ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2016) Volume 27, Issue 3
Beneficial effects of nerolidol on thioacetamide-induced damage of the reproductive system in male rats.
1Department of Urology, Faculty of Medicine, University of Inonu, Malatya, Turkey
2Department of Pharmacology, Faculty of Medicine, University of Inonu, Malatya, Turkey
3Department of Histology and Embryology, Faculty of Medicine, University of Inonu, Malatya, Turkey
4Department of Obstetrics and Gynaecology, Faculty of Veterinary Medicine, University of Firat, Elazig, Turkey
5Department of Emergency, Faculty of Medicine, University of Inonu, Malatya, Turkey
Accepted date: February 25, 2016
In this study, it was aimed to determinate beneficial effects of Nerolidol (NLR) against reproductive toxicity caused by Thioacetamide (TAA). Male, 3-4-months-old, rats (n=32) were divided into four groups. Group-1 was kept as control and given corn oil as carrier. Group-2 received TAA (200 mg/kg, intraperitoneal (i.p.), two times per week) for 3 weeks, in group-3; NRL was orally administered at the dose of 100 mg/kg per every other day by gavages, group-4; 200 mg/kg TAA and 100 mg/kg NRL were given. Thiobarbituric Acid Reactive Substances (TBARS) and reduced Glutathione (GSH) levels, Catalase (CAT), Superoxide Dismutase (SOD) and Glutathione Peroxidase (GPX), sperm parameters and reproductive organs weight were determined. TAA caused a significant rise in TBARS level and a significant reduce in GPX, CAT, SOD and GSH levels in the testicular tissues compared with the control group, while NLR led to significant reduce in lipid peroxidation via decreasing TBARS level and increasing the levels of GPX, CAT, SOD and GSH. Besides, sperm parameters significantly reduced, and pathologic testicular damage increased with TAA exposure. However, these effects of TAA on sperm parameters and histopathological changes were reversed by NLR treatment. In conclusion, our results demonstrate that the management of TAA induced the testicular damage and NLR prevented thioacetamide-induced testicular damage in rats.
Keywords
Nerolidol, Thioacetamide, Rat, Experimental, Testicular damage.
Introduction
The attempt to avoid diseases and toxicities by using natural herbal products is an old practice that has been the basis of treatment for many human afflictions. Medical plants are particularly useful, and antioxidants have obtained increasing attention in the fields of food and medicine [1]. Essential oils are highly enriched in combinations termed terpenoids, and have several biological specialities. Nerolidol is a sesquiterpene present in the essential oils of many plants and flowers, such as neroli, ginger, jasmine, lavender and tea tree [2]. Nerolidol is used as a fragrance additive in cosmetics (e.g., shampoos and perfumes) and non-cosmetics (e.g., detergents) and as a flavoring agent. It has also been studied as a topical skin penetration enhancer [3,4]. Various medicinal benefits of nerolidol have been identified; for instance, it has an antibacterial effects on S.aureus and E.coli by altering bacterial cell permeability [5,6]. Moreover, antifungal effect against Microsporum gypseum, antimalarial, antileishmanial, antioxidant and antiulcer activities [2,7-10].
Testes are rich in microtubule networks, which form the meiotic and mitotic spindles, and the sertoli cell cytoskeleton, and are of major significance in spermatogenesis [11]. The toxic effect of Thioacetamide (TAA) on the testes and spermatogenesis is known, but there are few studies available in the literature about the toxicity to the testis of TAA [12,13]. The present study was designed to investigate whether TAA causes male reproductive system toxicity and to examine the beneficial effects of NRL on such toxicity in an experimental rat model. To examine these effects, we evaluated histopathological findings, including apoptotic changes; biochemical analysis, including Thiobarbituric Acid Reactive Substances (TBARS), Catalase (CAT), Superoxide Dismutase (SOD), reduced Glutathione (GSH) and Glutathione Peroxidase (GPx); and sperm quality in adult male rats. Nerolidol was selected for this study due to its easy availability, its cost-effective nature and based on the fact that nerolidol is not well-known against toxicity. Furthermore, NRL is inherently available in many foods we eat, and is approved by the U.S. Food and Drug Administration as “Generally Recognized as Safe (GRAS)”. It has also been included by the Council of Europe in the list of substances granted approval [4,14].
