ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2022) Volume 33, Issue 7
Benefits to human health of experimental models by using laboratory mice and rats: Overview and attributes in microbiology medical research.
Giorgio Silva de Santana1,3*, Ana Luíza Mattos Guaraldi1,3, Kátia Calvi Lenzi Almeida2
1Health Science Center, Institute of Microbiology Paulo de Góes, Federal University of Rio de Janeiro, Rio de Janeiro (RJ) 21941-599, Brazil
2Department of Environmental Science and Conservation, Medical College, Federal University of Rio de Janeiro, Macaé, Rio de Janeiro (RJ) 27965-045, Brazil
3Laboratory of Diphtheria and Corynebacteria of Clinical Relevance, Faculty of Medical Sciences, University of the State of Rio de Janeiro, Rio de Janeiro (RJ) 20551-030, Brazil
- Corresponding Author:
- Giorgio Silva de SantanaHealth Science Center
Institute of Microbiology Paulo de Góes
Federal University of Rio de Janeiro
Rio de Janeiro (RJ) 21941-599
Brazil
Accepted date: September 19, 2022
The use of animals in scientific experiments became more frequent between the 16th and 18th centuries. With the advances in this area, the regulation of ethical norms and laws of animal protection were necessary to guide researchers in the correct use of procedures and the conscious use of animals. Currently, studies in the health field developed in human beings such as prophylactic measures and treatment for diseases must be previously conducted in laboratory animals. This study aimed to describe an overview of benefits to human health of experimental models by using laboratory mice and rats. Data included the use of laboratory of animals in scientific research and ethics procedures, addressing taxonomic aspects, and biological, physiological and reproductive parameters. Applicability of these laboratory of animals in microbiology medical research, including strain-dependent virulence mechanisms of human pathogens, also expressing multiresistance profiles, such as Staphylococcus aureus, was also verified. Mice and rats are the most used species in experiments. Laboratory animals are currently used in all fields of biological research. Although current trends advocate the use of alternative methods,in vivo studies still provide the most relevant results.
Keywords
Ethics, Experimental model, Microbiology, Mus musculus, Rattus norvegicus.
Introduction
Animal experimentation is an important aspect of life science and medical research. Animals have been used for the study of biological investigations and comparative diseases in laboratory research since the 16th century. It has been little more than a century and a half since Robert Koch made the discoveries that led Louis Pasteur to describe how small organisms called germs could invade the body and cause disease. In the final decades of the 19th century, Koch conclusively established that a particular germ could cause a specific disease. Even today, with all the advances in modern science, it would be impossible to prove that a specific germ is responsible for a disease without the use of laboratory animals, such as rabbits, guinea pigs, rats, mice and hamsters. Laboratory animals have been also contributing to research related to prevention of diseases such as AIDS, multiple sclerosis, and different types of cancer. Moreover, they may be used for the development of new surgical techniques, quality control of pharmaceutical products, and assessment of effectiveness, sterility, toxicity, and potency of therapeutic agents. Animals are essential in scientific research, development and production of vaccines or monoclonal antibodies, evaluation and control of biological products, and in studies on pharmacology and toxicology, bacteriology, virology and parasitology, basic immunology, immunopathology, organ transplants, immunosuppressive drugs, among others [1]. The discovery of insulin, the development of vaccines against various diseases avoiding epidemics and epizootics, production of serums, therapeutic use of antibiotics, the development of techniques of transplants of organs and the possibility of use of anesthetics and antidepressants drugs are successful examples of the contribution of studies performed in animals [2,3].
The Nuremberg Code determines that any experimentation in humans must be preceded by animal experimentation [4]. Despite the possibility of obtaining satisfactory results through alternative methods such as in vitro studies using cell cultures, animal models still allow achievements that are impossible by other methods, such as in the supply of information on the organism as a whole [5-7]. In order to guide researchers to promote safeguard of animals during scientific studies, protocols of ethical principles on animal experimentation societies of laboratory animal science are currently used in different countries, including Brazil (SBCAL/COBEA) [8-10]. Mice are extensively used in experimental studies due to the high (99%) genomic similarity with humans, which allows researchers to establish mechanisms involved in genetic disorders of both species. Mice carrying engineered genetic modifications have become an indispensable tool in the study of gene functioning, since they are still considered the most genetically manipulated species, whose modification reaches about 97% of their genes [2].
This study aimed to describe an overview of benefits to human health of experimental models by using laboratory mice and rats. Data included the use of laboratory of animals in scientific research and ethics procedures, addressing taxonomic aspects, and biological, physiological and reproductive parameters. Applicability in microbiology medical research was also verified, including studies reported by our research group related to pathogenicity of Staphylococcus aureus.
Animal model
In 1865, Claude Bernard established principles for animal use as a model of study and correspondence with the human physiology. He provoked physical and chemical situations which resulted in changes in animals that resembled human diseases, emphasizing the applicability of animal experimentation [3,4,11,12].
In the anthropocentric view of zoological scale organization, Homo sapiens put himself on top of evolutionary scale of species, creating a linguistic error adopted scientifically, distinguishing humans from animals, as if humans were not animals. Thus, the expression “animal model of disease” with the intention to designate “models in animals of diseases of human species”. Therefore, the term “animal model” is inappropriate. The proper nomenclature should be: “human model”. The terms “laboratory animal” or “experimental animal” are correlated much more to the human than to any other animal species [3,6,13,14].
An animal model should meet the following requirements: allow the study of biological phenomena or of behavior of the animal; enable a spontaneous or induced pathological process that can be investigated; ensure the phenomenon to be similar to phenomenon in human beings in one or more aspects [3] and enable its reproduction and obtain the same results by other researchers [13]. Results obtained from a successful study in animals are then applied to all the population [3,15].
Currently, there is a reference to four type’s pre-established models which use animals in the medical literature: induced spontaneous, negative and orphans. The induced and the spontaneous models are the most important ones [3,6,16]. The spontaneous models of human diseases use genetic variants that occur naturally. There are many lineages of animals with genetic characteristics that express similar pathologies to humans. These lines were characterized and preserved to serve as a model. An example of natural mutant used in research is the athymic “nude” mice used in the study of tumors hetero transplanted, enabling the first description of NK cells (Natural Killer). These animals were identified in the 60’s, even before its use in laboratories of immunological research [3,6,17].
