ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2012) Volume 23, Issue 3
Case Reports: Aripripazole Reduces Risperidone-Induced Hyperprolactinemia
1Mental Health Unit-District 44 – ASL Napoli
2Department of Pharmaceutical and Biomedical Science, University of Salerno, Italy
- *Corresponding Author:
- Anna Capasso
Department of Pharmaceutical and Biomedical Sciences
University of Salerno
Via Ponte don Melillo
84084 Fisciano (Salerno)
Italy
Accepted date: April 07 2012
Many schizophrenic patients treated with typical or atypical antipsychotics (AP) such as risperidone show significant clinical alterations as changes in sexual activity, menstrual cycle, amenorrhea, galattorrea and cardiovascular disorders because of AP-induced hyperprolactinemia. In the present paper we report two clinical cases showing, after switching with aripiprazole, a PRL normalization and a marked improvement of clinical events related to risperidone- induced hyperprolactinemia
Keywords
Aripiprazole, Hyperprolactinemia, Risperidone
Introduction
Hyperprolactinemia is an event common in patients treated with typical or atypical antipsychotics (AP) such as risperidone which acts by blocking dopamine D2 receptors in the tubuloinfundibular circuit (TIDA) [1,2]. High levels of prolactin (PRL) may induce significant clinical alterations, both short-term and long-term [3,4], as changes in sexual activity, menstrual cycle, amenor- /rhea, infertility, gynecomastia, galattorrea, cardiovascular disorders, osteoporosis, breast cancer and endometriosis [6-11].
A recent study [5] showed that, in schizophrenic and bipolar patients, the treatment with risperidone or with a typical AP induced hyperprolactinemia in 65.6% of childbearing age women and in 45% of women undergoing to postmenopausal status. Among the male patients, the prevalence of hyperprolactinemia was found in 42.4% of cases. These indices are considered significantly higher compared to the general population. The childbearing age women have an odds ratio 2.6 times greater of developing hyperprolactinemia compared to males and 2.2 times greater than women undergoing to postmenopausal status. In this study, the prevalence of hyperprolactinemia in risperidone-treated patients was significantly greater than women treated with a typical AP (88% versus 47.6%).
Here, we report two clinical cases showing, after switching with aripiprazole, a PRL normalization and a marked improvement of clinical events related to risperidoneinduced hyperprolactinemia. These clinical cases were followed by our MHU (Mental Health Unit).
Case Reports
Case 1
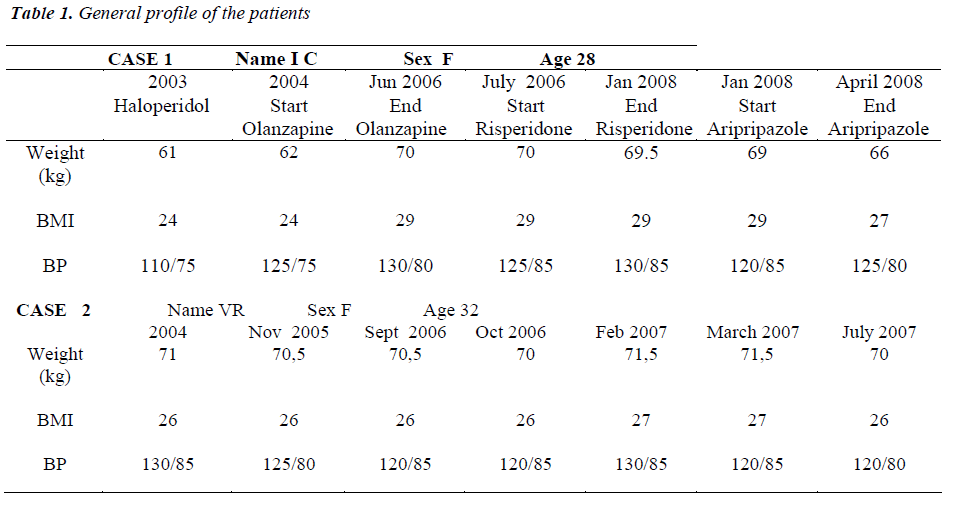
The woman patient named I.C, 28 years old, showed a diagnosis of schizophrenia, according to the criteria of the DSM IV. In 2003, I.C. was treated with haloperidol long acting 100 mg every 4 weeks for one year. Considering the no good results obtained, I.C. was treated with olanzapine 10 mg/day/ orally until June 2006. However, I.C. treated with olanzapine increased significantly her weight (+8 kg: from 62 kg to 70 kg and a BMI from 24 to 29), without changes in blood glucose and lipid level. Therefore, I.C. was treated with risperidone 6 mg/day/orally from July 2006 to January 2008.
