ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 4
Cardiac contractility modulation improves cardiac function in a rabbit model of chronic heart failure
Department of Cardiology Center, Hebei General Hospital, Shijiazhuang, Hebei Province, China
- *Corresponding Author:
- Xiao-Yong Qi
Department of Cardiology Center
Hebei General Hospital Hebei Province, China
Accepted on October 4, 2016
Cardiac Contractility Modulation (CCM) is a novel device-based therapy for chronic heart failure. This study aimed to explore the effects of CCM on electrophysiological characteristics of heart failure. Rabbits were randomly divided into sham group (n=10), HF group (n=10) and CCM group (n=10). The rabbit chronic heart failure model was induced after 12 weeks aortic constriction. Then CCM was delivered to the anterior wall of left ventricle lasting six hours per day for 4 weeks. Changes in cardiac function and electrophysiological characteristics were monitored and SERCA2a expression was detected by quantitative real time qRT-PCR and Western blot analysis. Compared to sham group, HF group showed decreases in LVEF, LVFS, E/A ratio, VFT and increase in QTc and ERP, as well as decreased mRNA and protein levels of SERCA2a (P<0.05). CCM restored LVEF, LVFS, E/A ratio, LVESD, LVEDD, VFT, QTc and ERP, and SERCA2a expression levels in rabbits with heart failure to sham group. In conclusion, CCM benefits cardiac function and electrophysiological characteristics in rabbit model of chronic heart failure, perhaps due to the up regulation of SERCA2a expression.
Keywords
Cardiac contractility modulation, Chronic heart failure, Cardiac function, SERCA2a.
Introduction
Chronic Heart Failure (CHF) is the terminal stage of a variety of cardiac diseases and has a high rate of incidence and mortality. According to American Heart Association, the prevalence of CHF is expected to increase by 25% from 2010 to 2030 [1]. A large number of patients with CHF are refractory to optimal standard medical therapy. Medical devices to treat heart failure, including Cardiac Resynchronization Therapy (CRT) and Ventricular Assist Devices (VADs), can improve patients’ symptoms, quality of life, and survival by providing favourable effects on myocardial remodeling. However, the majority of patients with heart failure are not candidate for CRT or VAD because they have no prolonged QRS duration or have severe symptoms [2]. Therefore, alternative therapy for patients with persistent symptoms is needed.
Cardiac Contractility Modulation (CCM) is an electrical device-based approach in which relatively high voltage electrical signals are applied during refractory period of the heart. These signals do not elicit a new contraction but rather increase the force of contraction without increasing the myocardial oxygen consumption [3]. CCM appears to reverse the molecular remolding associated with heart failure and normalize the expression of several calcium handling proteins and stretch response genes, such as phospholamban, sarcoendoplasmic reticulum Ca2+-ATPase (SERCA2a), Ryanodine Receptors (RYR), Calcium Binding Proteins (CBPs), Na-Ca exchanger-1 (NCX-1) [4-6]. Prospective randomized multicenter studies have demonstrated that CCM improved the symptoms, quality of life, and exercise capacity [7-9].
CHF leads to ventricular electrical remolding and the susceptibility to cardiac arrhythmia. Both CRT and VAD have been shown to alter the electrical characteristics of failing heart and reduce the incidence of ventricular arrhythmia [10]. From the hemodynamic perspective, CCM has significant beneficial effects similar to CRT and VAD. Recent clinical trial suggests that CCM improve cardiac function, prevent chronic ventricular depolarization delay in heart failure and reduce the mortality rates [11,12]. However, to our knowledge, the effect of CCM on cardiac electrophysiological characteristics during CHF heart has not been described. Therefore, in this study we investigated the effect of CCM on cardiac function and electrophysiological characteristics in a rabbit model of CHF.
Materials and Methods
Experimental animals and groups
All animal experiments were approved by The Institutional Animal Care and Use Committee of Hebei Medical University. Thirty healthy New Zealand white rabbits of both genders (6 months old and 2.5-3.5 kg weight) were provided by the Experimental Animal Center of the Hebei Medical University. The quality of the animals was first class standard (License No: SCXK-2008-1-003). Those animals were randomly divided into three groups (n=10): sham operation, heart failure, and CCM stimulation+heart failure (CCM group). In the sham operation group, rabbits underwent a thoracotomy only; in the heart failure group, rabbits underwent thoracotomy and ascending aortic cerclage without CCM; in the CCM group, rabbits underwent thoracotomy, ascending aortic cerclage and receive 4 weeks CCM after the formation of chronic heart failure.