The induced models are those in which situations under investigation are experimentally induced. For example, the induction of diabetes mellitus with alloxan and partial hepatectomy for the study of hepatic regeneration. In some cases of induction, the animal species should be evaluated. For instance, the short bowel syndrome can be simulated in some animals, making the resection of 80% of jejunum anastomosis of remaining stumps of small intestine. Studies have shown that the dogs initially used in this type of study could not stand the conditions of malabsorption, presenting high indices of mortality postoperatively, hindering the continuity of the study. Studies have then shown that the rat is the most suitable for this experiment type [3,18]. A special model for inducing disease used is related to the use of transgenic animals, since they can carry DNA inserted artificially in their genome. Mouse is the preferred species for transgenic models. These modified animals should receive special attention for presenting unknown disorders, possibly being unable to express signs of suffering and pain, which could make its use unethical [3,6,13,16]. Negative models use animals with no reactivity to a specific stimulus. They are applied in studies of resistance mechanisms, offering greater sharpness about the physiological basis [3,6,17]. Lastly, the orphan models of disease describe the conditions that naturally occur in non-human animal species but not yet found in humans, being are “adopted” when an illness similar in humans is identified posteriorly. We have, for example the Marek’s disease, papillomatosis and Bovine Spongiform Encephalopathy (BSE), also known as “mad cow disease” [3,6].
In animal models, the greater the phylogenetic similarity with humans, the better will be the obtained results. Nonetheless, the phylogenetic similarity not always guarantees validation of results. A classic example of this statement was demonstrated with unsuccessful results by choosing the chimpanzee model in studies related to AIDS. Likewise, pathological phenomena and the results of a disease or induced condition in the tested species should match with their respective pathological phenomena in the target species. Thus, the infection by virus of Feline Immunodeficiency (FIV) in cats may be a better model for the study of human AIDS than the infection by Human Immunodeficiency Virus (HIV) in apes [3,6,13,16].
It is impossible to get specific rules for choosing the best animal model, because many different aspects should be considered in each study, as well as the goals. The researcher must consider that “what is harmful or ineffective for nonhuman species can be innocuous or efficient in humans”. For example: in the 1960’s, thalidomide was prescribed as sedative and antiemetic for pregnant women, being subsequently also used in the treatment of allergies and as an anti-influenza medicine. The drug was then proved to be directly responsible for birth of babies with congenital malformations, when ingested by women in the first three months of pregnancy, disrupting the growth of arms and legs in human embryos, and increase of occurrence of stillborn children. These results were also observed in non-human primates; but were not found in rats or in other species [6,19].
The metabolism of small rodents is much faster than the human’s. The visceral organs that control and exercise the metabolism grow more slowly than body size as a whole. It was discovered that metabolism corresponds to about ? power of total body weight (the metabolic body weight). Therefore, experimental doses should, consequently, be estimated according to the metabolic body weight [3].
Phylogenetic correlation or anatomical compliance are factors influencing the choice of the proper animal model; however, studies have shown that it cannot always be reliable in parallel physiological behavior [3,6,13,16].
Mice and rats of laboratory
The researcher must choose the proper animal species based on literature data, in the genetic pattern and health status, so the doubts can be solved, providing reliable results and avoiding unnecessary animal deaths. Mice and rats are among the most used animals in experimentation. In rats, the genus Rattus has 137 species, originally from regions of Central Asia. Two species are of great laboratory importance: Rattus norvegicus (domestic rat or brown rat) and Rattus rattus (black rat) [20]. The albino lineage “Wistar”, from the species Rattus norvegicus, developed in the Wistar Institute in Philadelphia in 1906, is commonly used. This lineage was the first to be used as a model organism in a time in which researchers used primarily Mus musculus domesticus mice species [21]. They have as common characteristics the low tumor incidence and temporary alopecia. Heterogenic animals have been used with several scientific purposes and in surveys related to rheumatology, endocrinology, and orthopedics, among others [22].
Mice have origin in the Asian continent. The species Mus musculus domesticus was introduced as laboratory animal in the 19th century. In the beginning of 1900, it has also become an important experimental model for genetic studies [2,23]. It’s easy acceptance and great use in experimental studies was initially due to their small size, great prolificacy, and short gestation period. They are also of easy domestication and maintenance. The complete estrous cycle lasts four to five days, that is, ovulation occurs every five days. The proestrus starts with follicular phase of the ovary, which culminates in estrus. The metaestrous and diestrous are characterized by the luteinic phase of ovary. In restricted captivity, when only females are grouped, absence of estrous cycle may occur, the females remain no anestrus continuously until they are exposed to a male. The introduction of a male in the environment composed only by females in anestrous promotes the return of the estrous cycle within 48 hours, a phenomenon called Whitten Effect [2,24].
Mice can present some genes or even chromosomes of different species resulting from species crosses, for example the C57BL/6 lineage, with 6.5% of the genome originating from Mus spretus, and not of Mus musculus [25]. Therefore, the mice are considered excellent models for genetic studies, due to its genetic similarity with humans and easy manipulation of their genome. They present 20 pairs of chromosomes and a big number of mutant ones with hundreds of colonies genetically defined, which are obtained through systems of mating. In pharmacological studies, access to various administration routes of drugs is easy, including venous and intrathecal (intracerebral) via (Table 1) [2].
| Sanitary status | Microbiota | Sanitary barriers |
|---|---|---|
| Gnotobiotic | Known, non-existing or undetectable. | Absolute. |
| Germ-free (axenic) | Free from parasites, bacteria, fungi, protozoa and viruses. | Absolute. |
| Monoxenic, Dixenic and Polyxenic | Defined (intentionally contaminated with specific microorganisms or parasites). | Constant monitoring (identification of the presence of specific microorganism and absence of others). |
| Free of specific pathogens (heteroxenic) | Free of pathogenic microorganisms, allowing the presence of natural microbiota. | Rigorous. |
| Conventional | Indefinida. | Devoid of rigorous sanitary barriers. |
| Genetic status | Genetic constitution | Mating systems |
| Outbred or heterogenic | Elevated heterozygosity (genetic variability - several alleles). | Non-consanguineous. |
| Inbred or isogenic* | 99% homozygosity (identical; one common ancestor). | Consanguineous (crossbreeding between brothers, parents with children for 20 consecutive generations). |
| Transgenic | Incorporation of one segment of DNA from another species into the genome. | Implant of the embryo genetically modified in the uterus in the follicular phase of the ovary. |
*It may occur spontaneous mutations along the genome, may also occur hybrids through mating between two inbred lineages, obtaining heterozygous animals determining one pair of alleles to be analyzed.
Table 1. Classification of sanitary and biological status from laboratory mice and rats.