Materials and Methods
Chemicals
Nerolidol and all other chemicals for biochemical, histological and spermatological analysis were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The standard solutions were stored at 4°C. The pure water for solution preparation was from a Millipore Autopure WR600A system (Millipore, Ltd., USA). All substances were of analytical grade or of the highest grade available.
Animals and treatment
The experimental study protocol was approved by the Ethical Committee on Animal Research of Inonu University in Malatya-Turkey protocol no: 2015-114. The study was performed on the basis of The Guidelines for Animal Research from the National Institutes of Health publication. A total of 32 healthy adult male Wistar Albino rats (3-4 months old and 280-30 g in weight) were obtained from Inonu University Laboratory Animals Research Center. Animals were housed in sterilised polypropylene rat cages, in a 12 h light/dark cycle, at an ambient temperature of 21°C and at a humidity of 60% on a standard commercial pellet diet and water ad libitum (free-feeding).
The rats were randomly divided into four groups (n=8 in each group) as follows: Group 1-control, was given isotonic saline (intraperitoneal) and corn oil (orally; vehicles). Group 2- Thioacetamide (TAA), 200 mg/kg, Intraperitoneal (ip) TAA was given two times per week for three weeks, and 0.9% NaCl was administered i.p. as a single dose at the beginning; Group 3-NRL, 100 mg/kg NRL was given by gavages every other day, and corn oil was given by gavages daily; Group 4-TAA + NRL, 200 mg/kg TAA was given i.p. two times per week, and 100 mg/kg NRL, suspended in corn oil, was given by gavages every other day. The dosages of TAA and NRL were chosen according to the previous dose-response studies [4-13].
At four weeks treatment, the animals were sacrificed under ketamine (70 mg/kg) and xylazine (8 mg/kg) anaesthesia. Testis, epididymis, seminal vesicles and prostate tissue were removed immediately and dissected on ice-cold glass. Tissue samples were stored at -45°C until biochemical evaluations could be made.
Biochemical evaluation
The homogenization of tissues was performed in Teflon glass homogenizer with 150 mM KCl (pH 7.4) to obtain 1:10 (w/v) dilution of the whole homogenates. The homogenates were centrifuged at 18.000 Xg (4°C) for 30 min to determine TBARS, GSH levels and CAT, SOD, and GPx activities. The levels of homogenised 1 g testes tissue TBARS, as an index of lipid peroxidation, were determined by thiobarbituric acid reaction using the method of Yagi [15]. The product was evaluated spectrophotometrically at 532 nm, and results are stated as nmol/g tissue. The GSH content of the testis homogenate was measured at 412 nm using the method of Sedlak & Lindsay [16]. The GSH level was stated as nmol ml-1. Superoxide dismutase activity was determined by the inhibition of Nitroblue Tetrazolium (NBT) reduction due to O2 generated by the xanthine/xanthine oxidase system [17]. The product was measured spectrophotometrically at 560 nm, and results were stated as U/mg protein. Catalase activity of tissues was detected according to the method of Aebi [18]. The enzymatic decomposition of hydrogen peroxide (H2O2) was followed directly by the decrease in absorbance at 240 nm. The enzyme activities were given in k/mg protein. Glutathione peroxidase activity was detected by the method of Paglia & Valentine [19]. In the presence of glutathione reductase and NADPH, the oxidised glutathione (GSSG) is directly converted to the reduced form with a concomitant oxidation of NADPH to NADP. The decrease in absorbance at 340 nm was measured. GPx activity was expressed as IU mg-1 protein. Tissue protein content was determined according to the method of Lowry et al., using bovine serum albumin as standard [20].
Histological evaluation
For light microscopic evaluation, testis samples were fixed in 10% formalin and were embedded in paraffin. Paraffin-embedded specimens were cut into 5 μm thick sections, mounted on slides and stained with Hematoxylen-Eosin (H-E). Tissue samples were examined using a Leica DFC280 light microscope and a Leica Q Win Image Analysis system (Leica Micros Imaging Solutions Ltd., Cambridge, UK).
