ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 9
Blood mean platelet volume may be predictive for disease course in the cases with pemphigus vulgaris
Department of Dermatology, Cumhuriyet University School of Medicine, Sivas, Turkey
- *Corresponding Author:
- Melih Akyol
Department of Dermatology
School of Medicine
Cumhuriyet University, Turkey
Accepted on March 04, 2017
Pemphigus vulgaris (PV) is an autoimmune blistering disorder treated with immunosuppressive agents. The patients with pemphigus vulgaris have remission and relapses and there is a need to have easy accessible and cheap laboratory monitoring markers. The aim of this study was to investigate the relationship between the course of the disease and the changes of the levels of neutrophil/lymphocyte ratio (NLR), platelet/lypmhocyte ratio (PLR), mean corpuscular volume (MCV) and mean platelet volume (MPV) in the patients with PV. Pemphigus patients (n=43) and healthy controls (n=40) were included in the study, retrospectively. Clinical and laboratory data, including C-reactive protein, erythrocyte sedimentation rate, hemoglobin, hematocrit, red cell distribution width, lymphocyte count, neutrophil count, platelet count, MPV, of the patients and healthy controls were obtained from our institutional computerized medical database. NLR and MPV levels in patients are higher than health controls (p<0.05) MPV changes are consistent with disease course according to variance analysis. MPV cut-off level is 8.45 and the specificity and sensitivity of this cut-off level is 65% and 76% to predict the pemphigus attacks, respectively (p=0.427). NL ratio, PL ratio and MPV may be accepted as inflammatory markers. MPV levels may be recommended as a marker for the determination of relapses in the patients with pemphigus vulgaris.
Keywords
Pemphigus, Mean platelet volume, Neutrophil/lymphocyte ratio, Platelet/lymphocyte ratio, Course.
Introduction
Pemphigus vulgaris (PV) is an autoimmune disease characterized by blistering and erosions on skin and mucous membranes due to acantholysis. The patients with PV take immunsupressive drugs, i.e steroids, and are being followed. The aims of the follow up period for these patients are to provide disease control, to prevent complications and to avoid adverse events associated with the prolonged use of steroids and immunosuppressive drugs. The drugs can be stopped when a state of no new lesions are observed during follow up period. If necessary, treatment may be resumed. Treatment regimens determine follow up period and management of the patients with PV [1].
Although PV is an example of skin-specific autoimmune disorders, it is well known the role of cytokine activation and inflammation in the pathogenesis of PV [2]. IgG antibodies in the patients with PV are detected in the intercellular substance and in the serum of the patients by direct and indirect immunofluorescence tests. However, in some cases, there are no strong correlation between the titre of pemphigus antibodies and the disease activity [3]. Additionally, these tests are expensive in some countries and less accessible. Therefore,there is a need for easier and cheaper laboratory tests during the follow up period of the patients with PV.
The neutrophil to lymphocyte ratio (NLR) has been suggested as a simple index of systemic inflammatory response in ill patients [4,5]. Also, platelet to lymphocyte ratio (PLR) and mean platelet volume (MPV) are also accepted as novel indirect potential inflammatory markers [6-8]. These tests in routine hameatologic laboratory analysis which are cheaper, easier accessible could be possible to reflect drug requirements and a suspected activation in patients with an inflammatory disease.
The aim of this study was to investigate the relationship between the course of the disease and the changes of the levels of NLR, PLR and MPV in the patients with PV.
Materials and Methods
Participants
After approval from the local human ethics committee, we included the patients with pemphigus vulgaris in this retrospective study (between 2008-2016 years). Healthy adult subjects admitted to our institution for a routine check-up were included as a control group. Patients with known additional inflammatory disorders and/or comorbidities, patients with missing data were not included the study. The cases not having a disease severity score were excluded.
Study design
This study was designed as a retrospective cohort study. However, a healthy control group was used to determine the differences of the initial haematological parameters in the patients and healthy controls.
Clinical and laboratory data, including C-reactive protein, erythrocyte sedimentation rate, hemoglobin, hematocrit, red cell distribution width, lymphocyte count, neutrophil count, platelet count, mean platelet volume, mean corpuscular volume of the patients and healthy controls were obtained from our institutional computerized medical database.