Table 2 shows some of the main biological, physiological, and reproductive parameters influencing the choice of mice and rats as a model of research. Currently, the geneticists have available animal models carrying numerous organic and systemic dysfunctions to research on, reproducing the same diseases observed in humans. Among these dysfunctions we can cite the nude, hairless, obese, diabetic and muscular dystrophy, described in Table 3 [2,23].
| Parameters | Rats | Mice |
|---|---|---|
| Taxonomy | ||
| Phylum | Chordata | Chordata |
| Sub-phylum | Vertebrata | Vertebrata |
| Class | Mammalia | Mammalia |
| Order | Rodentia | Rodentia |
| Family | Muridae | Muridae |
| Genus | Rattus | Mus |
| Specie | norvegicus | musculus |
| General Characteristics | ||
| Weight at birth | 4-7 g | 1-2 g |
| Breastfeeding | 19-21 d | 19-21 d |
| Weaning | 18-24 d | 18-21 d (ob) 28 d (ib) |
| Weight at weaning | 35-60 g | 10-15 g (ob) 8-10 g (ib) |
| Male weight (ad) | 300-500 g | 20-50 g |
| Female weight (ad) | 250-350 g | 25-45 g |
| Chromosomes | 42 dp | 40 dp |
| Body temperature | 36.0°C -37.5°C | 35.2°C -37.9°C |
| Rectal temperature | 38.2°C | 37.4C |
| Water intake/d | 20-45 mL | 3-7 mL |
| Feed intake/d | 10-20 g | 3-6 g |
| Life expectancy | 3 yrs | 2-4 yrs |
| Cardiovascular and respiratory aspects | ||
| Heart rate (ab.r) | 250-480 bpm | 500-780 bpm |
| Respiratory rate | 70-115 mov.min | 163 mov.min (t.r) |
| Arterial pressures (ab.r) | 187-138 mmHg | 147-106 mmHg |
| Background reproductive | ||
| Estral cycle (pl) | 4-5 d | 4-5 d |
| Gestation period | 19-22 d | 19-21 d |
| Reproductive life | 10-12 mn | 8-12 mn (male) 6-8 childbirth (female) |
| Male puberty | 50-70 d | 40-45 d |
| Female puberty | 35-80 d | 35-56 d |
| Sexual maturity | 56-70 d | 42-60 d |
| Litter size | 8-16 pp | 8-10 pp (ob) 5 pp (ib) |
| Environmental conditions | ||
| Temperature | 20-25°C (± 2) | 21-22°C (± 2)* |
| Humidity | 50-55% | 50-55% |
| Space in cage /animal | 150 cm2 i.gr 450 cm2 ad 800 cm2 female (w.pp) |
65 cm2 i.gr 100 cm2 ad 300 cm2 female (w.pp) |
*Study conducted by Gaskill et al. (2009) using lineage C57BL/6J demonstrated that the lineage, maintained in conditions from creation in the Vivarium, has a predilection by temperatures higher (female around 30°C and male between ~25-30°C). This preference can be related to hormonal difference between the male and female genus, by the changes in the light and dark cycles, which will affect the activities and in behavior during the day [41].
Abbreviations: adult (ad), year(s) (yrs), beat(s) per minute (bpm), with puppie(s) (w.pp), day(s) (d), diploid(s) (dp), in growth (i.gr), puppie(s) (pp), inbred (ib), month(s) (mn), millimeter(s) of mercury (mmHg); respiratory movements per minute (mov.min), outbred (ob), polyestrous (pl), thermal regulation (t.r), absolute rest (ab.r).
Table 2. Taxonomy, biological, physiological and reproductive parameters of rats and mice.
| Genotype | Laboratory applicability | Phenotype |
|---|---|---|
| Mice | ||
| Diabetes (LEPRDB) Chromosome 4 |
Pathophysiology of insulin resistance associated with arterial hypertension and obesity; treatment of skin wounds caused by diabetes. | Elevation of plasmatic insulin (10 to 14 days) and glycose (fourth week) present polyphagia, polydipsia and polyuria. C57BLKS: pancreatic cell destruction (mortality after 10 months). C57BL/6: compensatory hyperplasia in the pancreas with hyperinsulinemia (mortality after 20 months). |
| Obesity (LEPOB) Chromosome 6 |
Treatment of human obesity by low leptin protein production. | They are sub-fertile, with absence of leptin and increased adipocytes (body weight three times normal), present hyperphagia, hyperglycemia. They are glycose intolerant, have high plasmatic insulin and increased hormonal production of the pituitary and adrenal glands. |
| Muscular dystrophy (LAMA2DY) Chromosome 10 |
Human muscular dystrophy. | Usually are sterile, with absence of laminin-alpha 2 (merosine) in cardiac, skeletal muscles and peripheral nerves, muscle weakness and paralysis (½ and 3 weeks of life) with mortality after 6 months. |
| Nude (HFH11NU) Chromosome 11 |
Immune response great dependent, growth and metastasis of human tumors, development of antitumor pharmacus and autoimmune diseases (Lupus erythematosus). | Absence of hair, lower fertility and slow growing, rudimentary or absent thymus (T lymphocyte deficiency), absence of transplant rejection, sensitivity to infections, failures at ossification. |
| Beige (LYSTBG) Cromossomo 13 |
Chediak-Higashi syndrome and organ transplantation. | Presence of giant vesicles produced by lysosomes, enzyme concentration in erroneous locations in cell, natural killer (NK) cell deficiency and platelets, enlarged and underdeveloped alveoli. |
| Hairless (HR) Chromosome 14 |
Toxicity of products chemists, allergic reactions to cosmetics, development of pharmacus for skin infections. | Hair and vibrissae loss (tenth day) with curved nails, helper T-cell deficiency; stratified epithelium hyperkeratosis and cyst development. |
| Scid (PRKDCSCID) Chromosome 16 |
Immune response dependent on the thymus, lymph nodes and spleen, organ transplantation, growth and metastasis of tumors. | DNA repair failed by deficiency on activation of protein kinase, underdevelopment of the thymus, lymph nodes and spleen (one tenth of normal), absence of mitosis in the spleen not producing B and T lymphocytes, low levels of IgA, IgM and IgG, absence of transplant rejection. |
| Rats | ||
| Biobreeding (BBDP) | Pathogenesis of type 1 diabetes mellitus (T1DM). | Prone to develop autoimmune diabetes type 1, similar to human, because of susceptibility of RT1u MHC class II haplotype on chromosome 20 and one mutation in GIMAP5 gene on chromosome 4. The mutation in Gimap5 gene results in lymphopenia T cells, contributing to T1D pathogenesis. |
| Brattleboro | Precursor of knockout rats. | Natural mutant unable to produce the vasopressin hormone (antidiuretic) which increases the arterial pressure, decreasing urine volume. |
| Hairless: rnu (rowett), fz (fuzzy), shn (shorn) | Immune response deficit, susceptibility to genetic kidney disease, respiratory tract infections. | Rowett: absent from thymus; Fuzzy and Shorn: progressive renal insufficiency. |
| Knockout | Genetic diseases as Parkinson's, Alzheimer's, hypertension and diabetes. Beyond cardiovascular disease, arthritis, autoimmune and neurological disorders. In the toxicity of therapeutic compounds. | Genetically modified, with absence of expression from one gene by specific mutations. Many of your detoxifying enzymes are similar to those of humans assisting in manufacturing of therapeutic drugs. |
| Lewis | Organ transplants, arthritis and inflammation induction, experimental allergic encephalitis and streptozotocin-induced diabetes (STZ). | Albino coloring, docile behavior, low fertility. Spontaneous pathologies as incidence of neoplasms: pituitary adenoma, adrenal cortical carcinoma, mammary gland tumor, endometrial carcinoma in females, adenoma/carcinoma of C cells of the thyroid gland and tumors of the hematopoietic system in males. Prone to develop spontaneous transplantable lymphatic leukemia. In old age may develop spontaneous glomerular sclerosis. |
| Long-Evans | Multipurpose in behavioral study and obesity. | White coloring with black/brown hood. |
| RCS | Macular degeneration related the age. | Inherited retinal degeneration by mutation in Mertk gene, resulting in phagocytosis of retinal epithelium containing pigment, in the external segments of photoreceptors. |
| Shaking rat Kawasaki (SRK) |
Development cerebral and central nervous system. | Autosomal recessive mutant with deletion of the RELN gene, resulting in reduced expression of Reelin protein. Small cerebellum, frequent absence of vermis and parafloccule. Histology notes neuron malposition in the cerebral cortex, hypothalamus and cerebellum. |
| Sprague Dawley | Multipurpose in nutritional studies. | Docile behavior of easy manipulation. |
| Wistar | Medical biology. | First rat developed as a model organism, are more active with hypertension propensity. |
| Zucker | Genetic model for obesity and hypertension human. | Obese Zucker has recessiveness (fa/fa) leptin receptor, enabling high levels of lipids and cholesterol in the bloodstream, are insulin resistant without hyperglycemia, gain weight with increased adipocyte, being able to weigh 1 kg. Obesity is related to its hyperphagic nature and excessive hunger. Skinny zucker presents dominance (Fa/Fa) or (Fa/fa) from the receiver of leptin. |
Table 3. The more common types of mutations in mice and rats used as potential models of human diseases and main features.