Measurement of seminiferous tubule diameter and germinal epithelial cell thickness
In each section, the diameters of 20 separate circular seminiferous tubules were randomly measured using a 20x objective. We totally measure 100 tubule diamater in each group. The Mean Seminiferous Tubular Diameter (MSTD) and Germinal Epithelial Cell Thickness (GECT) of each testis was determined in micrometers (μm). We measured MSTD and GECT using a Leica DFC280 light microscope and a Leica Q Win Image Analysis system (Leica Micros Imaging Solutions Ltd., Cambridge, UK).
Evaluations of sperm parameters
The epididymal sperm concentration was defined with a haemocytometer using a modified method shortly expressed by Sonmez et al. [21]. The right epididymis was slim minced by medical scissors in 1 ml of isotonic saline in a Petri container and later it was allowed to incubate at room temperature. Then, the supernatant fluid including all epididymal sperm cells was counted with a light microscope at 200x enlargement. As Sonmez et al. described, the percentage of forward progressive sperm motility was measured visually using a light microscope at 400x enlargements [22]. Motility evaluations were made from three different areas in each sample. The mean of the three successive evaluations was used as the ultimate motility score. The slides stained with eosin-nigrosin were prepared so as to detect the percentage of morphologically abnormal spermatozoa, and later viewed under a light microscope at 400x enlargement. A total of 300 sperm cells (2100 cells in each group) were investigated on each slide. By the method of Sonmez et al. the head, tail and total abnormality rates of spermatozoa were stated as a percentage [22].
Statistical analysis
Data were analysed using the SPSS/PC (version 22.0; SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) and post hoc Tukey’s honestly significant difference test were performed to determine the difference among the groups in terms of the sperm and biochemical parameters. The values were presented as mean Standard Deviation (SD). After a significant Kruskal-Wallis H test, a Conover test was also carried out for the histopathological results. The results were stated as median (min-max). The degree of significance was set at P<0.05.
Results
Biochemical result
The levels of SOD, GPx, CAT, GSH and TBARS are given in table 1. The results showed that TAA treatment caused a significant increase in TBARS levels compared to other groups. Additionally, GSH, CAT, SOD and GPx levels were significantly decreased with TAA treatment compared to control.
| Testis | TBARS | GSH | CAT | SOD | GPx |
|---|---|---|---|---|---|
| Control | 4.18 ± 1.01a | 162.1 ± 14.5a | 0.033 ± 0.006a | 24.16 ± 1.68a | 225.8 ± 9.95a |
| TAA | 7.64 ± 1.23b | 105.6 ± 15.2b | 0.021 ± 0.003b | 15.27 ± 1.34b | 154.5 ± 9.38b |
| NRL | 4.12 ± 0.98a | 167.9 ± 13.7a | 0.034 ± 0.004a | 25.32 ± 2.09a | 238.9 ± 10.7a |
| TAA+NRL | 3.94 ± 0.92a | 158.7 ± 9.1a | 0.027 ± 0.007c | 19.91 ± 1.04c | 189.6 ± 7.16c |
| Different superscript letters a, b and c within same column showed significant (P<0.05) differences between all groups. | |||||
Table 1: The levels of SOD, GPx, CAT, GSH and TBARS in the rat testis tissue (Mean ± SEM).
On the other hand, NRL administration together with TAA lead to a significant decrease in elevated TBARS levels and a significant increase in GSH, CAT, SOD and GPx levels in comparison to the only given TAA group and these values was nearly control level.
Result about organ weights and sperm parameters
The effects of TAA and NRL on the testis, epididymis, seminal vesicles, prostate weight, epididymal sperm concentration, sperm motility and abnormal sperm rate are given in table 2. The results indicate that there was no significant change in organ (testis epididymis, seminal vesicles, prostate) weights and abnormal sperm rate with TAA or NRL treatment compared with control group. However, TAA causes a significant (p<0.05) decrease in sperm motility and sperm concentration compared to other groups. On the other hand, sperm motility and sperm concentration was numerically increased in NRL group compared to TAA groups. But, these values did not closely control value with NRL treatment.