Four measurements for haematological parameters (HP) at the four different period of the disease were evaluated: 1) the levels of HP at the time of the first diagnosis before treatment; 2) the levels of HP at the time of clinical response and tapering prednisolone dose; 3) the levels of HP at the time of the first maintenance dose of prednisolone; and 4) the levels of HP at the time of the first relapse after stopping the treatment for each cases.
In this retrospective cohort study, assessment of disease severity was based on Pemphigus Severity Scale, and disease severity was classified as mild, moderate, or severe based on a severity score of ≤ 2+, 3 to 6+, or ≥ 7+, respectively [9].
Statistical analysis
Data was assessed by using SPSS programme. (SPSS 20.0; SPSS Inc., Chicago, IL, USA). Results expressed as mean ± SD. Variables are conducted with either independent samples t test. A spearman correlation analysis was used to determine the correlations between haematological parameters and pemphigus disease severity. A variance analysis and Bonferoni test A were used to determine the changes and differences in the repeated measurements. Roc analysis was used to determine cut-off value with sensitivity and specificity for MPV levels. p value of <0.05 is considered as statistically significant.
Results
Between January 2008 to July 2016, 43 outpatients with a diagnosis of pemphigus vulgaris attending the Dermatology Department (27 male and 16 female), based on clinical examination, histopathology, and direct immunofluorescence and 40 healthy controls (20 male and 20 female) were included in the study.
The mean age of the patients and controls were 51.2 years (SD=13.02; range, 27-80 years) and 50.5 years (SD=12.7; range, 25-70), respectively. There were no significant differences in the mean age between sexes.
The most frequent phenotype was mucocutaneous in 13 cases (30%); mucosal and cutaneous phenotypes were seen in 10 (23%) and 20 (47%) patients, respectively. The number of the moderate cases was 33 (77%), and the number of the severe cases was 10 (23%).
24 of the patients (56%) had received prednisolone, and 19 (44%) had received a combined therapy with prednisolone and azathiopirine. After treatment is stopped, the median remission period was of 6 months (3 months- 1 year).
Table 1 shows the comparisons of laboratory variables of the patients and healthy controls. There is no significant relationship and correlation between other haematological parameters and pemphigus disease severity (Spearman correlation, p>0.05) in our patients group.
| Patients (n=43) | Healthy controls (n=40) | p value | |
|---|---|---|---|
| C-reactive protein (mg(L) | 17.1± 36.5 | 4.2 ± 0.3 | 0.02 |
| Eryhtrocyte sedimentation rate (mm/h) | 22.5 ± 18.1 | 7.3 ± 4.2 | 0.002 |
| Hemoglobin (g/L) | 13.7 ± 1.7 | 14.8 ± 1.5 | 0.001 |
| Hematocrit | 41.0 ± 4.8 | 44.2 ± 3.9 | 0.001 |
| Red cell distribution width (RDW) | 13.9 ± 2.0 | 13.3 ± 0.6 | 0.06 |
| Lymphocyte (L) count (109/L) | 1.0 ± 0.6 | 2.2 ± 0.6 | 0.25 |
| Neutrophil (N) count (109/L) | 5.2 ± 2.9 | 3.7 ± 0.8 | 0.002 |
| Platelet count (P) (109/L) | 275.5 ± 73.6 | 240.2 ± 56 | 0.013 |
| Mean platelet volume (MPV) (fL) | 9.2 ± 1.3 | 9.8 ± 1.2 | 0.029 |
| Mean corpuscular volume (MCV) | 86.1 ± 9.9 | 90 ± 4.0 | 0.017 |
| N/L rate | 2.9 ± 2.4 | 1.9 ± 1.4 | 0.017 |
| P/L rate | 151.9 ± 114.6 | 118.3 ± 65.8 | 0.09 |
| Pemphigus severity score | 5.6 ± 1.2 (mean ± SD) |
_ | _ |
Table 1. Comparison of laboratory variables of pemphigus patients (before treatment) and healthy controls at baseline.