Microbiological research in vivo
In general, pathogenic microorganisms may be endowed with an array of virulence factors that facilitate their ability to invade and survive within host tissues and confer resistance to clearance by immune mechanisms and antimicrobial killing. The risk factors for bacteremia include ongoing chemotherapy and/or advanced age. The use of indwelling medical devices among other invasive procedures is also a matter of concern.
Various studies have been carried out by our research group using laboratory rats and mice, in the evaluation of pathogenicity of S. aureus resulting from invasion, mediated by bacterial virulence factors of clinical isolates susceptible and resistant to β-lactams, allowed us to verify data that had not yet been reported and impossible to be obtained by alternative methods to investigate mechanisms during occurrence of bacterial infections in human hosts.
S. aureus is one of the most common Gram-positive bacteria described as pathogens in both hospital and community environments. S. aureus strains may cause nosocomial human infections in primary colonization sites, and reach surrounding areas, in addition to severe invasive illness, especially when natural defense barriers are compromised by trauma and/or surgical procedures, as skin and mucosal surfaces. Depending on clinical conditions of the patient, S. aureus strains can cause bacteremia leading to severe infections such as meningitis, endocarditis, including sepsis, a leading cause of acute renal failure. Moreover, Methicillin-Resistant S. aureus (MRSA) have been commonly reported as etiologic agents of nosocomial infections in various countries and as a major problem in cardiovascular surgical units associated to catheter insertion.
During the last decades, several studies have been performed in attempt to elucidate virulence mechanisms of multiple natures expressed by S. aureus that promote strain-dependent and/or independent interaction processes with host cells allowing local biofilm formation and leading to bacteremia and/or sepsis and other systemic infections. S. aureus strains have evolved a wide range of strategies to colonize and invade human organs, despite the presence of multiple host defense mechanisms. Inserting a contaminated vascular catheter during a surgical procedure is a factor that might promote biofilm formation on the catheter, possibly leading to bacteremia in patients. The use of in vivo experimental models, by using mice and rats, allowed a better understanding of infectious heterogenic processes of multiple organs.
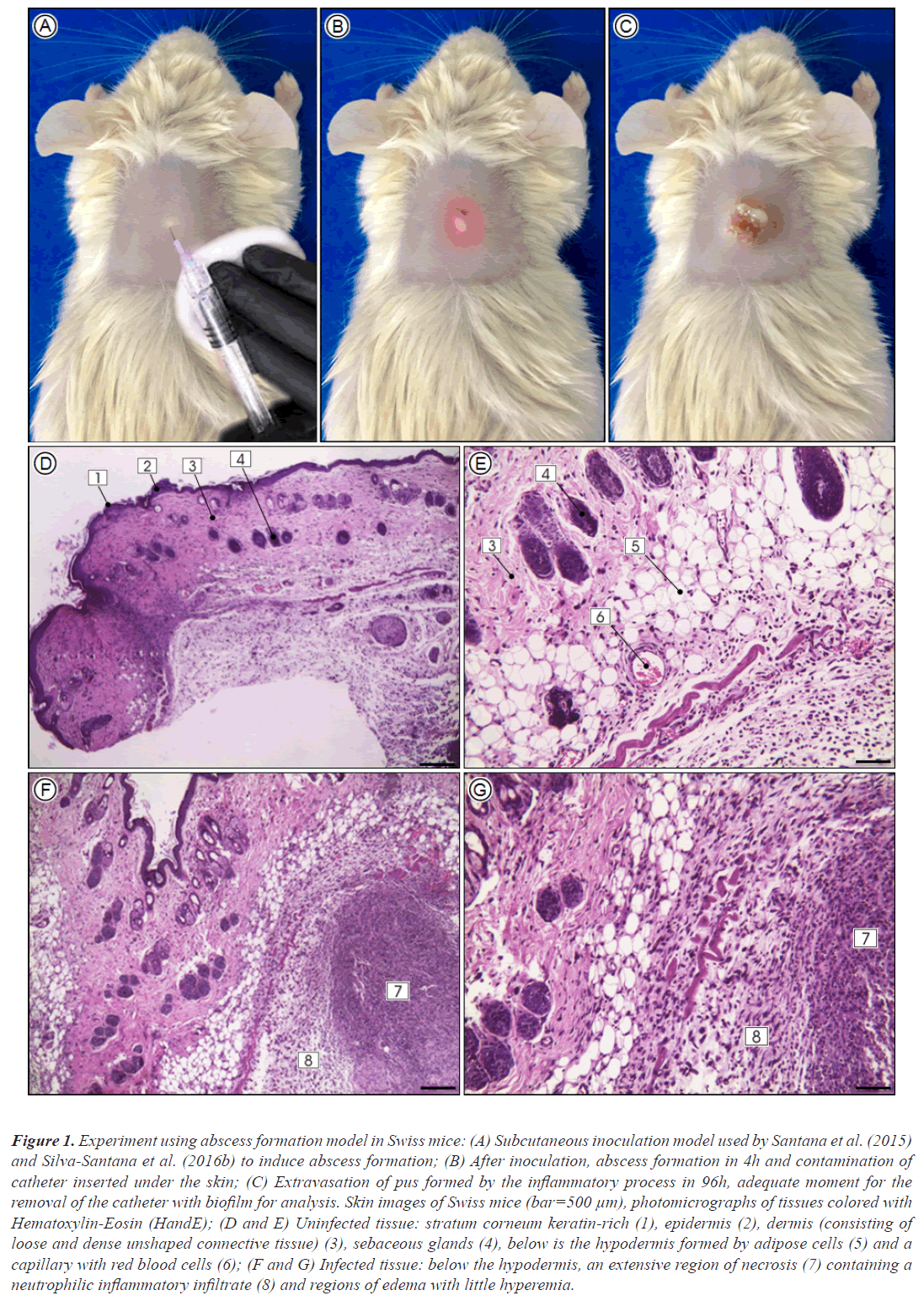
The studies using rats and mice done by our research group, elucidated previously unknown issues. In a murine model applied to Rattus norvegicus (Wistar) for evaluation of biofilm formation by resistant and susceptible S. aureus to β-lactams, in subcutaneous implanted polystyrene catheter fragment in the back of the animals, we observed the formation of strong edema, hyperemia with skin scaling and dermonecrosis at the insertion site. The explanted catheters showed biofilms formed by the inoculated microorganisms, biofilms allowed the invasion of S. aureus to tissues and, through it were able to cause bacteremia with infection of multiple vital organs. Additionally, in a similar study applied to Mus musculus (Swiss) with the aim of evaluating the biofilm formation by clinically isolated MRSA clones, possessing virulence genes responsible for the production of Panton-Valentine Leukocidin (PVL), it was possible to observe PVL-positive strains causing associated severe skin infections, suggesting that PVL production may be associated with increased skin and soft tissue infections. PVL-positive strains were also able to produce biofilm, suggesting greater expression of the icaC gene, indicating a higher virulence potential of MRSA clones expressing leucocidin (Figure 1).