Rabbit model of CHF
Rabbits were anesthetized with 3% sodium pentobarbital at 1 ml/kg body weight. The chest was open between intercostals 2 and 4 at the left sternal border area and the heart was exposed. The ascending aorta was dissected for about 4-5 mm at 1.0 cm distal to aortic root. The ascending aortic diameters were measured by M-mode echocardiography before surgery. Aortic circumference was calculated and occluded to make cerclage constriction to 60% of the original circumference. The electrode was sutured to left ventricular anterior wall and bare wires were ensured to have good contact with myocardial tissue. The other ends of the electrode were punctured subcutaneously to the neck for later use. 12 weeks later, the symptoms of heart failure such as loss of appetite, reduction of activities, breathing acceleration occurred in the rabbits. Left ventricular ejection fraction ≤ 40% indicated that the heart failure model was successfully established.
CCM protocol
The free ends of the electrode on the neck were connected to an R-wave triggering stimulation instrument (BARD Micro Pace EPS320 Cardiac Stimulator, USA) to deliver CCM stimuli. CCM signals were applied to the epicardial surface of the left ventricular anterior wall through the electrode as a biphasic square-wave pulses (phase duration=2 ms, stimulus amplitude=7 V, 30 ms delay after R-wave sensing). CCM stimuli were delivered to the absolute refractory period of cardiac excitations under sinus rhythm 6 h per day for consecutive 4 weeks.
Surface ECG recording
Standard lead II ECG was recorded before and after CCM. Needle electrocardiographic leads were attached under the skin of each limb at optimized position to obtain maximalamplitude recordings, enabling accurate measurements of QT intervals. RR intervals were simultaneously recorded. QTc was calculated using Carlsson’s formula: QTc=QT-0.175 (RR-300) [13].
Echocardiography measurement
Left Ventricular (LV) function was assessed by echocardiography (1-5 MHz probe, GE, VIVID7, USA). The anterior chest area was shaved, two-dimensional images and M-mode tracings were recorded. Teichholz’s formula was adapted to measure LV Endsystolic Diameter (LVESD), LV End-Diastolic Diameter (LVEDD), Interventricular Septal Thickness (IVSd), LV Posterior Wall Thickness (LVPWd), LV Shortening Fraction (LVFS) and Ejection Fraction (LVEF). The Doppler sampling probe was placed between diastolic mitral tip to measure peak flow during early diastolic period of mitral values (E) and peak flow of late diastole (A). The E/A ratio were calculated, and all data represented the average of three consecutive measurements.
Electrophysiological assay
Programmed electrical stimulation protocols were used to assess the electrophysiological characteristics. The Diastolic Threshold (DT) was measured using S1-S1 stimuli. The pacing rhythm was 10 times faster than its own heart rate. DT was defined as the minimum voltage required for capturing the heart. The Effective Refractory Period (ERP) was estimated using an extra-stimulus protocol consisting of an 8-beat drive train (S1) followed by an extra-stimulus (S2) at progressively shorter CLs (2 ms steps). ERP was defined as the longest S1- S2 interval that failed to capture ventricular. Pacing stimuli were applied at twice the diastolic threshold intensity and 2 ms in duration. The interval between two drives was 1 min.
To eliminate variations of vulnerability to fibrillation associated with the slowing or acceleration of heart rate, Vortex Formation Time (VFT) measurements were performed using S1-S1 stimuli at the same heart rate. Electric stimulation was supplied at a stable paced cycle length of 100 ms. The interval between each episode of stimulation was 1 min. The initial pacing voltage was 2 V, and progressively increased by a step of 1 V. The VFT was defined as the smallest amount of voltage required to elicit ventricular fibrillation [14].
Measurement of serum level of brain natriuretic peptide (BNP)
Serum BNP levels were measured using double-antibody sandwich ABC-ELISA kit (West tang Biotech, Shanghai, China) according to the manufacturer’s protocol.