| Examing organs | Control | Thioacetamide | Nerolidol | TAA + NRL | |
|---|---|---|---|---|---|
| Testes Weight (g) | Right | 1.428 ± 0.054 | 1.267 ± 0.03 | 1.477 ± 0.06 | 1.324 ± 0.03 |
| Left | 1.380 ± 0.048 | 1.304 ± 0.028 | 1.415 ± 0.040 | 1.317 ± 0.030 | |
| Epididymis Weight (g) | Right | 0.667 ± 0.047 | 0.641 ± 0.036 | 0.625 ± 0.557 | 0.557 ± 0.021 |
| Left | 0.680 ± 0.008 | 0.495 ± 0.025 | 0.650 ± 0.028 | 0.578 ± 0.022 | |
| Seminal Vesicules (g) | 1.204 ± 0.096 | 0.728 ± 0.121 | 1.395 ± 0.143 | 0.597 ± 0.031 | |
| Prostate (g) | 0.766 ± 0.048 | 0.392 ± 0.068 | 0.520 ± 0.057 | 0.228 ± 0.022 | |
| Sperm Concentration (million/cauda) | 266.14 ± 5.509a | 146.85 ± 6.836b | 267.71 ± 5.348a | 158.00 ± 6.233bc | |
| Sperm Motility (%) | 89.99 ± 1.855a | 74.041.919b | 93.57 ± 0.673a | 76.90 ± 2.038bc | |
| Abnormal Sperm Rate (%) | Head | 3.15 ± 0.41 | 7.51 ± 0.37 | 3.28 ± 0.35 | 4.57 ± 0.36 |
| Tail | 3.21 ± 0.38 | 6.73 ± 0.59 | 3.00 ± 0.30 | 4.71 ± 0.60 | |
| Total | 6.36 ± 0.52 | 14.24 ± 0.85 | 6.28 ± 0.35 | 9.28 ± 0.80 | |
| Data expressed as mean ± SEM. The differences among values bearing differnt cases-a,b(p<0,05)and A,B,C,D(p<0,01) at, in the same line are istatistically significant. | |||||
Table 2: Reproductive organ weights, epididymal sperm concentration, sperm motility, abnormal sperm rate in each rat group.
Histological result
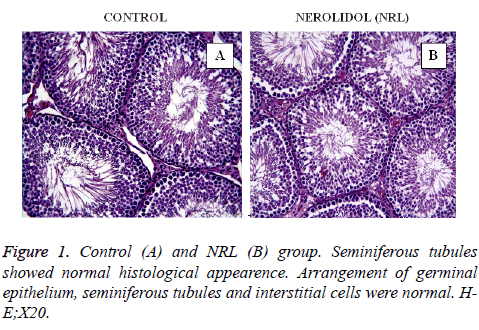
In this study, all the figures demonstrate the histological findings of each group in testis. In the control (Figure 1A) and Nerolidol (NRL) (Figure 1B) groups testis tissue demonstrated a normal histological appearance. Arrangement of germinal epithelium, seminiferous tubules and interstitial cells were normal.
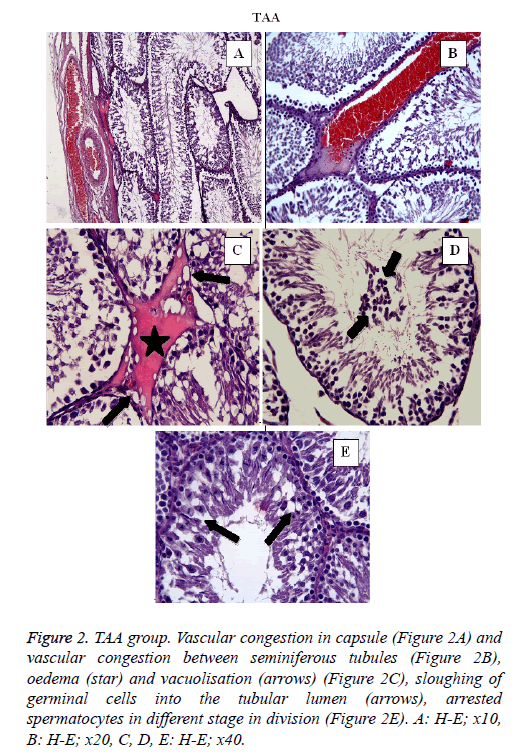
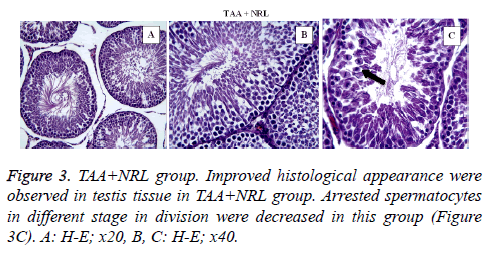
In TAA group (Figure 2) some histological changes were seen such as vascular congestion in capsule (Figure 2A), vascular congestion between seminiferous tubules (Figure 2B), oedema (star) and vacuolisation (arrows) (Figure 2C), sloughing of germinal cells into the tubular lumen (arrows) (Figure 2D), arrested spermatocytes in different stage in division (Figure 2E). In TAA+NRL group (Figures 3A, 3B and 3C), we detected an ameliorated histological appearence in testis tissue. Histopathological changes were decreased in TAA+NRL group compared with TAA group. NRL treatment can prevent histopathological effects of TAA and can reverse side effects of TAA. NRL treatment with TAA may prevent histological damages.