Figure 1: Experiment using abscess formation model in Swiss mice: (A) Subcutaneous inoculation model used by Santana et al. (2015) and Silva-Santana et al. (2016b) to induce abscess formation; (B) After inoculation, abscess formation in 4h and contamination of catheter inserted under the skin; (C) Extravasation of pus formed by the inflammatory process in 96h, adequate moment for the removal of the catheter with biofilm for analysis. Skin images of Swiss mice (bar=500 μm), photomicrographs of tissues colored with Hematoxylin-Eosin (HandE); (D and E) Uninfected tissue: stratum corneum keratin-rich (1), epidermis (2), dermis (consisting of loose and dense unshaped connective tissue) (3), sebaceous glands (4), below is the hypodermis formed by adipose cells (5) and a capillary with red blood cells (6); (F and G) Infected tissue: below the hypodermis, an extensive region of necrosis (7) containing a neutrophilic inflammatory infiltrate (8) and regions of edema with little hyperemia.
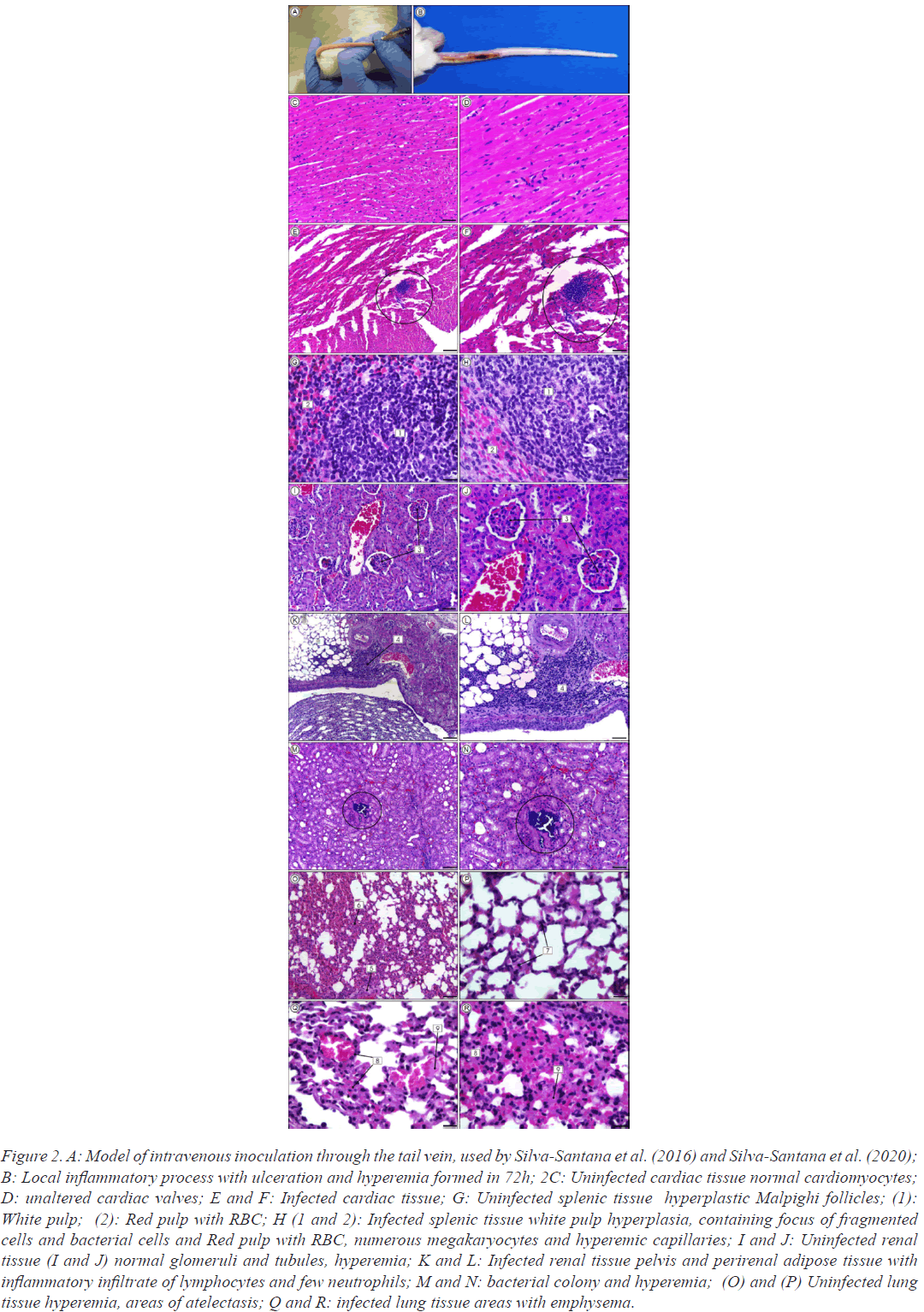
In a previous research a systemic infection model was also used by inoculation of β-lactams-resistant S. aureus strains isolated from infections and nasal colonization through the tail vein in Mus musculus (Swiss) mice. Data showed 80% of mice mortality by inoculation of MRSA pvl-positive strains. Moreover, bacterial colonies within intravascular clots characteristic of sepsis were found in cardiac tissue. The presence of acute infection the kidneys, with focal pyelitis and pyelonephritis, progressing to tubular necrosis affecting the glomeruli. Bacterial colonies of MRSA pvl-positive strains were also observed inside glomeruli. Splenic tissue with fragmented cells apoptosislike feature was also observed. In all cases the lung tissue was the most injured, containing severe inflammatory processes, mainly caused by MRSA pvl-positive strains isolated from human infections (Figure 2).