Real-time PCR
Total RNA was isolated from cardiac tissue using TRIzol (Invitrogen, USA). Reverse transcription was performed using the First-Strand cDNA Synthesis system (Promega, USA), and quantitative real-time PCR was performed on an ABI PRISM 7300 PCR System (Applied Bio systems, USA) using Syber Green I GoTaq® qPCR Master Mix (Promega, USA). Primer sequence were as follows: SERCA2a, forward 5’- ACGCCAAACAAACCAAGC-3’ and reverse 5’- ACTTCCAACCCGAATGTGG-3’ GAPDH, forward 5’- TGAACGGGAAGCTCACTGG-3’ and reverse 5’- GCTTCACCACCTTCTTGATGTC-3’. The mRNA level was calculated using the comparative cycle threshold (Ct) method (2-ΔΔCt).
Western blot analysis
Protein concentration was determined by BCA protein assay reagent (Pierce, USA). Equal amounts of protein were subjected to SDS-PAGE, and then transferred onto a PVDF membrane. After blocking with 5% fat-free milk for 1 h, the membrane was incubated with primary antibody SERCA2a (1:200 dilution; Santa Cruz Biotechnology, USA) overnight at 4˚C. The membranes was washed three times in TTBS, and incubated with secondary antibody (1:200 dilutions; Santa Cruz Biotechnology, USA) at 4˚C for 1 h. Protein bands were detected on an X-ray film using Pierce ECL Western Blotting Substrate (Santa Cruz, USA). GAPDH was used as loading control.
Statistical analysis
All statistical analyses were performed using SPSS 16.0 software. The data were presented as mean ± standard deviation (x ± s). Differences among multiple groups were compared with single factor analysis of variance while the comparison between two groups was detected with LSD method. A p value<0.05 was considered statistically significant.
Results
The establishment of heart failure model
There were no deaths in sham operation group. In heart failure group, one rabbits died during aortic constriction surgery due to pneumothorax caused by injury to the parietal pleura. In CCM group, one rabbits died as a result of artery rupture. Rabbits that survived and met the criteria of heart failure 12 weeks post-aortic constriction included 10 in the sham group, 9 in the heart failure group, and 9 in the CCM group. After 12 weeks, animals in both heart failure group and CCM group met the criterion of heart failure with symptoms of accelerated breathing, fastened heart rate, and loss of appetite. These 28 rabbits were used for the subsequent experiments.
Comparison of echocardiography indicators in three groups
After 12 weeks of aortic constriction, LVEF, LVFS, E/A ratio were markedly decreased, while LVESD and LVEDD were increased in HF group and CCM group compared to sham group (P<0.05, Table 1). IVS and LVPW showed no significant differences among the three groups (P>0.05). 4 weeks therapy with CCM increased LVEF, LVFS and E/A ratio, and decreased LVESD and LVEDD compared to HF group (P<0.05, Table 2).
| Group | IVSd (mm) | LVPWd (mm) | LVESD (mm) | LVEDD (mm) | LVEF (%) | LVFS (%) | E (cm/s) | A (cm/s) | E/A |
|---|---|---|---|---|---|---|---|---|---|
| Sham (n=10) | 2.08 ± 0.22 | 2.02 ± 0.18 | 9.37 ± 0.35 | 11.19 ± 0.55 | 68.26 ± 5.03 | 34.86 ± 3.97 | 75.64 ± 5.40 | 54.47 ± 5.51 | 1.39 ± 0.11 |
| HF (n=9) | 2.02 ± 0.27 | 2.12 ± 0.16 | 11.67 ± 0.52* | 14.27 ± 0.78* | 37.23 ± 1.98* | 18.69 ± 1.91* | 64.01 ± 3.87* | 55.3 ± 3.22 | 1.16 ± 0.11* |
| CCM (n=9) | 2.09 ± 0.16 | 2.19 ± 0.19 | 11.43 ± 0.50* | 14.05 ± 0.76* | 37.22 ± 1.71* | 18.74 ± 1.64* | 64.52 ± 3.88* | 55.9 ± 6.73 | 1.16 ± 0.93* |
Note: *P<0.05 compared with sham group.