Figure 2: TAA group. Vascular congestion in capsule (Figure 2A) and vascular congestion between seminiferous tubules (Figure 2B), oedema (star) and vacuolisation (arrows) (Figure 2C), sloughing of germinal cells into the tubular lumen (arrows), arrested spermatocytes in different stage in division (Figure 2E). A: H-E; x10, B: H-E; x20, C, D, E: H-E; x40.
The value of MSTD and GECT was given in table 3. The results indicated that the MSTD and GECT were significantly decreased in TAA groups compared with control and the others. On the other hand the decrease level of MSTD and GECT were normally against TAA group. Besides, they were not significantly differences between control and only given NRL group.
| Groups | MSTD | GECT |
|---|---|---|
| 1-Control | 89.35 ± 10.79a | 314.40 ± 24.68a |
| 2-Thioacetamide (TAA) | 40.36 ± 7.73b | 255.28 ± 23.42b |
| 4-Nerolidol (NRL) | 84.64 ± 11.67a | 306.57 ± 21.39a |
| 3-TAA+NRL | 62.91 ± 5.99c | 288.31 ± 14.57c |
| The mean differences the values bearing different superscript letters within the same column are statistically significant. (p≤0,0001) SD: Standart Deviation | ||
Table 3: The effects of thioacetamide and nerolidol on the Mean Seiniferous Tubular Diameter (MSTD) and Germinal Epithelial Cell Thickness (GECT). (Mean ± SD).
Discussion
Many chemicals and diseases can disrupt the male reproductive system via changes in physiological and biochemical functions. Thioacetamide is widely used in classical qualitative inorganic analysis as an in situ source for sulfide ions; it is known to produce marked hepatotoxicity in exposed experimental animal models [23,24]. TAA regarding the effects on the testes are noted in only a few reports in the literature. These studies reported that the TAA have an adverse effect on sperm morphology [12,13]. In the present study, sperm counts and motility in the TAA group were significantly decreased, compared to the control group (p<0.05), and the morphology abnormality in the TAA group was significantly increased, compared to other groups (p<0.05).
Recent increased interest in alternative medicine and disease prevention in light of the use of dietary supplements and herbal products makes this study significant. Nerolidol is a sesquiterpene present as an essential oil in some plants with a floral odour, and it is used as a fragrance ingredient, a flavouring agent and in detergents [4,14]. Nerolidol has shown antioxidant, antinociceptive and antiulcer properties [2,9,25]. It is also active against bacteria, fungi and malaria [5,7,8].
Thiobarbituric acid reactive substances, an indicator of lipid peroxidation; it is produced with peroxidation by reactive oxygen species (ROS) of fatty acids and causes irreversible cell damage, so it reduces the capacity of antioxidant defence system consisting of SOD, CAT, GPx activities and GSH levels [26,27]. The testes are the major target organs for oxidative stress because they contain a high concentration of polyunsaturated membrane lipids cells equipped with an antioxidant system [28]. Our results showed that TAA exposure induced lipid peroxidation via an increase in TBARS levels and decreased the efficacy of the antioxidant defence system via a reduction of CAT, SOD, GPx activity and GSH levels. Previous studies have shown that TAA causes significant oxidative damage in liver tissue, but there are few studies on the testes [12,13]. These studies are in agreement with our findings.