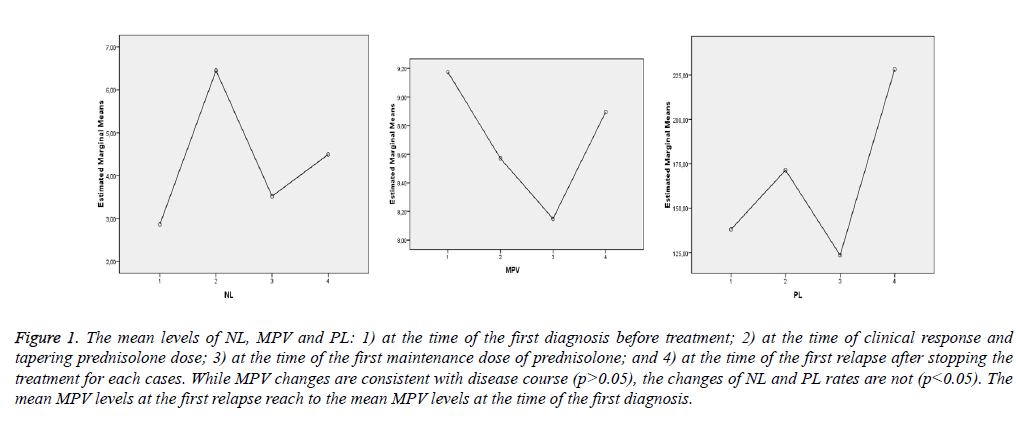
Table 2 and Figure 1 show that MPV changes are consistent with the disease course, but the changes of NL and PL rates are not consistent.
| (I) NL rate | (J) NL rate | Mean Difference (I-J) | Std. Error | Sig.a | 95% Confidence Interval for Differencea | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| 1 | 2 | -3.588* | 1.099 | 0.013 | -6.631 | -0.544 |
| 3 | -0.658 | 0.512 | 1 | -2.076 | 0.76 | |
| 4 | -1.631* | 0.573 | 0.041 | -3.217 | -0.046 | |
| 2 | 1 | 3.588* | 1.099 | 0.013 | 0.544 | 6.631 |
| 3 | 2.929 | 1.172 | 0.098 | -0.315 | 6.173 | |
| 4 | 1.956 | 1.179 | 0.627 | -1.308 | 5.221 | |
| 3 | 1 | 0.658 | 0.512 | 1 | -0.76 | 2.076 |
| 2 | -2.929 | 1.172 | 0.098 | -6.173 | 0.315 | |
| 4 | -0.973 | 0.504 | 0.361 | -2.368 | 0.422 | |
| (I) PL rate | (J) PL rate | Mean difference (I-J) | Std. error | Sig.a | 95% Confidence ınterval for differencea | |
| Lower bound | Upper bound | |||||
| 1 | 2 | -33.260 | 24.090 | 1.000 | -99.966 | 33.446 |
| 3 | 14.508 | 9.508 | 0.807 | -11.821 | 40.837 | |
| 4 | -89.943* | 23.534 | 0.003 | -155.109 | -24.777 | |
| 2 | 1 | 33.260 | 24.090 | 1.000 | -33.446 | 99.966 |
| 3 | 47.768 | 24.602 | 0.353 | -20.355 | 115.890 | |
| 4 | -56.683 | 33.808 | 0.606 | -150.299 | 36.932 | |
| 3 | 1 | -14.508 | 9.508 | 0.807 | -40.837 | 11.821 |
| 2 | -47.768 | 24.602 | 0.353 | -115.890 | 20.355 | |
| 4 | -104.451* | 25.439 | 0.001 | -174.892 | -34.010 | |
| (I) MPV | (J) MPV | Mean difference (I-J) |
Std. error | Sig.a | 95% confidence interval for differencea | |
| Lower bound | Upper bound | |||||
| 1 | 2 | 0.604* | 0.175 | 0.008 | 0.119 | 1.089 |
| 3 | 1.026* | 0.201 | 0.000 | 0.470 | 1.581 | |
| 4 | 0.279 | 0.209 | 1.000 | -0.299 | .0857 | |
| 2 | 1 | -0.604* | 0.175 | 0.008 | -1.089 | -0.119 |
| 3 | 0.422 | 0.173 | 0.114 | -0.057 | 0.900 | |
| 4 | -0.325 | 0.180 | 0.469 | -0.823 | 0.173 | |
| 3 | 1 | -1.026* | 0.201 | 0.000 | -1.581 | -0.470 |
| 2 | -0.422 | 0.173 | 0.114 | -0.900 | 0.057 | |
| 4 | -0.747* | 0.171 | 0.001 | -1.221 | -0.272 | |
1 means “before treatment; 2 means “at the first time of tapering dose of prednisone”; 3 means at the time of “the first maintenance dose of prednisone”; and 4 means “at the time of relapse”
Based on estimated marginal means
*. The mean difference is significant at the 0,05 level
a. Adjustment for multiple comparisons: Bonferroni.