Figure 2: A: Model of intravenous inoculation through the tail vein, used by Silva-Santana et al. (2016) and Silva-Santana et al. (2020); B: Local inflammatory process with ulceration and hyperemia formed in 72h; 2C: Uninfected cardiac tissue normal cardiomyocytes; D: unaltered cardiac valves; E and F: Infected cardiac tissue; G: Uninfected splenic tissue hyperplastic Malpighi follicles; (1): White pulp; (2): Red pulp with RBC; H (1 and 2): Infected splenic tissue white pulp hyperplasia, containing focus of fragmented cells and bacterial cells and Red pulp with RBC, numerous megakaryocytes and hyperemic capillaries; I and J: Uninfected renal tissue (I and J) normal glomeruli and tubules, hyperemia; K and L: Infected renal tissue pelvis and perirenal adipose tissue with inflammatory infiltrate of lymphocytes and few neutrophils; M and N: bacterial colony and hyperemia; (O) and (P) Uninfected lung tissue hyperemia, areas of atelectasis; Q and R: infected lung tissue areas with emphysema.
Later, we investigated hematological and histopathological aspects caused by susceptible and resistant S. aureus to β-lactams and positive for lukF/S-PV (MSSA and MRSA) by using the systemic infection model in Swiss mice. Laboratorial and microscopic analysis verified quantitative and qualitative changes of blood elements including erythrocytes, leukocytes and platelets resulting from systemic infection. Infected animals presented reduction in intracellular hemoglobin similar to bone marrow degenerative anemia, thrombocytosis with the presence of cells characteristic of hemolytic anemia. Intense immune response mediated by reactive lymphocytes and monocytes, accompanied by inflammation, cell damage and tissue, commonly observed during bankruptcy multiple organ associated with bacterial sepsis. In the serum, high concentrations of C-reactive protein have been identified, an important marker of the acute inflammatory response, participating in the defense against bacterial infections and in the removal of necrotic material, the rise of its indexes may be related to systemic infection and septic shock. Increased levels of nitrogen compounds (urea and creatinine) observed are associated with reduction of the renal blood flow, commonly caused by nephritis and tubular necrosis with loss or damage to the nephron, being confirmed by histopathology, the kidneys showed intraglomerular congestion associated with bacterial colonization. Liver, kidney and heart damage have been associated with increased alanine aminotransferase levels. Changes in the spleens with increased production of follicular lymphocytes and macrophages common in inflammatory processes; it can lead to the development of splenomegaly due to the high immune stimulation. The lungs presented blockage of vessels by bacterial colonization leading to blood stasis, neutrophil influx, and bronchiolar epithelial necrosis present mainly in animals inoculated with MRSA pvl-positive strains.
These studies reinforce the importance of using laboratory animals in several medical researches providing us more details and/or complete data, including the understanding about the behaviour of bacterial pathogens and other microorganisms during invasion of human and animal bodies trying to subsist within the metabolism and the immune system during infectious processes.
Ethics in the use of animals in scientific research
Ethics can be defined as the science of morals. It comes from logic thinking and is related to the “right” and “wrong”; it is a cultural attitude, and criticism regarding values and positions are relevant in an act upon nature.
In opposition to the Cartesian criterion of Descartes, the philosopher Voltaire (1694-1778) defended that the language would be one of the major differences between the man and the “animal”: “It’s just for me to be gifted of speech for what you judge that I have feeling, memory, ideas?”. In charge of an active posture in the protection of animals, the English philosopher Jeremy Bentham (1748-1842) in the 18th century was historically responsible for the systematization of utilitarianism, proposing limits to the use of non-humans by humans, based on the criterion of sensitivity, ethical doctrine which determines if the action is correct and if the intrinsic benefit is exercised to the community. The bigger the benefit the better the action will be. This benefit must be applied to all the sensitivitygifted beings, being legit to include all the animals in the morality of an act. In his work-An Introduction to principles of morality and of legislation – Bentham states that “(...) the problem does not consist to know if animals can reason; it does not matter either they talk or not; the real problem it’s this one: can they suffer?” The first actions of animal protection emerged based on his ideas. Thus, in 1824, the Royal Society for the Prevention of cruelty to animals was created in England, being the first protective society for the animals. The first society aimed at the protection of laboratory animals was created in 1860 by Marie Françoise Bernard (1819-1901)-wife of Claude Bernard (1813-1878), known as the father of contemporary experimental physiology-dissatisfied by her husband using her daughter’s pet dog for demonstrations to his students.
With the discovery and practice of surgical anesthesia using ether, by William T. G. Morton in 1846, the animals deserved the same benefits conquered and applied to humans, mainly in the realization of operative pain acts, thus occurring the first attempts to promote standards in use of animals in research. Subsequently, in London (1876), ethical principles were developed and applied to the benefit of animal experimentation, as well as the first law regulations for the use of animals in research, by the British Cruelty to Animals Act, which is still in force.
Only in 1909 emerged the first North-American publication on ethical aspects of animal use in experiments, based on the following principles: a) it will only be authorized the use of animals in scientific experiments if it is for direct benefit of human and animal lives, same which indirectly, by searching knowledge of the structures, functions and behaviors of living beings; b) this practice will not be ethically valid when reliable alternative methods for the knowledge that is sought is available, and cannot be based on the satisfaction of individual desires. The ethical principle for life requires that you can get yourself a “gain” greater than knowledge with a lower “cost” in the number of animals used and with as little suffering as possible. These aspects have many ethical problems, once they try to both justify animal use for their own benefit and avoid animal pain and suffering-an inevitable conflict that can only be solved by balancing such opposite values.
In 1926, Charles Hume (1886-1981) founded the University of the London Animal Welfare Society aimed at the well-being of the animal used in research, in an attempt to raise awareness of scientists to think rationally about their attitudes towards the animals, stating that “what the animal well-being needs is educated people with cold heads and hot hearts prepared to see the suffering of the animals and looking for practical means of relieving them”.
In Canada, the Animal’s Well-Being movement was based on two main purposes: reducing both the animal pain and suffering and the number of animals used in research, sensitizing scientists to make them reflect on the need to use animals in their experiments. Current surveys are being sponsored to develop in vitro what was previously conducted in living animals. However, it is questioned if the technology used to replace animals can prejudice the evolution of medical science.