Many experimental studies emphasise the role of oxidative stress in the mechanisms of testicular damage [29,30]. Oxidative stress, which happens due to an overproduction of ROS and the reduction in the antioxidant level, is prevented or depressed by antioxidant defence mechanism, with the inclusion of GSH, CAT, SOD and GPx in organisms. In this study, the levels of these antioxidants were significantly reduced in the rats treated with TAA, but nerolidol management caused a significant increase in these antioxidants in the testicular tissue of rats. The reducing effect of TAA on these antioxidants has been shown in previous studies [23]. Some studies have suggested that catalase and glutathione peroxidase, in particular, were decreased with TAA [13]. In our study, the protective effect of NRL against TAA-induced oxidative stress in the blood plasma of rats was demonstrated by the improvement of their antioxidant defence mechanisms.
Spermatozoa are affected negatively by oxidative stress. This causes to the release of more ROS and anti-inflammatory cytokines, which may trigger an inflammatory response [31]. In previous experimental studies has been noted that testicular atrophy results form the suppression of spermatogenesis in the toxicity of TAA [13]. In our study, we detected that TAA caused slight reduction in the weights of both testes and the epididymis, compared to the control group, but NLR treatment raised the weights of these organs compared to TAA group. But the results were not statistically significant. In addition, in this study, NLR also induced important development in the sperm parameters, including sperm count, sperm motility and sperm abnormality, which were worsened by the TAA’s effect. Previously, Kang and colleagues reported the negative effect of TAA on the testes [13]. Our study is a more exhaustive experimental one concerned with its toxicity on male reproductive systems.
In the current study, the diameter of seminiferous tubule, germinal epithelium thickness layers, mean score and injured tubule numbers in the TAA group were significantly lower than in the control group. The changes in group TAA+NLR were also significantly different from the control however, these changes were smaller than in the TAA group. It is supposed that the histopathological toxic effects of TAA on testes may be owing to oxidative effects, which may lead to infertility in rats. According to these histopathological results, we argue that TAA causes important testicular damage in rats, and NLR has a comparatively protective effect against TAA-induced histological changes. In this sense, we suggest that NLR treatment can be used against morphological changes caused by TAA.
Conclusion
This experimental study showed that TAA damages testicular tissue and highlighted negative effect of TAA on sperm parameters and oxidative stress. In addition to this, NLR treatment reformed the toxic effect of TAA on male reproductive organs by diminishing lipid peroxidation, increasing the levels of antioxidants (CAT, SOD, GSH and GPx), the beneficial effect on sperm parameters and histological alterations as well as reproductive organ weights. According to our results, after more experimental and clinical trials, nerolidol can be used to prevent male reproductive organs against TAA toxicity.
References
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 2012; 75: 311-335.
- Pacifico S, D’Abrosca B, Golino A, Mastellone C, Piccolella S. Antioxidant evaluation of polyhydroxylated nerolidols from redroot pigweed (Amaranthus retroflexus) leaves. Food Sci Technol 2008; 41: 1665-1671.
- Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev 2004; 56: 603-618.
- Lapczynski A, Bhatia SP, Letizia CS, Api AM. Fragrance material review on nerolidol (isomer unspecified). Food Chem Toxicol 2008; 46: S247-S250.
- Brehm-Stecher BF, Johnson EA. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob Agents Chemother 2003; 47: 3357-3360.
- Inoue Y, Shiraishi A, Hada T, Hirose K, Hamashima H, Simada J. The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol Lett 2004; 237: 325-331.
- Lee SJ, Han JI, Lee GS, Park MJ, Ghoi IG, Na KJ, Jeung EB. Antifungal effect of eugenol and nerolidol against Microsporum gypseum in a Guinea pig model. Biol Pharm Bull 2007; 30: 184-188.
- Lopes NP, Massuo MJ, Andrade EHA, Maia JGS, Yoshida M, Planchart AR, Katzin AM. Antimalarial use of volatile oil from leaves of Virola surinamensis (Rol.)Warb by Waiãpi Amazon Indians. J Ethnopharmacol 1999; 67: 313-319.