Table 1. Comparison of echocardiography indicators in three groups before modulation.
| Group | IVSd (mm) | LVPWd (mm) | LVESD (mm) | LVEDD (mm) | LVEF (%) | LVFS (%) | E (cm/s) | A (cm/s) | E/A |
|---|---|---|---|---|---|---|---|---|---|
| Sham (n=10) | 2.05 ± 0.19 | 2.03 ± 0.18 | 9.38 ± 0.39 | 11.27 ± 0.44 | 68.56 ± 4.31 | 34.76 ± 3.24 | 76.98 ± 4.31 | 56.57 ± 3.68 | 1.36 ± 0.72 |
| HF (n=9) | 2.05 ± 0.25 | 2.12 ± 0.16 | 11.73 ± 0.44* | 14.23 ± 0.43* | 36.44 ± 1.94* | 18.61 ± 1.16* | 62.80 ± 2.83* | 54.57 ± 4.81 | 1.16 ± 0.13* |
| CCM (n=9) | 2.04 ± 0.19 | 2.14 ± 0.22 | 10.02 ± 0.28*# | 13.13 ± 0.22*# | 49.22 ± 2.95*# | 24.52 ± 1.97*# | 68.12 ± 2.89*# | 53.69 ± 4.45 | 1.27 ± 0.12*# |
Note: *P<0.05 compared with sham group; #P<0.05 compared with HF group.
Table 2. Comparison of echocardiography indicators in three groups 4 weeks later.
Comparison of electrophysiological characteristics in three groups
After 12 weeks of aortic constriction, QTc and ERP were extended, DT and VFT were decreased in HF group and CCM group compared to sham group (P<0.05). CCM administrations significantly restored QTc, ERP, DT and VFT compared to HF group (Table 3).
| Group | QTc (ms) | DT (V) | ERP (ms) | VFT (V) | ||||
|---|---|---|---|---|---|---|---|---|
| Beginning | 4 weeks later | Beginning | 4 weeks later | Beginning | 4 weeks later | Beginning | 4 weeks later | |
| Sham (n=10) | 140.70 ± 4.42 | 141.30 ± 4.24 | 1.66 ± 0.13 | 1.65 ± 0.14 | 121.20 ± 9.50 | 121.50 ± 9.54 | 12.34 ± 0.99 | 12.32 ± 0.98 |
| HF (n=9) | 152.00 ± 4.80* | 152.11 ± 5.49* | 0.99 ± 0.11* | 1.05 ± 0.23 * | 139.44 ± 5.55* | 140.56 ± 5.10* | 9.62 ± 1.78* | 9.35 ± 0.99* |
| CCM (n=9) | 151.44 ± 3.97* | 144.67 ± 3.84# | 1.02 ± 0.81* | 1.27 ± 0.94*# | 143.33 ± 3.78* | 133.33 ± 6.12*# | 8.72 ± 1.12* | 10.19 ± 0.42*# |
Note: *P<0.05 compared with sham group; #P<0.05 compared with HF group.
Table 3. Comparison of electrophysiological characteristics in three groups before and after 4 weeks of modulation.
Comparison of serum BNP level in three groups
As a marker of HF, serum level of BNP was significantly increased after 12 weeks of aortic constriction in HF group and CCM group compared to sham group (P<0.05). CCM decreased serum BNP level in CCM group compared to HF group (P<0.05) (Table 4).
| Sham group (n=10) | HF group (n=9) | CCM group (n=9) | |
|---|---|---|---|
| Beginning | 29.32 ± 3.03 | 61.55 ± 5.82* | 64.29 ± 5.16* |
| 4 weeks later | 28.83 ± 2.90 | 61.69 ± 5.51*# | 50.16 ± 3.44* |
Note: *P<0.05 compared with sham group; #P<0.05 compared with HF group.
Table 4. Comparison of serum BNP level (ng/ml) in three groups before and after 4 weeks of modulation.
Comparison of SERCA2a expression in three groups
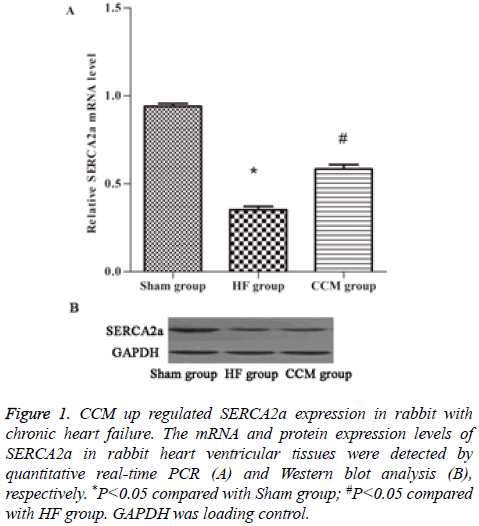
SERCA2a is a key regulator of cardiac contractile force. By PCR we examined SERCA2a mRNA expression levels in each group. The results showed that SERCA2a mRNA level was significantly decreased in HF group compared to sham group (P<0.05), but was significantly increased in CCM group compared to HF group (P<0.05) (Figure 1A). Similar changes of SERCA2a protein expression levels were observed in the three groups (Figure 1B).
