ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 4
Bacteria induced extrinsic and intrinsic apoptotic pathways in the rat gastrointestinal system
1Department of Medical Microbiology, Medical Faculty, Pamukkale University, Denizli, Turkey
2Department of Histology and Embryology, Medical Faculty, Pamukkale University, Denizli, Turkey
3Animal Research Center, Medical Faculty, Pamukkale University, Denizli, Turkey
4Department of Anatomy, Medical Faculty, Pamukkale University, Denizli, Turkey
5Department of Biophysics, Medical Faculty, Pamukkale University, Denizli, Turkey
- *Corresponding Author:
- Gulcin Abban Mete
Department of Histology and Embryology, Medical Faculty
Pamukkale University, Turkey
E-mail: gabban@pau.edu.tr
Accepted date: September 14, 2016
Apoptosis is a key factor in the death of organ-specific cells. Developing a clear understanding of the effect that bacterial translocation has on initiating apoptotic pathways that induce multiple organ dysfunction syndrome (MODS) is essential for developing effective treatment modalities. Translocation does not occur naturally and apoptotic pathways remain unclear. The aim of this study is to investigate the effect of bacterial translocation on apoptotic pathways in the spleen, small intestine, colon, liver, and mesenteric lymph nodes (MLN) of rats. We randomly divided 12 healthy Wistar-Albino rats into two groups and induced bacterial translocation in the experiment group by clamping the superior mesenteric arteries (SMA) for comparison of test results to the control group. Samples from both groups were collected under sterile conditions and an inoculation procedure was performed. Caspase 3, 8, 9 and p53 were evaluated by immunohistochemistry, and cell expressions were counted. Klebsiella and E. coli colonies were observed in the bacteria cultures associated with the experiment group. Bacterial translocation-activated caspase 8 and 3 were found in all tissues of the experiment group; however, activated p53 was identified only in the colon and liver and activated caspase 9 was seen in small intestine, mesenteric lymph nodes and liver. We concluded that translocated bacteria stimulated extrinsic and intrinsic signaling pathway in gastrointestinal systems.
Keywords
Apoptosis, Bacteria translocation, Caspases, P53, Pathway, Sepsis
Introduction
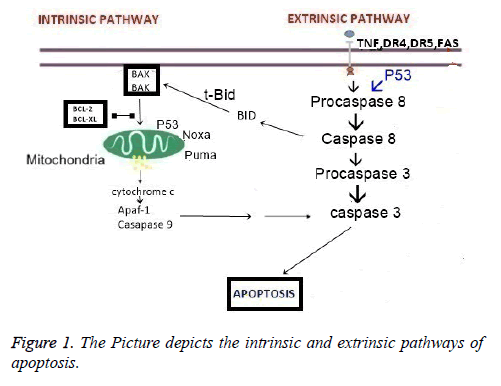
Bacterial translocation (BT) is the passage of microflora through the lamina propria to local mesenteric lymph nodes (MLN) and to other organs, such as the liver and spleen [1]. Translocation does not occur under normal conditions. In conditions such as burns; starvation; intestine obstruction; surgical trauma; anesthesia; obstruction of the gall bladder; and shock, bacterial translocation impairs intestinal barrier function and the mucosal structure in the GI tract [1,2]. Following translocation, enteric bacteria pass into the systemic circulation and spread to the entire body, which may cause sepsis; shock; multiorgan failure; and death [1-3]. Multiorgan failure is a chief concern due to the associated mortality. Multiple organ failure is often caused by Multiple Organ Dysfunction Syndrome (MODS). MODS is most commonly found in patients hospitalized in intensive care units [2-4]. Apoptosis also is an important player in the death of organ-specific cells and in multiorgan failure. In the presence of bacterial translocation, particularly in sepsis, the retardation and delay in neutrophil apoptosis deteriorates the immune response and worsen the cascade that underpins MODS [1-3]. There are two major pathways in apoptosis: Extrinsic and intrinsic pathway (Figure 1).