In Declaration of Helsinki, adopted at the 18th world Medical Assembly in Helsinki (Finland), in 1964, the item 1 of the Basic Principles was stated: “Clinical research must conform to the moral and scientific principles that justify medical research and should be based on laboratory and animal experiments or other scientifically established facts”. In 1975, the publication of the book Animal Liberation by professor Peter Singer, containing reports of the conditions animals were subjected, generated several debates on the use of animals in research and in other activities such as slaughterhouses, creation, transportation and cosmetics industry. In the same year, the publication contributed to the inclusion of one safeguard use of animals in the first revision of the Declaration of Helsinki, adopted in 29th World Medical Assembly, in Japan.
Some Ethics Committees for Research in Animals were then created. Sweden was the first country to create commissions, in 1979. The United States of America adopted this practice in 1984, whereas in Brazil the committees were constituted in the 90’s. In this context, it was of great importance that the assigned members were able to evaluate the nature and consequences of particular experiments. The members of the Ethics Committees in Animal Experimentation work as legislators and have as their duty to reconcile the ethical aspects with the scientific, legal, economic, and commercial interests [7].
The Brazilian College of Animal Experimentation (COBEA), currently denominated Brazilian Society of Science of Laboratory Animals (SBCAL), an entity affiliated with the International Council for Laboratory Animal Science (ICLAS), searches for the enhancement of the discussion in animal experimentation in Brazil, elaborated by the Ethical Principles of Animal Experimentation [7].
In addition to being built upon ethics, an experiment involving animals requires an important justification to be approved. Thus, the greater the suffering that the experiment may cause to animals, the harder it is to justify their use. In this context, the English zoologist William M. S. Russell and the microbiologist Rex L. Burch in 1959 published a book in which they synthesized in three words the Humanitarian Principle of Animal Experimentation, called the “3 R’s”: Replacement, Reduction and Refinement [8,19].
Animal protection laws
In the United States, laws aim to protect animal rights. In this sense, animals have rights against abuse and illtreatment, cruel actions, and sufferings, even when perpetuated by their owners. However, there are no specific laws concerning laboratory animals. In United Kingdom, animal protection laws are not related to animals themselves, since they impose duties to men [1]. In an assembly in Brussels on January 27/1978, the United Nations Educational, Scientific and Cultural Organization (UNESCO) proclaimed the Universal Declaration of Animal Welfare, which demands that all animals must be respected by men.
In Brazil, the Federal Law no. 6.638, of May 8/1979 establishes rules for didactic-scientific practice of vivisection of animals and determines other measures. In 1998, the Law of Environmental Crimes was sanctioned in Brazil. It establishes crime and defines traffic fines and penalty for those that abuse, mistreat, injure or maim wild, domestic or domesticated, native or exotic animals. They include the same penalties for those that perform painful or cruel experiences in living animals, even for teaching or scientific purposes, if alternative resources are available. Currently, the Law nº 11.794, from October 8/2008 regulates subsection VII of § 1º of art. 225 of the Federal Constitution, establishing procedures for the scientific use of animals, repealing the Law nº 6.638, of May 8 from 1979. However, only on July 15/2009, the Decree nº 6.899, which regulates the Arouca Law, was published, establishing the creation of National Council of Control of Animal Experimentation (CONCEA) and making mandatory the constitution of the Animal Ethics Commission (CEUAs) to ensure the ethical service and humanitarian use of animals for scientific purposes [8]. The decree of Law nº 113/2013, from August 7, transposed almost entirely the European Commission Directive 2010/63/EU, from September 22, for this reason, Brazilian legislation has shown great concern in the protection of animals for scientific purposes.
Current animal protection legislation strives to put in balance the human and animal interests to decide whether an animal experiment is morally justified or not. An ethical evaluation process is conducted based on the harm-benefit assessment of the experiment. The researcher has to implement the 3Rs (Replacement, Reduction, Refinement) to minimize the harms to the animals and make sure that the outcomes are scientifically significant, and that the quality of the science is high, in order to maximize benefits to humans and animals.
Health status and genetic classification
Vivarium is an installation that meets the requirements to raise or maintain animals, providing well-being and health, so they can develop and reproduce, satisfactorily responding to the tests that will be performed. There must be essential conditions for preservation of life, such as water, specific food for each species, constant ambient temperature and appropriate artificial lighting. Knowing the species and analyzing both physiological and behavioral factors are essential to evaluate their well-being, since any physiological change (reduction of the immune response and increase of heart rate, for example) and abnormal behavior may indicate unfavorable conditions. The animals raised for an experimental research are kept in an animal hospital and should be of easy handling, prolificacy, docility, small size, with low food consumption, known physiology and short reproductive cycle. In the Vivarium, the animals can be classified according to their health status or microbiota. They can be classified as observed in Table 3.
Analgesia in animals for scientific practice
The specific symptoms of pain can be observed by both behavioral and physiological changes, which vary in mice and rats according to the different lineages. They usually present changes in motor activity, appearance such as curved posture, piloerection, ocular or nasal secretion and increase in sleep time. Regarding their behavior, changes in temperament with an increase of aggressiveness, reluctance to interact with insulation of the group, increased vocalization when touched, hitting, or grinding of teeth, weight loss due to reduction of appetite or dehydration, leading to the decrease of excretion of urine and feces, changes in heartbeat, respiratory rates, blood pressure, oxygen saturation levels and in skin coloration can be listed among the physiological changes. The researcher can also evaluate changes of the surgery site, checking seeking for erythema, edema, and others [2].
Opioid analgesics and Non-Steroidal Anti-Inflammatory Drugs (NSAID) can be used for pain control. The opioids such as morphine, meperidine, fentanyl, etorphine, buprenorphine and tramadol are employed in cases of moderate to severe pain, causing side effects such as respiratory depression and hypotension. NSAIDs such as aspirin, naproxen, ketoprofen are used in control of mild to moderate pain, also being useful when opioids are not recommended.
Humanitarian finalization
Euthanasia means “good death” or death without pain, suffering and anguish. It is indicated as way to remove the pain or suffering, when it cannot be avoided by means of sedatives and analgesics, or the survival of an animal threatens public health. However, gathering of biological material for analysis usually leads to animal’s death. Although various techniques are accepted, the recommended methods are those that produce death in a humanitarian way that is the entire process since animal housing and physical restraint must be carefully conducted to minimize the suffering and fear, prevent terror, anxiety and apprehension, with a minimum time so that the animal lose consciousness. Moreover, the researcher must chose a euthanasia method that exclude changes that may jeopardize the interpretation of scientific results, is easy to apply, with a fast action and low cost. The procedure must also be safe, prevent contamination of the site, either from blood or any other body fluid, avoiding the spread of infectious diseases. The most recommended methods produce a humanitarian death; however, some methods can be accepted with some restrictions, given their risk of not producing a humanitarian death due to their technical nature, the greater potential of error in the execution, or safety problems. Such methods can be employed only if the use of the methods recommended in Resolution nº 714 of 2002 is impossible. The unacceptable methods do not produce humanitarian death under no circumstances, since they may involve risk for both the animal and the executor. Adjuvant methods grant humanitarian death when combined with other techniques; if used alone, they are considered unacceptable.