Table 2. Changes of MPV, NL rates and PL rates on repeated measurements in patients group.
Figure 1: The mean levels of NL, MPV and PL: 1) at the time of the first diagnosis before treatment; 2) at the time of clinical response and tapering prednisolone dose; 3) at the time of the first maintenance dose of prednisolone; and 4) at the time of the first relapse after stopping the treatment for each cases. While MPV changes are consistent with disease course (p>0.05), the changes of NL and PL rates are not (p<0.05). The mean MPV levels at the first relapse reach to the mean MPV levels at the time of the first diagnosis.
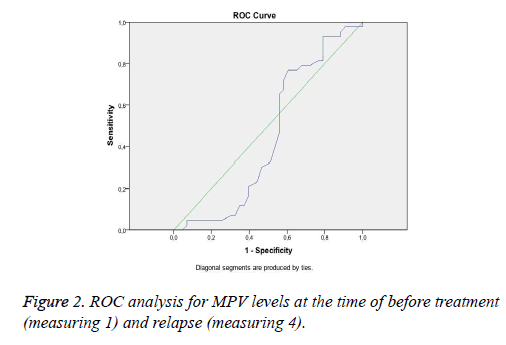
Figure 2 shows ROC-curve for MPV levels at the time of before treatment (measuring 1) and relapses (measuring 4).
According to ROC analysis, that MPV cut-off level is 8.45 and the specify and sensitivity of this cut-off level is 65% and 76% to predict the pemphigus attacks, respectively (p=0.427).
Pemphigus vulgaris patients require systemic corticosteroids and/or immunosuppressive therapies. These agents may put the patients into remission. According to different treatment protocols, immunsupressive drugs may be stopped. However, relapses can be observed within an indefinite period of time. Anti-desmoglein antibodies (anti-Dsg1 and anti-Dsg3) are correlated with the course of the disease in patients with Pemphigus vulgaris [10]. However, in some cases, these correlations may be weak [3]. Additionally these test are not cheap and easy accessible in some countries. Therefore, there is a need for cheaper and easier accessible laboratory tests when following the patients with pemphigus vulgaris during the period of remission and relapse.
Although pemphigus vulgaris is an autoimmune bullous skin disease, inflammatory pathways may play a role in pemphigus vulgaris as well [11]. Proinflammatory cytokines are typically considered to be pathogenic in some autoimmune diseases [12].
N/L ratio may be considered a systemic inflammatory marker. N/L ratio was studied in various dermatologic diseases including psoriasis, Behcet's disease and lichen planus [13-15]. It was reported that NL rates of the patients with pemphigus vulgaris were higher than the controls in a study, but were not related to disease severity [16]. In systemic inflammation, NL rates increase and the severity of the clinical status and clinical outcome may correlate well with the N/L ratio [4]. While NL rates in the patients group are higher than the controls, there is not a correlation between NL rates and disease severity in our study.
MPV is a parameter detected during routine blood count, and known to be a marker determined from megakaryocytes during platelet production, which is associated with platelet function and activation. There is an inverse relationship between platelet size and number in normal conditions. Platelet activation is a link in the pathophysiology of diseases prone to thrombosis and inflammation and MPV may be considered as a prognostic and therapeutic marker [8].
We know that MPV is decreased in some inflammatory bowel diseases such as ulcerative colitis and it could be used for determination of disease activity [17]. Likewise, high-grade inflammatory diseases, such as active rheumatoid arthritis or attacks of familial Mediterranean fever, present with low levels of MPV, and anti-inflammatory therapy may reverse the levels of MPV into normal range. Low-grade inflammatory conditions prone to arterial and venous thromboses, and high MPV associates with cardio- and cerebrovascular disorders [8]. Even though pemphigus vulgaris is an autoinflammatory disease, we can say that it has some characteristics with high grade inflammation according to our results.