Extrinsic pathway
The extrinsic apoptosis pathways were classified as type I and type II. In type I, cells’ capacity to activate direct caspase 8 apoptosis pathways was via death receptors (TNF-R, FAS, DR3, DR4, DR5, DR6) [5]. Activated caspase 8 initiates a caspase cascade. Cleavage of caspases 3, 6 and 7 leads to the characteristic morphological and biochemical features of apoptosis [6]. In type II, the signal coming from the activated receptor needs to be amplified via mitochondria-dependent apoptotic pathways. In this case, caspase 8 is activated later, Bid is cleaved by caspase 8 and in its truncated form (tBid) translocates to the mitochondria to induce the release of cytochrome c into the cytosol [7]. Cytosolic cytochrome c binds to monomeric Apaf-1, which then triggers the activation of the initiator procaspase 9 [8,9]. Activated caspase 9 subsequently initiates a caspase cascade involving downstream effector caspases such as caspase 3, caspase 7, and caspase 6 [9]. In this type II response, Bid is the key protein that facilitates the cross-talk between the extrinsic and intrinsic pathways. In so-called Type I cells stimulation of the extrinsic pathway is sufficient to drive apoptosis, whereas in Type II cells required further amplification by Bid for commitment to apoptosis.
Intrinsic pathway
In the intrinsic or mitochondrial pathway, The Bcl-2 family of proteins have either pro-or anti-apoptotic activities and regulate apoptosis by controlling mitochondrial permeability. In response to many types of stress or damage the pro-apoptotic Bcl-2-associated X protein (Bax) or Bcl-2 antagonist/killer-1 (Bak) are activated and they promote the release of cytochrome c. The association of cytochrome c with apaf-1 recruit procaspase 9, cleavage of activated caspase 9, and the activation of caspase 3 which terminate the cell death [10]. In the literature, there are few studies showing the induction of apoptosis through the investigation of bacterial translocation. More significantly, the pathways of apoptosis were not clear in previous similar studies. In this study our aim was to show the apoptotic pathways in gastrointestinal system. In order to do caspase 3, 8, 9 and p53 expressions were investigated immunohistochemically.
Materials and Methods
Ethics
This experiment was conducted according to the Turkish “Experiments on Animals Act” and was approved by the Institutional Animal Ethics Committee of the Central Animal Laboratory of Pamukkale University (01.31.2012- PAU.HDEK-2012/007).
Animals
Twelve healthy Wistar-Albino rats were used in this study. The mean weight of the rats was 230 g (200-250 g) and the age of the rats was 20 weeks. Care for the animal care use was provided in an Experimental Animal Laboratory in Pamukkale University. The rats were humanely treated and divided by four rats per cage in a well-ventilated, 12 h dark and light cycle room at the temperature of 24°C throughout the experiment.
Groups
The rats were randomly divided into two groups. Group I was assigned as the control and Group II was assigned as the experiment group.
Interventions and surgical procedures
The rats were anesthetized with intraperitoneal ketamine (50 ml/kg). Following the skin shave, the skin was cleaned by povidone iodide and median laparotomies were performed under sterile conditions. The superior mesenteric artery (SMA) was clamped on the rats in the experimental group and ischemia was produced. Forty-eight hours after the experiment, samples of liver, spleen, small intestine, and colon were taken into sterile tubes. These samples were homogenized by adding 1 ml saline. The inoculation was performed on blood agar and eosine methylene blue (EMB) agar, and colonies were counted on the plaques. The blood samples of 0.5 ml were taken under sterile conditions and 5 ml brain-heart infusion was placed into Broth medium. The blood cultures were incubated for 7 days at 37°C and subcultured in blood agar and EMB agar mediums and the production was evaluated.
Apoptosis procedure and histology
Mesenteric lymph nodes, liver, spleen, small intestine, and colon tissues were left in 10% formaldehyde for 72 h. The fixed tissues were processed with light microscopy procedure and 5 μm sections from the paraffin-embedded tissues were taken onto positive loaded slides.