Euthanasia may occur by three mechanisms: direct or indirect hypoxia; direct depression of neurons related to vital functions or physical disruption of brain activity. The most used methods can be divided into physical and chemical ones. The physical methods, such as cervical dislocation, decapitation and exsanguination, are methods not widely used and should only be employed when other methods may invalidate certain information or research, especially those related to the biochemical processes of the animal. The chemical methods are the most commonly used, such as inhalant pharmacological agents (anesthetics and gases), or non-inhalant pharmacological agents (pentobarbital sodium or chloral hydrate). These methods provide better resolution, avoiding animal trauma [2].
For the proper choice of a euthanasia method and preservation of the quality of research results, the following criteria should be considered: species of animal to be euthanized, means available for containment of the animal, ability of the person who will perform the euthanasia, number and size of the animals that will be subjected to procedure.
The inhalant agents used for euthanasia include the anesthetic gases (halothane, enflurane, sevoflurane and isoflurane) and non-anesthetic gases (carbon dioxide, nitrogen, argon and carbon monoxide). In both cases, euthanasia is performed with excessive administration of the chosen gas. Chloroform and ether are no longer accepted as methods of euthanasia, ether due to its carcinogenic, hepatotoxic and nephrotoxic potential; the chloroform, for its cumulative effects for the executor. In addition to these risks, both inhalants are unacceptable due to their flammable and explosive nature.
The non-inhalant agents are the injectable pharmaceuticals. They are considered the most reliable and fastest agents for euthanasia, being mostly used to induce overdose (administration of double or triple of the recommended anesthetic dose). There are the barbiturates, which lead first to a depression of the respiratory system and then a cardiac arrest, when it reaches a deep plane of anesthesia. If the access to the venous circulation for the administration of the agent is difficult, the intraperitoneal route can be used, however with the utilization of a non-irritant substance and without neuromuscular-blocking action.
In mice and rats, the euthanasia methods accepted are the inhalation of anesthetics in a special chamber, potassium chloride preceded by general anesthesia and irradiation with microwave. Other acceptable methods are the animal’s contact with a mixture of carbon dioxide and oxygen, and its exposure to carbon monoxide. The inhalation of a concentration of 70% of CO2 and 30% of O2 results in rapid depression of the Central Nervous System (CNS). Barbiturates, in turn, cause CNS depression by overdose, leading to an irreversible unconsciousness and death. The cervical dislocation (<200 g) occurs by applying pressure at the upper neck, dislocating the spine, thus separating it from the skull. The decapitation by guillotine (<200 g) allows the researcher to obtain anatomical sample of brain and tissues without changes and chemical contamination. The Resolution nº 714/2002 of the Federal Council of Veterinary Medicine cites as unacceptable methods of euthanasia gas embolism, cranial trauma, incineration in vivo, chloral hydrate (for small animals), chloroform or sulfuric ether, cyanide gas, decompression, drowning, exsanguination (without prior sedation), immersion in formaldehyde or any fixing substance, isolated use of neuromuscular blockers (isolated use of nicotine, magnesium sulfate, potassium chloride and all curarizes), strychnine, electrocution without numbing or prior anesthesia and any other method considered without scientific background.
For the confirmation of death, the executor must be sure if the vital signs are ceased, especially before discarding the carcass. The professional who will execute euthanasia must check if the animal is really dead, by the absence of respiratory movement (apnea); absence of cardiac electrical impulses (asystole) preferably referenced by the use of stethoscope, or equipment such as doppler ultrasound; absence of pulse; absence of corneal reflex to touch the eyeball; purplish of the mucosa indicating anoxia and permanent loss of brain function.
Disposal of contaminating waste
Biosafety is the term for the safety of life and involves a set of practical conditions directed to prevention, minimization or elimination of inherent risks to production activities, teaching and research, that may affect the health of human beings, animals and cause damage to the environment and compromise the quality of work. Although sometimes neglected, the appropriate disposal of carcasses is part of the research project. Either contaminated by pathogenic agents or not, they are considered solid waste and classified as A group, according to the legislation in force in Brazil, expressed through Resolution nº. 5, of August 1993-the National Council of the Environment. Solid wastes from A group are, by definition, those which present potential risk to public health and the environment due to the presence of “biological agents”. The carcasses of animals naturally dead or killed by euthanasia must be destroyed right after necropsy and collection of material, avoiding risk of environment contamination from fluids and secretions by cadavers, which may become medium of culture. After conditioning in plastic bags, the carcasses must be kept in cold chambers or freezers at -18°C for up to 24 hours [2,16,14]. The carcasses can be discarded by three means: in sanitary landfills, by autoclaving and incineration [2,5]. When the carcass is contaminated by pathogenic agents, autoclaving is required for being the safest method, sterilizing the carcass before the transportation from the laboratory to the disposal site.
Conclusion
Although the search for alternative methods that reduce the use of animals in scientific experiments has been progressive and continuous, a complete replacement may never be possible. Experimental disease models that use mice and rats have been the most used vertebrates in scientific studies. Additional analysis of these techniques remains applicable to ensure animal welfare in biological experiments. Studies using laboratory animals require careful planning and respect to animal rights by complying with ethics procedures, laws and country guidelines enabling the choice of the most appropriate research model and species, to minimize the number of animals used in projects or protocols and refine methods and procedures to avoid pain or distress. The empathy with these animals during experiments advocates anesthetic methods, humane euthanasia, and dispose of carcasses properly so as not to contaminate the environment.
This study reinforced the significance of using laboratory animals in several scientific research, since it discussed various benefits to human health of experimental models by using laboratory mice and rats. Data also highlighted the applicability of these laboratory of animals in microbiology medical research, including strain-dependent virulence mechanisms of human pathogens, also expressing multiresistance profiles, including S. aureus.
Acknowledgement
We would like to thank Institute of Microbiology Paulo de Góes/UFRJ, Laboratory of Diphtheria and Corynebacteria of Clinical Relevance/UERJ. This study was financed in part by the National Council for Scientific and Technological Development (CNPq), Coordination for the Improvement of Higher Level Personnel-Brazil (CAPES)- Finance Code 001 and Research Support Foundation for the State of Rio de Janeiro- Brazil (FAPERJ).
Author’s Contribution
All authors made substantial contributions to the development of the study, drafting the manuscript with critical revision, and providing final approval of the version to be published.
Conflict of Interest
The authors declare that there are no conflicts of interest.