There was no relationship between disease severity and MPV levels. However, in our cases, during treatment and maintenance period, the levels of MPV decreased at the time of remission and maintenance of the therapy. The levels of MPV re-increased again at the time of relapse. This finding suggests that there may be a relationship between MPV levels and disease activity. The mean MPV levels at the time of relaps reach to the mean MPV levels at the time of the first diagnosis in patients with pemphigus vulgaris. According to ROC analysis in our study, there is conformity of MPV levels at the time of first diagnosis and the first relaps after stopping treatment. When MPV level is above 8.45 (cut-off level), a clinical attack may be expected in patients with pemphigus vulgaris.
Our study has some limitations. Present study was designed retrospectively. Because pemphigus vulgaris is a rare autoimmune disease, the study population is small. We could not performed any comparisons between MPV levels and anti- Dsg1 and anti-Dsg3 antibodies. However, to the best of our knowledge, this is the first report on the relationship between MPV and the course of pemphigus vulgaris. The results of the study need to be confirmed with further studies.
Conclusion
NL ratio, PL ratio, MCV and MPV may be accepted as inflammatory markers. MPV levels may be recommended as a marker for the determination of relapses in the patients with pemphigus vulgaris according to the results of our study. MPV index can be of any use in the patients with pemphigus vulgaris.
References
- Cholera M, Chainani-Wu N. Management of Pemphigus Vulgaris.Adv Ther 2016; 33: 910-958.
- Shamsabadi RM, Basafa S, Yarahmadi R, Goorani S, Khani M. Elevated expression of NLRP1 and IPAF are related to oral pemphigus vulgaris pathogenesis.Inflammation 2015; 38: 205-208.
- Raj D, Brash DE, Grossman D. Keratinocyte apoptosis in epidermal development and disease.J Invest Dermatol 2006; 126: 243-257.
- Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill.Bratisl Lek Listy 2001; 102: 5-14.
- Sen BB, Rifaioglu EN, Ekiz O, Inan MU, Sen T. Neutrophil to lymphocyte ratio as a measure of systemic inflammation in psoriasis.Cutan Ocul Toxicol 2014; 33: 223-227.
- Akboga MK, Canpolat U, Balci KG, Akyel A, Sen F. Increased Platelet to Lymphocyte Ratio is related to Slow Coronary Flow.Angiology 2016; 67: 21-26.
- Wang Q, Yi H, Liu Y, Wang L, Cao W, Zhao H, Yan Y, Li H, Ding J, Luo L, Ma X. Increased platelet to lymphocyte ratio is related to inflammation in patients with Takayasu arteritis. Int J Clin Exp Med 2016; 9: 6363-6367.
- Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des 2011; 17: 47-58.
- Herbst A, Bystryn JC. Patterns of remission in pemphigus vulgaris.J Am Acad Dermatol 2000; 42: 422-427.
- Abasq C, Mouquet H, Gilbert D, Tron F, Grassi V. ELISA testing of anti-desmoglein 1 and 3 antibodies in the management of pemphigus.Arch Dermatol 2009; 145: 529-535.
- Giordano CN, Sinha AA. Cytokine networks in Pemphigus vulgaris: An integrated viewpoint.Autoimmunity 2012; 45: 427-439.
- Kim EY, Moudgil KD. Regulation of autoimmune inflammation by pro-inflammatory cytokines.Immunol Lett 2008; 120: 1-5.
- Imtiaz F, Shafique K, Mirza SS. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med 2012; 26: 2.
- Sen BB, Rifaioglu EN, Ekiz O, Inan MU, Sen T. Neutrophil to lymphocyte ratio as a measure of systemic inflammation in psoriasis.Cutan Ocul Toxicol 2014; 33: 223-227.
- Atas H, Cemil BÇ, Kurmus GI. Assessment of systemic inflammation with neutrophil-lymphocyte ratio in lichen planus Threatte GA. Usefulness of the mean platelet volume. Clin Lab Med 1993; 13: 937-50.
- Uçmak D, Akkurt M, Uçak H. The relationship of neutrophil to lymphocyte ratio with pemphigus vulgaris. Konuralp Tip Derg 2015; 7: 88-92.
- Kapsoritakis AN, Koukourakis MI, Sfiridaki A, Potamianos SP, Kosmadaki MG. Mean platelet volume: a useful marker of inflammatory bowel disease activity. Am J Gastroenterol 2001; 96: 776-781.