Antibodies and staining procedure
Endogenous peroxidase activity was blocked in 3% hydrogen peroxidase for 10 min, and the sections were incubated with saponin to facilitate binding of the primary antibody to the antigenic areas. Epitopes were stabilized by application of serum blocking solution (goat serum; Lot# 20570999; Zymed Laboratories Inc., South San Francisco, CA, USA) for 60 min at room temperature. Sections were incubated in phosphatebuffered saline (PBS) at room temperature for 60 min using the following primary antibodies: caspase 8 (1:200 sc-5263; Lot# H0911 Santa Cruz Biotechnology), caspase 9 (1:100, Ab-4 Lot# H0911 Neomarker Biotechnology), caspase 3 (1;200, Sc-1226 Lot# F0206, Santa Cruz Biotechnology), p53 (1:200, sc-1311; Lot# D1911, Santa Cruz Biotechnology). In the next step, secondary antibodies were applied to tissue slides: antirabbit IgG and avidin-biotin-complex-peroxidase (ABC; Lot# 903532A; Invitrogen, Carlsbad, CA, USA). Diaminobenzidine (DAB; Lot# 100M16942; Sigma-Aldrich, St. Louis, MO, USA) was used as the chromogen. In the following step, the slides were counterstained with hematoxylin for 1 min, dehydrated in graded ethanol, and mounted in conventional medium. The immunoperoxidase reaction was determined to be negative (-) or positive (+). The findings were observed and photographed under OLYMPUS BX51 microscope.
Statistical analysis
In each slide, five areas and 100 cells/areas were evaluated under 40X magnification. The percentages of the positive cells within these areas were determined at different times by two investigators blinded to the source of the samples, and the mean was calculated. The data were analysed in an SPSS 17 program. In addition, P<0.05 was estimated as significant for the non-parametric Mann-Whitney U test.
Results
Microbiological results
The significant reproduction was observed in six rats where translocation had been applied. Three of the six were found in the liver, five in the mesenteric lymph nodes, and two in the spleen. Klebsiella and E. coli reproduction were observed, throughout.
Histological results
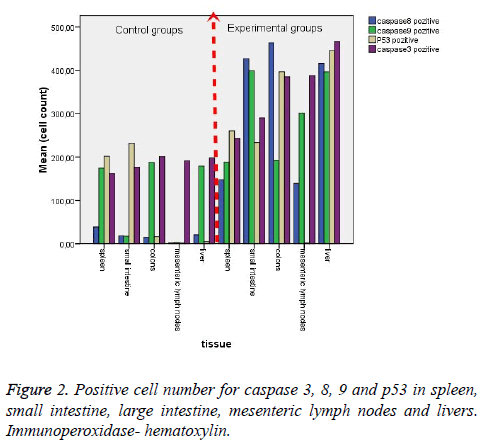
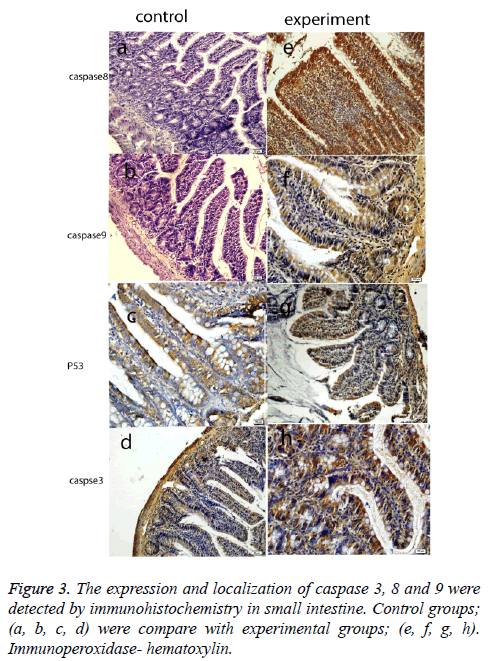
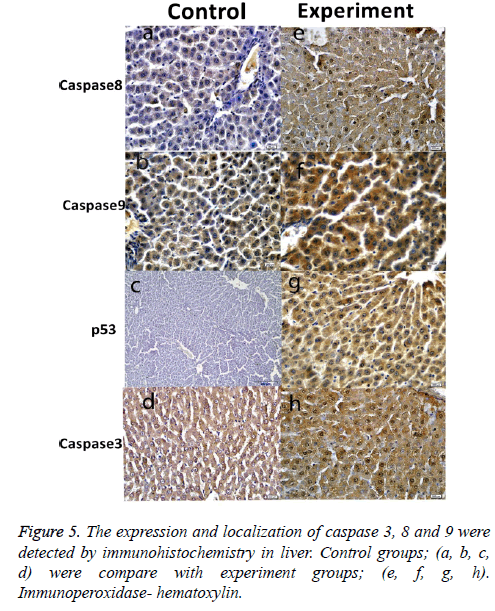
