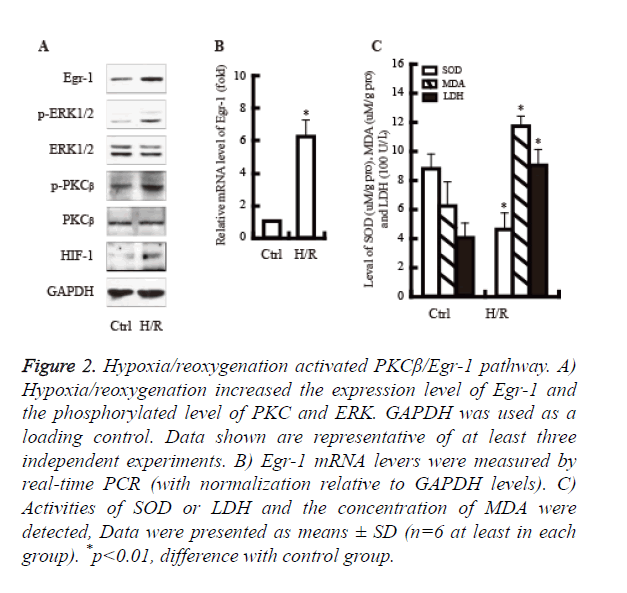
It well known that PKCβ/Egr-1 pathway is involved in the hypoxia/reoxygenation injury. We next examined whether hypoxia/reoxygenation would activate PKCβ/Egr-1 pathway. As shown in Figure 2, hypoxia/reoxygenation significantly increased the expression of PKCβ, Egr-1 and ERK protein. Moreover, the activity of LDH and MDA were up-regulated obviously; By contrast, the activity of SOD declined significantly.
Figure 2. Hypoxia/reoxygenation activated PKCβ/Egr-1 pathway. A) Hypoxia/reoxygenation increased the expression level of Egr-1 and the phosphorylated level of PKC and ERK. GAPDH was used as a loading control. Data shown are representative of at least three independent experiments. B) Egr-1 mRNA levers were measured by real-time PCR (with normalization relative to GAPDH levels). C) Activities of SOD or LDH and the concentration of MDA were detected, Data were presented as means ± SD (n=6 at least in each group). *p<0.01, difference with control group.
Astragaloside IV attenuated hypoxia/reoxygenation induced cardiac myocytes death via down-regulating PKCβ/Egr-1 pathway
Astragaloside IV exerts beneficial effects on hypoxia/ reoxygenation-induced cardiomyocyte injury. However, the exact mechanisms remain unknown. We aimed to investigate the role of PKCβ/Egr-1 pathway in the protective effect of Astragaloside IV on hypoxia/reoxygenation-induced cardiomyocyte injury.
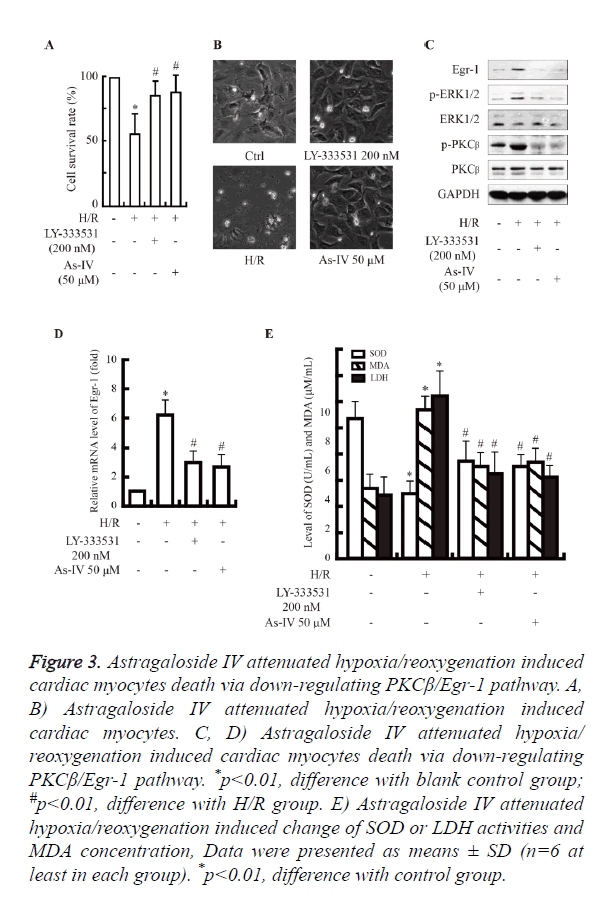
As shown in Figure 3A, the cells with Astragaloside IV preconditioning increase cell survival rate compared with hypoxia/reoxygenation alone (~80% vs. ~60%, P<0.05).