Figure 1 : CCM up regulated SERCA2a expression in rabbit with chronic heart failure. The mRNA and protein expression levels of SERCA2a in rabbit heart ventricular tissues were detected by quantitative real-time PCR (A) and Western blot analysis (B), respectively. *P<0.05 compared with Sham group; #P<0.05 compared with HF group. GAPDH was loading control.
Discussion
Chronic heart failure is a significant health burden. Approximately half of the deaths are secondary to arrhythmic cause, mainly because of lethal ventricular arrhythmias such as ventricular tachycardia and ventricular fibrillation. With the progression of CHF, the heart adapts through a complex process of remolding, resulting in adverse effect on cardiac function and electrophysiological characteristics [15]. An appropriate CHF animal model would be useful to develop novel preventative and reparative therapies. In the study, we presented a novel rabbit model of chronic heart failure and investigated the effects of CCM on cardiac function and electrophysiological characteristics using this model. The reasons for the choice of rabbit as the model in this study are as follows: (1) Rabbit closely mimics the alterations of myocardial function in the end-stage failing human myocardium. (2) The cardiac electrophysiological characteristics of rabbit are similar to those of human. (3) The heart size of rabbit is large enough to implant electrode [16,17].
The heart failure model induced by aortic constriction led to increased left ventricular afterload. After 12 weeks of aortic constriction, the heart failure model was confirmed based on echocardiography examination and serum BNP levels. The animals in the HF group with both systolic and diastolic dysfunctions and structure remolding exhibited similar pathological changes to human, such as the increases in LVEDD, LVESD and BNP, and the decreases in LVEF, LVFS and E/A. Compared with shame group, electrophysiological remolding was observed in HF group, QTc and ERP were extended, DT and VFT were decreased. Those changes can increase the risk for life-threatening arrhythmias.
CCM indicates the application of non-excitatory electrical signals to the myocardium to elicit a positive inotropic effect during the absolute refractory period of the action potential [18]. CCM could benefit symptomatic patients with HF who are not candidates for CRT. Preclinical studies have demonstrated that electromagnetic field produced by CCM can induce the changes in gene expression, which will help restore the ability of failing myocytes to handle calcium cycling and contraction. Chronic CCM signal application can reverse ventricular remodeling both globally and at the cellular and molecular levels [19]. Recent study further showed that CCM can reduce mortality rates in heart failure population estimated from the MAGGIC score [11]. In present study we showed that CCM improved LVEF, LVFS, E/A ratio and decreased LVESD , LVEDD and BNP levels, indicating that CCM therapy enhances systolic and diastolic function and reverses the remolding of left ventricle. Furthermore, we demonstrated that the underlying mechanism of CCM therapy may be related to the up regulation of SERCA2a expression at mRNA and protein levels. SERCA2a is a critical ATPase responsible for Ca2+ reuptake during excitation-contraction coupling, and impaired Ca2+ uptake consequent to decreased expression and activity of SERCA2a is a hallmark of heart failure characterized by severe contractile dysfunction. Normalization of SERCA2a expression has been shown to restore cardiac function and ameliorate associated symptoms [3].