Histological results are summarized in Table 1. We also analyzed positive cell number for caspase 3, 8, 9 and p53 in spleen, small intestine, colon, mesenteric lymph nodes and livers in Figure 2 and Table 2. As can be seen in the results for the small intestines in Table 1, while caspase 8 and caspase 9 were negative in the control group, these two proteins were positive in the experimental group. The same results were also found for p53 expression in the experimental and the control groups; however, caspase 3 expression was increased in the experimental groups (Figure 3). In the colon, in the control group, expressions of caspase 8, 9, and p53 were negative while in the experiment groups they were positive. P53 expression was different from the results of the small intestine. Similarly, caspase 3 expression was increased in the experiment group when compared with the control group (Figure 4). Likewise, the liver also showed increased expression of caspase 3, 8, and 9 in the experiment group when compared with the control group; however, while the p53 expression was negative in control group, the results had changed to a positive in the experiment group (Figure 5).
| C | EXP | C | EXP | C | EXP | C | EXP | ||
|---|---|---|---|---|---|---|---|---|---|
| Caspase 8 | Caspase 9 | P53 | Caspase 3 | ||||||
| spleen | White pulp | - | + | - | + | + | + | + | ++ |
| Red pulp | + | ++ | + | ++ | + | + | + | + | |
| small intestine | Epithelium | - | +++ | - | ++ | ++ | ++ | ++ | +++ |
| Connective tissue | - | +++ | - | ++ | ++ | ++ | ++ | +++ | |
| Muscle tissue | +++ | - | ++ | ++ | ++ | ++ | +++ | ||
| colons | Epithelium | - | +++ | - | ++ | - | +++ | ++ | +++ |
| Connective tissue cells | - | +++ | - | ++ | - | +++ | - | + | |
| Muscle tissue | +++ | - | ++ | +++ | ++ | +++ | |||
| MNL | Lenf nodules | - | ++ | - | ++ | - | - | - | +++ |
| Medulla | - | + | - | + | - | - | - | + | |
| liver | Hepatocyte cytoplasm | + | +++ | ++ | +++ | - | +++ | ++ | +++ |
| Hepatocyte nuclei | - | +++ | - | - | - | - | - | +++ | |
Table 1: The localization and expression of caspase 3, 8, 9 and p53 in spleen, liver, small intestine and MNL.
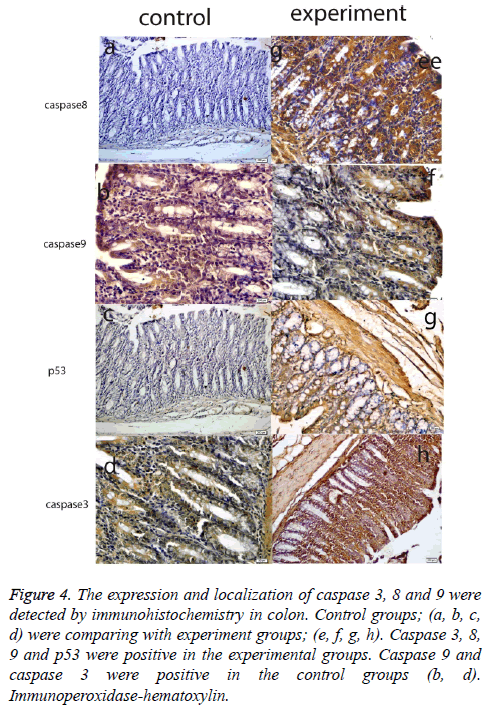
Figure 4: The expression and localization of caspase 3, 8 and 9 were detected by immunohistochemistry in colon. Control groups; (a, b, c, d) were comparing with experiment groups; (e, f, g, h). Caspase 3, 8, 9 and p53 were positive in the experimental groups. Caspase 9 and caspase 3 were positive in the control groups (b, d). Immunoperoxidase-hematoxylin.