- Arruda DC, D’Alexandri FL, Katzin AM, Uliana SRB. Antileishmanial activity of the terpene nerolidol. Antimicrob Agents Chemother 2005; 49: 1679-1687.
- Klopell FC, Lemos M, Sousa JP, Comunello E, Maistro EL, Bastos JK, Andrade SF. Nerolidol, an antiulcer constituent from the essential oil of Baccharis dracunculidolia DC (Asteraceae). Z Naturforsch C 2007; 62: 537-542.
- Russel LD, Malone JP, MacCurdy DS. Effect of the microtubule disrupting agents, colchicine, and vinblastine, on seminiferous tubule structure in the rat. Tissue Cell 1981; 13: 349-367.
- Mazzanti L, Lopez M, Del Tacca M. Hyperlipidic diet as a factor allowing alpha-naphtyl-isothiocyanate and thioacetamide toxicity on the albino rat testis. Experientia 1971; 27: 169-170.
- Kang JS, Morimura K, Toda C, Wanibuchi H, Wei M, Kojima N, Fukushima S. Testicular toxicity of DEHP, but not DEHA, is elevated under conditions of thioacetamide-induced liver damage. Reprod Toxicol 2006; 21: 253-259.
- McGinty D, Letizia CS, Api AM. Addendum to Fragrance material review on Nerolidol (isomer unspecified). Food Chem Toxicol 2010; 48: 43-45.
- Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol 1998; 108: 101-106.
- Sedlak J, Lindsay RH. Estimation of total, proteinbound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 1968; 25: 192-205.
- Sun Y, Oberley LW, Li YA. Simple method for clinical assay of superoxide dismutase. Clin Chem 1988; 34: 497-500.
- Aebi H. Catalase In: Methods of Enzymatic Analysis. Bergmeyer HU (ed). Academic Press, New York, 1974; 673-677.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967; 70: 158-169.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RI. Protein measurement with folin phenol reagent. J Biol Chem 1951; 193: 265-275.
- Sönmez M, Yüce A, Türk G. The protective effects of melatonin and vitamin E on antioxidant enzyme activities and epididymal sperm characteristics of homocysteine treated male rats. Reprod Toxicol 2007; 23: 226-231.
- Sönmez M, Türk G, Yüce A. The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male Wistar rats. Theriogenology 2005; 63: 2063-2072.
- Czechowska G, Celinski K, Korolczuk A, Wojcicka G, Dudka J, Bojarska A, Reiter RJ. Protective effects of melatonin against thioacetamide-induced liver fibrosis in rats. J Physiol Pharmacol 2015; 66: 567-579.
- AbouLaila M, Sivakumar T, Yokoyama N, Igarashi I. Inhibitory effect of terpene nerolidol on the growth of Babesia parasites. Parasitol Int 2010; 59: 278-282.
- Koudou J, Abena AA, Ngaissona P, Bessière JM. Chemical composition and pharmacological activity of essential oil of Canarium schweinfurthii. Fitoterapia 2005; 76, 700-703.
- Montjean D, Ménézo Y, Benkhalifa M, Cohen M, Belloc S, Cohen-Bacrie P, de Mouzon J. Malonaldehyde formation and DNA fragmentation: two independent sperm decays linked to reactive oxygen species. Zygote 2010; 18: 265-268.
- Schiller HJ, Reilly PM, Bulkley GB. Tissue perfusion in critical illnesses. Antioxidant therapy. Crit Care Med 193; 21: 92-102.
- Chainy GB, Samantaray S, Samanta L. Testosteroneinduced changes in testicular antioxidant system. Andrologia 1997; 29: 343-349.
- Ciftci O, Beytur A, Vardi N, Ozdemir I. Evaluation of reproductive toxicity in male rats treated with novel synthesized ruthenium (II) and gold (I)-NHC complexes. Drug Dev Ind Pharm 2012; 38: 40-46.
- Altintas R, Ciftci O, Aydin M, Akpolat N, Oguz F, Beytur A. Quercetin prevents docetaxel-induced testicular damage in rats. Andrologia 2014; 47: 248-256.
- Iwasaki A, Gagnon C. Formation of reactive oxygenspecies in spermatozoa of infertile patients. Fertil Steril 1992; 57: 409-416.