Cardiac remodeling includes structural remodeling and electrophysiological remolding. During electrophysiological remolding, the changes of a variety of ion channels, sarcoplasm reticulum calcium cycle and the gap junctions between cells lead to reduced cardiac electrical stability, slowing of conduction, prolonged duration of the action potential and make the heart prone to arrhythmia [20]. In the present study we found that CCM restored the prolonged QTc and ERP, increased VFT, and reduced the susceptibility of arrhythmia. The effects of CCM on electrophysiological remolding can be interpreted as follows based on previous studies. First, improved cardiac mechanics could reduce the incidence of arrhythmias and reverse electrophysiological remolding. It have been documented that both CRT and Left Ventricular Assist Devices (LVAD) achieve significant beneficial effect on mechanical remolding [10,17]. QTc interval was significantly decreased with LVAD [21]. Second, a reduction in SERCA2a can predispose to Ca2+ and electrical alternans in CHF, which will change electrophysiological characteristics and increase arrhythmia susceptibility [22]. Preclinical study showed that enhanced SERCA2a levels in CHF model reduced the propensity for both Ca2+ and APD alternans. As a result, increased expression of SEECA2a in HF model reduces arrhythmia [23]. Increased expression of SERCA2a in cardiomyocytes produced larger SR Ca2+ release, leading to a reduction in prolonged ADP [24]. In our study we found that extended QT interval and ERP in CHF rabbits were rescued with the up regulation of SERCA2a expression. Third, CCM may restore the function of ion channel in CHF cardiomyocytes. CCM could shorten the ADP duration in isolated rabbit heart partially dependent on the activation of Iks [25]. Electrical stimulation (CRT) also manifests a partially restoration of the reduction Ik1 and Ik in dogs with HF [26]. Therefore, we speculate that CCM could restore electrophysiological characteristics in CHF and improve the remolding of ion channel by some unknown mechanism.
The present study has some limitations. First, in our model CCM was applied to epicedial, which was different from the endocardial typically used in clinical practice. It is unclear whether this difference affects the extrapolation of our results to human. Second, patch clamp analysis is needed to explore the effects of CCM on the function of ion channels.
In conclusion, ascending aortic constriction is an effective method to establish a rabbit model of chronic heart failure. CCM can improve cardiac function; reverse structural remodeling and electrophysiological changes in heart failure rabbit model, perhaps by up regulating the expression of SEECA2a. These findings provide insights into the mechanism of CCM therapy and support the application of CCM in the clinical treatment of CHF.
Acknowledgments
This work was supported by Natural Science Foundation of Hebei Province, China (No. H2015307037).
Declaration of Conflict of Interest
None.
References
- Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl S, Sacco RL, Saver JL, Trogdon JG. Stroke C: Forecasting the future of stroke in the United States: A policy statement from the American Heart Association and American Stroke Association. Stroke 2013; 44: 2361-2375.
- Kahwash R, Burkhoff D, Abraham WT. Cardiac contractility modulation in patients with advanced heart failure. Expert Rev CardiovascTher 2013; 11: 635-645.
- Ning B, Qi X, Li Y, Liu H, Zhang F, Qin C. Biventricular pacing cardiac contractility modulation improves cardiac contractile function via up regulating SERCA2 and miR-133 in a rabbit model of congestive heart failure. Cell PhysiolBiochem 2014; 33: 1389-1399.
- Butter C, Rastogi S, Minden HH, Meyhofer J, Burkhoff D, Sabbah HN. Cardiac contractility modulation electrical signals improve myocardial gene expression in patients with heart failure. J Am CollCardiol 2008; 51: 1784-1789.
- Gupta RC, Mishra S, Wang M, Jiang A, Rastogi S, Rousso B, Mika Y, Sabbah HN. Cardiac contractility modulation electrical signals normalize activity, expression, and phosphorylation of the Na+-Ca2+ exchanger in heart failure. J Card Fail 2009; 15: 48-56.
- Gupta RC, Mishra S, Rastogi S, Wang M, Rousso B, Mika Y, Remppis A, Sabbah HN. Ca (2+)-binding proteins in dogs with heart failure: effects of cardiac contractility modulation electrical signals. ClinTranslSci 2009; 2: 211-215.
- Kadish A, Nademanee K, Volosin K, Krueger S, Neelagaru S. A randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. Am Heart J 2011; 161: 329-337.
- Borggrefe MM, Lawo T, Butter C, Schmidinger H, Lunati M. Randomized, double blind study of non-excitatory, cardiac contractility modulation electrical impulses for symptomatic heart failure. Eur Heart J 2008; 29: 1019-1028.
- Abraham WT, Lindenfeld J, Reddy VY, Hasenfuss G, Kuck KH, Boscardin J, Gibbons R, Burkhoff D. A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation in patients with moderately reduced left ventricular ejection fraction and a narrow QRS duration: study rationale and design. J Card Fail 2015; 21: 16-23.