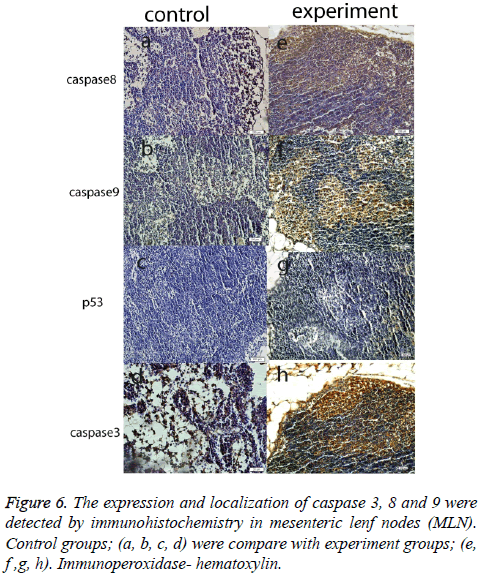
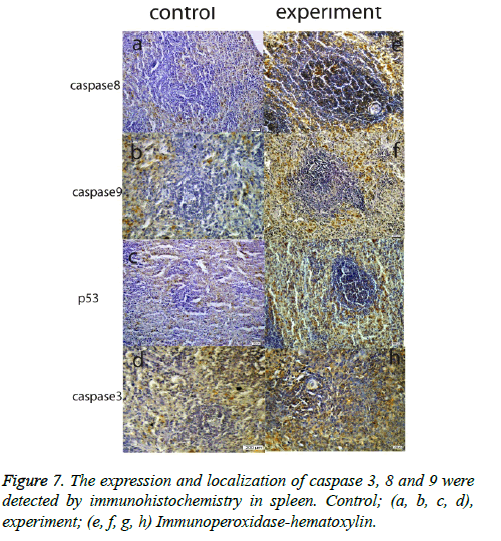
Another important finding across all organ tissues from the experimental group was a positive expression of caspase 8 and caspase 3 in the nuclei and cytoplasm. In MLN tissue groups, while caspase 8, 9, and 3 expressions were negative in the control groups, those proteins were positive in the experiment groups; and p53 expression was negative, too. (Figure 6). In the spleen, caspase 8, 9, 3, and p53 were expressed positively in both the experiment and control groups, but the expression was more elevated in the experimental group (Figure 7). According to the cell number for expression, caspase 8 and caspase 3 were increased significantly (p<0.05) in all experimental tissue groups when compared to the control tissue groups. Caspase 9 was high significantly (p<0.05) in the small intestine, MLN, and liver, but not in the spleen and colon. Although p53 was positive, the expression was poor in almost all tissues, but a significant difference was observed in colon and liver tissue specimens for the experiment group (p<0.05) (Table 2 and Figure 2).
| Tissue | Caspase8 positive cell number | Caspase9 positive cell number | p53Positive cell nuber | Caspase3 positive cell number | |||||
|---|---|---|---|---|---|---|---|---|---|
| spleen | Mean | 39 | 148 | 175 | 188 | 202 | 261 | 162 | 243 |
| Std. Deviation | 14 | 12 | 14 | 9 | 55 | 6 | 7 | 10 | |
| small intestine | Mean | 18 | 427 | 18 | 399 | 232 | 234 | 177 | 290 |
| Std. Deviation | 3 | 33 | 3 | 7 | 11 | 3 | 6 | 14 | |
| colons | Mean | 15 | 464 | 187 | 193 | 16 | 397 | 201 | 385 |
| Std. Deviation | 3 | 41 | 9 | 9 | 3 | 9 | 4 | 10 | |
| mesenteric lymph nodes | Mean | 2 | 140 | 3 | 301 | 2 | 2 | 192 | 388 |
| Std. Deviation | 1 | 19 | 2 | 8 | 1 | 1 | 11 | 12 | |
| liver | Mean | 21 | 415 | 179 | 397 | 5 | 445 | 198 | 467 |
| Std. Deviation | 3 | 7 | 27 | 5 | 2 | 8 | 7 | 24 | |
Table 2: Positive cell number for caspase 3, 8, 9 and p53 in spleen, small intestine, colon, mesnteric lymph nodes and livers.