Figure 3. Astragaloside IV attenuated hypoxia/reoxygenation induced cardiac myocytes death via down-regulating PKCβ/Egr-1 pathway. A, B) Astragaloside IV attenuated hypoxia/reoxygenation induced cardiac myocytes. C, D) Astragaloside IV attenuated hypoxia/ reoxygenation induced cardiac myocytes death via down-regulating PKCβ/Egr-1 pathway. *p<0.01, difference with blank control group; #p<0.01, difference with H/R group. E) Astragaloside IV attenuated hypoxia/reoxygenation induced change of SOD or LDH activities and MDA concentration, Data were presented as means ± SD (n=6 at least in each group). *p<0.01, difference with control group.
Hypoxia/reoxygenation injury activated the PKCβ/Egr-1 pathway (Figures 2A and 2B). While, the expression of PKCβ, Egr-1 and ERK was decreased at mRNA and protein levels in Astragaloside IV group, compared with H/R group. Subsequently, the up-regulation of LDH and MDA induced by hypoxia/reoxygenation was decreased significantly in Astragaloside IV preconditioning group (Figures 3B-3E).
Discussion
In the present study, we showed that hypoxia/reoxygenation induced cardiac myocytes death in primary cultured neonatal rat cardiac myocytes, possibly through activation of PKCβ/ Egr-1 pathway. Astragaloside IV could inhibit hypoxia/ reoxygenation-induced cardiac myocytes death by blocking PKCβ/Egr-1 Pathway. Similar results were found in PKCβ inhibitor group.
Ischemia and reperfusion injury is a primary cause of cardiac failure, morbidity, and mortality after cardiac operations or heart infarctions [16]. Determining how to salvage the viable myocardial tissue and restore its electrical and mechanical functions has become a primary focus in clinical settings [17,18]. There are many powerful strategies to limit ischemia and reperfusion injury. Astragalus membranaceus, which is a Chinese traditional medicine, has long been used for the management of heart diseases. Astragaloside IV is one of the main active constituents of astragalosides. The protective effects of Astragaloside IV against myocardial ischemia and reperfusion injury have been reported in different models. However, the underlying mechanism of Astragaloside IV as a potential protective agent against ischemia and reperfusion injury in the heart has not been well investigated. Here, we confirmed that hypoxia/reoxygenation could induce cardiac myocytes death in primary cultured neonatal rat cardiac myocytes, possibly through activation of PKCβ/Egr-1 pathway [19,20]. And then we first showed that Astragaloside IV could inhibit hypoxia/reoxygenation-induced cardiac myocytes death by blocking PKCβ/Egr-1 pathway.
In conclusion, our data provide more insights into Astragaloside IV’s role in caridioprotection. This will help us to understand the mechanism of Astragaloside IV as a protective agent against ischemia and reperfusion injury.
Funding
This work was supported by the Zhejiang Provincial Traditional Chinese Medicine Science program (grant numbers, 2014ZA006)
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Che X, Wang Q, Xie Y, Xu W, Shao X, Mou S, Ni Z. Astragaloside iv suppresses transforming growth factor-beta1 induced fibrosis of cultured mouse renal fibroblasts via inhibition of the mapk and nf-kappab signaling pathways. Biochem Biophys Res Commun 2015; 464: 1260-1266.
- Zhao Z, Wang W, Wang F, Zhao K, Han Y. Effects of Astragaloside IV on heart failure in rats. Chin Med 2009; 4: 6.
- Tu L, Pan CS, Wei XH, Yan L, Liu YY, Fan JY, Mu HN, Li Q, Li L, Zhang Y, He K, Mao XW, Sun K, Wang CS, Yin CC, Han JY. Astragaloside i.v. protects heart from ischemia and reperfusion injury via energy regulation mechanisms. Microcirculation 2013; 20: 736-747.