- Saba S, Mehdi H, Mathier MA, Islam MZ, Salama G, London B. Effect of right ventricular versus biventricular pacing on electrical remodeling in the normal heart. CircArrhythmElectrophysiol 2010; 3: 79-87.
- Kuschyk J, Roeger S, Schneider R, Streitner F, Stach K. Efficacy and survival in patients with cardiac contractility modulation: long-term single center experience in 81 patients. Int J Cardiol 2015; 183: 76-81.
- Roger S, Michels J, Heggemann F, Stach K, Rousso B. Long term impact of cardiac contractility modulation on QRS duration. J Electrocardiol 2014; 47: 936-940.
- Li X, Wang T, Han K, Zhuo X, Lu Q. Bisoprolol reverses down-regulation of potassium channel proteins in ventricular tissues of rabbits with heart failure. J Biomed Res 2011; 25: 274-279.
- Liu Y, Li H, Xia W, Yu S, Huang C. Electrophysiological effect of rotigaptide in rabbits with heart failure. Arch Med Sci 2014; 10: 374-380.
- Kessler EL, Boulaksil M, van Rijen HV, Vos MA, van Veen TA. Passive ventricular remodeling in cardiac disease: focus on heterogeneity. Front Physiol 2014; 5: 482.
- Zaragoza C, Gomez-Guerrero C, Martin-Ventura JL, Blanco-Colio L, Lavin B, Mallavia B, Tarin C, Mas S, Ortiz A, Egido J. Animal models of cardiovascular diseases. J Biomed Biotechnol 2011; 2011: 497841.
- Saba SA, Mathier M, Mehdi H, Gursoy E, Liu T, Choi BR, Salama G, London B. Prevention of adverse electrical and mechanical remodeling with biventricular pacing in a rabbit model of myocardial infarction. Heart Rhythm 2008; 5: 124-130.
- Lyon AR, Samara MA, Feldman DS. Cardiac contractility modulation therapy in advanced systolic heart failure. Nat Rev Cardiol 2013; 10: 584-598.
- Imai M, Rastogi S, Gupta RC, Mishra S, Sharov VG, Stanley WC, Mika Y, Rousso B, Burkhoff D, Ben-Haim S, Sabbah HN. Therapy with cardiac contractility modulation electrical signals improves left ventricular function and remodeling in dogs with chronic heart failure. J Am CollCardiol 2007; 49: 2120-2128.
- Coronel R, Wilders R, Verkerk AO, Wiegerinck RF, Benoist D. Electrophysiological changes in heart failure and their implications for arrhythmogenesis. BiochemBiophysActa 2013; 1832: 2432-2441.
- Ambardekar AV, Buttrick PM. Reverse remodeling with left ventricular assist devices: a review of clinical, cellular, and molecular effects. Circ Heart Fail 2011; 4: 224-233.
- Sikkel MB, Hayward C, MacLeod KT, Harding SE, Lyon AR. SERCA2a gene therapy in heart failure: an anti-arrhythmic positive inotrope. Br J Pharmacol 2014; 171: 38-54.
- Cutler MJ, Wan X, Laurita KR, Hajjar RJ, Rosenbaum DS. Targeted SERCA2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. CircArrhythmElectrophysiol 2009; 2: 686-694.
- Lyon AR, Bannister ML, Collins T, Pearce E, Sepehripour AH, Dubb SS, Garcia E, OGara P, Liang L, Kohlbrenner E, Hajjar RJ, Peters NS, Poole-Wilson PA, Macleod KT, Harding SE. SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. CircArrhythmElectrophysiol 2011; 4: 362-372.
- Winter J, Brack KE, Ng GA. The acute inotropic effects of cardiac contractility modulation (CCM) are associated with action potential duration shortening and mediated by beta1-adrenoceptor signalling. J Mol Cell Cardiol 2011; 51: 252-262.
- Aiba T, Hesketh GG, Barth AS, Liu T, Daya S, Chakir K, Dimaano VL, Abraham TP, ORourke B, Akar FG, Kass DA, Tomaselli GF. Electrophysiological consequences of dyssynchronous heart failure and its restoration by resynchronization therapy. Circulation 2009; 119: 1220-1230.