Discussion
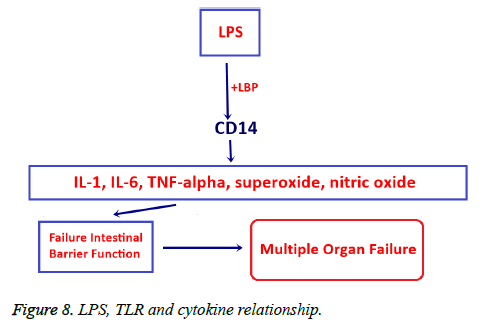
We investigated the effects of bacterial translocation caused by mesenteric artery obstruction on apoptosis in the small intestine, colon, mesenteric lymph nodes, liver, and spleen tissues using both inferential and descriptive statistical measures. Significant reproduction was observed in applied bacterial translocation for 6 rats in the experiment group. The most common translocated microorganisms were E. coli and Klebsiella spp. Markers of apoptosis: caspase 3, 8 and 9 were activated in small intestine, MLN, Liver samples from the experiment group, but p53 activation was seen only in colon and liver tissue samples. Bacteria generally induce apoptosis with the secretion of protein synthesis inhibitors, the production of pore proteins, and the activation of death machinery molecules such as lipopolysaccharides (LPS), or other super antigens [11-13]. LPS which is component of the outer wall of gram-negative bacteria such as E. coli binds to LBP, a soluble acute-phase protein, LPS+LPB can induced expression of CD14, TNF-related apoptosis inducing ligand, and TLR14 (the toll like receptor for LPS) thus stimulating nuclear transcription factor kappa B (NF-κB) which results in the release of many types of inflammatory factors and cellular toxic substances. Consequently, LPS impairs intestinal barrier function by activating chemical mediators, i.e. eicosanoids, interleukin-1, interleukin- 6, TNFα, superoxide, and nitric oxide. Impaired intestinal barrier leads to multiple organ failure (Figure 8) [14-21].
In our study the expression of caspase 8 and caspase 3 were increased in all experimental tissue groups when compared to the control tissue groups (p=0.004). The caspase 9 expression was intense and the increase in positive cell in the small intestine, MLN, and in liver was statistically significant (p=0.004), but it was not in the spleen (p=0.065) and colon (p=0.336). Although p53 was positive, the expression was poor in almost all tissues, but a significant difference was observed in colon and liver tissue specimens for the experiment group (p<0.05) (Table 2 and Figure 2). In experimental sepsis models there was a correlation between increase of apoptosis in paranchmal tissue and increase of TNFα level in serum [22]. We argue further that increased caspase 8 and 9 expressions can be linked to tumor necrosis factor-alpha (TNFα) due to the inflammatory factors that would accompany TNFα induce apoptosis. TNFα binds to membrane-bound death receptors, such as TNF- receptor 1 (TNF-R1). TNF-R activates the caspase 8 and then pro-apoptotic BCL-2 family proteins are activated, leading to changes in the integrity of the mitochondrial membrane so that pro-apoptotic Bcl 2 family members Bax and Bak can induce the release of cytochrome c and active caspases 9 and 3 (Figure 1) [7-11]. In our study, there is a significant increase of p53 positive cell in liver while there was no p53 activation in small intestine and MLN suggesting that apoptotic pathways in liver are different from small intestine and MLN (p=0.004). We think that apoptotic pathway in small intestine and in MLN might be due to TNF alpha.