- Zhao M, Shao D, Yu L, Sun X, Wang Y, Hu H, Feng R, Gao Q, Guo F, Hao L. Electrophysiological effect and the gating mechanism of astragaloside i.v. on l-type ca (2+) channels of guinea-pig ventricular myocytes. Eur J Pharmacol 2015; 760: 27-35.
- Belmonte SL, Blaxall BC. PKC-ing is believing: targeting protein kinase C in heart failure. Circ Res 2011; 109: 1320-1322.
- Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. Pkc-alpha regulates cardiac contractility and propensity toward heart failure. Nature Med 2004; 10: 248-254.
- Yabe K, Tanonaka K, Koshimizu M, Katsuno T, Takeo S. A role of PKC in the improvement of energy metabolism in preconditioned heart. Basic Res Cardiol 2000; 95: 215-227.
- Sun S, Ning X, Zhai Y, Du R, Lu Y, He L, Li R, Wu W, Sun W, Wang H. Egr-1 mediates chronic hypoxia-induced renal interstitial fibrosis via the pkc/erk pathway. Am J Nephrol 2014; 39: 436-448.
- Malakooti J, Sandoval R, Amin MR, Clark J, Dudeja PK, Ramaswamy K. Transcriptional stimulation of the human nhe3 promoter activity by pma: Pkc independence and involvement of the transcription factor egr-1. Biochem J 2006; 396: 327-336.
- Liu QF, Yu HW, Sun LL, You L, Tao GZ, Qu BZ. Apelin-13 upregulates egr-1 expression in rat vascular smooth muscle cells through the pi3k/akt and pkc signaling pathways. Biochem Biophys Res Commun 2015.
- Chen M, Xiong F, Zhang L. Promoter methylation of egr-1 site contributes to fetal hypoxia-mediated pkcepsilon gene repression in the developing heart. Am J Physiol Regulatory Integr Compar Physiol 2013; 304: 683-689.
- Ramadas N, Rajaraman B, Kuppuswamy AA, Vedantham S. Early growth response-1 (egr-1) - a key player in myocardial cell injury. Cardiovasc Hematol Agents Med Chem 2014; 12: 66-71.
- Zhang Y, Shi G, Zheng J, Lv Y, Gao P, Huang Z, Gao F, Zhou Y. The protective effect of egr-1 antisense oligodeoxyribonucleotide on myocardial injury induced by ischemia-reperfusion and hypoxia-reoxygenation. Int J Exp Cell Physiol Biochem Pharmacol 2008; 22: 645-652.
- Simpson P, Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circulation Res 1982; 50: 101-116.
- Qin J, Liu ZX. Fak-related nonkinase attenuates hypertrophy induced by angiotensin-ii in cultured neonatal rat cardiac myocytes. Acta Pharmacologica Sinica 2006; 27: 1159-1164.
- Solaro RJ, Arteaga GM. Heart failure, ischemia/reperfusion injury and cardiac troponin. Adv Exp Med Biol 2007; 592: 191-200.
- Vijayalakshmi K, Kunadian B, Wright RA, Sutton AG, Hall JA, de Belder MA. Successful thrombus extraction with the rescue thrombus management system during acute percutaneous coronary intervention improves flow but does not necessarily restore optimal myocardial tissue perfusion. Catheterization Cardiovascular Interventions J Society Cardiac Angiography Interventions 2006; 67: 879-886.
- Shi CZ, Zhang XP, Lv ZW, Zhang HL, Xu JZ, Yin ZF, Yan YQ, Wang CQ. Adipose tissue-derived stem cells embedded with enos restore cardiac function in acute myocardial infarction model. Int J Cardiol 2012; 154: 2-8.
- Yan SF, Harja E, Andrassy M, Fujita T, Schmidt AM. Protein kinase c beta/early growth response-1 pathway: A key player in ischemia, atherosclerosis, and restenosis. J Am Coll Cardiol 2006; 48: 47-55.
- Pines A, Romanello M, Cesaratto L, Damante G, Moro L, DAndrea P, Tell G. Extracellular atp stimulates the early growth response protein 1 (egr-1) via a protein kinase c-dependent pathway in the human osteoblastic hobit cell line. Biochem J 2003; 373: 815-824.