p53 expression that had been negative in liver and colon in the control group changed to a positive expression in the experiment group in our study. In spleen, an increased p53 expression was observed in the experiment group when compared with the control group. However, this increase was not statistically significant (p=0.574). Intrinsic (mitochondrial) pathway are often used by p53; however, at the same time, this protein can activate the extrinsic apoptotic pathway through the induction of genes by encoding three trans-membranic proteins: Fas, DR5, and PERP [23,24]. In other studies, p53- dependent Fas mRNA induction has been demonstrated in the spleen, thymus, kidney, and lung [25,26]. Figure 1 shows the p53 mediated extrinsic pathway [26,27]. Statistically, in liver caspase 8, caspase 9 and p53 positive cell number was increased in experiment group compared to control (p=0.004) however, in colon caspase 8 and p53 positive cell number was increased significantly between experiment and control (p=0.004). On the other hand, there was no significance in the number of caspase 9 positive cells (p=0.336).
Our findings obtained from the small intestine, MLN and liver demonstrate that activation of caspase 8 may be related to an increased TNFα expression. However, we view this connection only appears plausible because the mesenteric lymph nodes and remained negative in both the control and experiment groups in small intestine p53 expression was similar in both the control and experiment groups. On the other hand, in the small intestine and MLN groups the caspase 9 increase without an accompanying change in p53 expression may indicate crosstalk between intrinsic and extrinsic pathways. In addition to that in liver caspase 8, 9 and p53 increase suggests that there is a cross talk between intrinsic and extrinsic pathways and there is also a p53 activation. On the other hand, there is a non-significant caspase 9 increase and there is only significant caspase 8 increase suggests that there is only Type I extrinsic pathway activation in spleen. Our results indicate that translocates bacteria created by hypoxia in the tissue due to clamping of the SMA activated the extrinsic apoptosis pathway type II in the small intestine and mesenteric lymph nodes, extrinsic apoptosis pathway type II and p53 activation in liver, extrinsic apoptosis pathway type I and p53 activation (intrinsic apoptotic pathway) in colon and extrinsic apoptosis pathway type I in spleen.
Conclusions
In this study we detected both extrinsic and intrinsic apoptotic pathways in the gastrointestinal systems (GIS) of the experimental group rats. Despite numerous intensive treatment modalities, an effective method for treating MODS has not yet been found. This study is important because it expands the current body of knowledge on the effect of bacterial translocation on apoptotic pathways; however, further exploration of apoptosis and cell survival mechanisms will facilitate development of new, more effective strategies for MODS treatment. We believe that the prevention of apoptosis in the intestinal epithelium is the most effective, leading edge intervention to pursue for MODS treatment in the future.
References
- Alexander JW, Boyce ST, Babcock GF, Gianotti L, Peck MD, Dunn DL, Pyles T, Childress CP, Ash SK. The process of microbial translocation. Ann Surg 1990; 212: 496-510.
- Wolochow H, Hildebrand GJ,Lamanne C. Translocation of microorganisms across the intestinal wall of the rat: effect of microbial size and concentration. J Infect Dis 1966; 116: 523-528.
- Yigitler C, Gulec B, Aydogan H, Ozcan A, Kilinc M, Yigit T. Effect of mesalazine, metroinidazole and gentamicin on bacterialtranslocation in experimental colitis. J Gastroenterol Hepatol 2004; 19: 1179-1186.
- Louis K, Netea MG, Carrer DP, Kotsaki A, Mylona V, Pistiki A, Savva A, Roditis K, Alexis A, Van der Meer JW, Giamarellos-Bourboulis EJ. Bacterial translocation in an experimental model of multiple organ dysfunctions. J Surg Res, 2013; 183: 686-94.
- Yang R, Miki K, Oksala N, Nakao A, Lindgren L, Killeen ME, Mennander A, Fink MP, Tenhunen J. Bile high-mobility group box 1 contributes to gut barrier dysfunction in experimental endotoxemia. Am J Physiol Regul Integr Comp Physiol 2009; 297: R362-R369.
- Kucuk C, Sozuer E, Gursoy S, Canoz O, Artis T, Akcan A, Akyildiz H, Muhtaroglu S. Treatment with Met-RANTES decreases bacterial translocation in experimental colitis. Am J Surg 2006; 191: 77-83.
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science 1998; 281: 1305-1308.
- Scaffidi C, Fulda S, Srinivasan A,Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J 1998; 17: 1675-1687.
- Acehan D, Jiang X, Morgan DG, Heuser JE, Wang X,Akey CW. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol Cell 2002; 9: 423-432.
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 1998; 94: 481-490.
- Grassme H, Jendrossek V, Gulbins E. Molecular mechanisms of bacteria induced apoptosis. Apoptosis 2001; 6: 441-445.
- Weinrauch Y, Zychlinski A. The induction of apoptosis by bacterial pathogens. Annu Rev Microbiol 1999; 53: 155-187.
- Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell 1993; 75: 653-660.
- Feng M, Li J, Wang J, Ma C, Jiao Y, Wang Y, Zhang J, Sun Q, Ju Y, Gao L, Zhao Y. High glucose increases LPS-induced DC apoptosis through modulation of ERK1/2, AKT and Bax/Bcl-2. BMC Gastroenterol 2014; 14: 98.
- Liu D, Yi B, Liao Z, Tang L, Yin D, Zeng S, Yao J, He M. 14-3-3γ protein attenuates lipopolysaccharide-induced cardiomyocytes injury through the Bcl-2 family/mitochondria pathway. IntImmunopharmacol 2014; 21: 509-515.
- Bielaszewska M,Rüter C, Kunsmann L, Greune L,Bauwens A, Zhang W, Kuczius T, Kim KS, Mellmann A, Schmidt MA, Karch H.Enterohemorrhagicescherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis. PLoS Pathog. 2013; 9: e1003797.
- Enomoto N, Takei Y, Yamashina S, Fukuda T, Suzuki S, Ikejima K, Kitamura T, Sato N. Burn injury sensitizes rat Kupffer cells via mechanisms dependent on gut-derived endotoxin. J Gastroenterol 2004; 39: 1175-1181.
- Crouser ED, Julian MW, Weinstein DM, Fahy RJ, Bauer JA. Endotoxin-induced ileal mucosal injury and nitric oxide dysregulation are temporally dissociated. Am J Respir Crit Care Med 2000; 161: 1705-1712.
- Opal SM, GlckT. Endotoxin as a drug target. Crit care Med2003; 31: 57-64.
- Pahl HL. Activators and target genes of Rel/NFkappaB transcription factors. Oncogene, 1999; 18: 6853-6866.
- Burns TF, Bernhard EJ, El-Deiry WS. Tissue specific expression of p53 target genes suggests a key role for KILLER/DR5 in p53- dependent apoptosis in vivo. Oncogene 2001; 20: 4601-4612.
- Bouvard V, Zaitchouk T, Vacher M, Duthu A, Canivet M, Choisy- Rossi C, Nieruchalski M, May E. Tissue and cell-specific expression of the p53-target genes: bax, fas, mdm2 and waf1/p21, before and following ionizing irradiation in mice. Oncogene 2000; 19: 649-660.
- Fu J, Wang Y, Zhang J, Wu W, Chen X, Yang Y. Anti-inflammatory and anti-apoptotic effects of oxysophoridine on lipopolysaccharide-induced acute lung injury in mice. Am J Transl Res 2015; 15: 2672-2682.
- Deng W, Deng Y, Deng J, Wang DX, Zhang T. Losartan attenuated lipopolysaccharide-induced lung injury by suppression of lectin-like oxidized low-density lipoprotein receptor-1. Int J Clin Exp Pathol 2015; 8: 15670-1576.
- Li J, Kandatsu N, Feng GG, Jiang JZ, Huang L, Kinoshita H, Okada S, Fujiwara Y. Propofol reduces liver dysfunction caused by tumor necrosis factor-α production in Kupffer cells. J Anesth 2016; 1-7.
- Bennett M, Macdonald K, Chan SW, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science 1998; 282:290-293.
- Haupt S, Berger M, Goldberg Z, Haupt Y. Apoptosis – the p53 network. J Cell Sci 2003 116: 4077-4085.