During risperidone therapy, I.C, showed firstly oligomenorrhea and then amenorrhea with galattorrea associated with very high blood levels of PRL (November 2007: PRL 55 mcg/ml; December 2007: PRL 88 mcg/ml). Starting by January 2008, I.C. was treated with aripiprazole (15 mg/day) and PRL levels resulted timedependently reduced: 36 mcg/ml at the end of January 2008, 24 mcg/ml in February 2008 and 22 mcg/ml in March 2008. By February 2008 I.C. did not show any clinical hyperprolactinemia symptoms as galattorrea and the normal menstrual cycles was restored, with progesterone levels compatible with ovulation in the second part of the mestrual cycle. During the first 4 months of treatment with aripiprazole (January-April 2008) the I.C. lost 3 kg with a BMI from 29 to 27.
Case 2
The women patient named V.R., 32 years old, in 2004 showed a diagnosis of schizophrenia, according to the diagnostic criteria of DSM IV, and until November 2005 she was treated with haloperidol at doses ranging between 20 and 30 mg/day/orally and then with haloperidol long acting 100 mg/monthly until September 2006. By October 2006, V.R. was followed by our MHU, and the haloperidol long acting therapy was replaced with risperidone (long acting injection) 25 mg vials i.m. every 15 days. Before risperidone treatment, V.R. had regular menstrual cycles and the PRL values were 28 mcg/ml. Starting by December 2006, V.R. showed an abnormal menstrual cycles (2 menstruation in 4 months) and then also galattorrea from February 2007. The PRL values resulted increased in a time dependent manner: 65 mcg/ml in January 2007; 73 mcg/ml in February 2007; 82 mcg/ml in March 2007. Given the above oligomenorrhea and galattorrea, the treatment with risperidone (long acting injection) was replaced with aripiprazolo 10 mg/day (April 2007). After the switch with aripiprazole (April 2007), PRL values resulted reduced in a time dependent manner: 45 mcg/ml in May 2007; 28 mcg/ml in June 2007; 19 mcg/ml in July 2007. Also, the galattorrea clinical symptoms were interrupted at the end of April 2007 and mestrstual cycles resulted regular since June 2007. Table 1 showed the general profile of the patients
Conclusions
The results of the present study indicate that the switch with aripiprazole (both orally and long acting injection) induce a PRL normalization and a marked improvement of clinical events (oligomenorrhea, amenorrhea and galattorrea) related to risperidone-induced hyperprolactinemia. Although aripiprazole is a partial agonist of dopamine D2 receptors, it does not block the D2 receptors of TIDA circuit and consequently it does not cause a sharp reduction in dopamine levels in the hypophysial area [14,15]. At this level, dopamine is able to inhibit the prolactin inhibitor factor (PIF) a modulator of PRL secretion. Thus, considering that aripiprazole does not block the PIF, it does not induce an increased production and release of PRL by the hypophysial area.
In the above patients, aripiprazole also showed a good effect on clinical symptoms of psychosis, so that the scores on the PANSS [12] and on the CGI [13] were substantially overlapped both during treatment with the risperidone and during treatment with aripiprazole. In addition, the aripiprazole was well tolerated in both cases, without adverse side effects, and in one of the patients, the body weight resulted reduced of 3 kg in 4 months of treatment.
In conclusion, the present data suggest that aripiprazole is able to reduce the increased PRL levels induced by risperidone and to improve the clinical events related to hyperprolactinemia. However, we need further cases which may confirm these very suggestive data.
References
- Haddad M, Wieck A. Antipsychotic-induced hyperprolactinemia. Drugs 2004; 64(20): 2291-2314.
- Maguire GA. Prolactin elevation with antipsychotic medications: mechanisms of action and clinical consequences J Clin Psychiatry 2002; 63(suppl 4): 56-62.
- Halbreich U., Kahn L. Hyperprolactinemia and schizophrenia: mechanisms and clinical aspect” 2003 J Psychiatric Practive 9: 344-353.
- Halbreich U, Kinon BJ, Gilmore JA, Kahn LS. Elevated prolactin levels in patients with schizophrenia: mechanisms and related adverse effects” Psychoneuroendocrinology 2003 28: 53-67.
- Kinon BJ. Prevalence of hyperprolactinemia in schizophrenic patients treated with conventional antipsychotic medications or risperidone Psychoneuroendocrinology 2003; 28: 55-68.
- Hummer M., Malik P, Gasser RW, Hofer A, Kemmler G, Moncayo Naveda RC, Rettenbacher MA, Fleischhacker WW. Osteoporosis in patients with scizophrenia” Am J Psychiatry 2005 162: 162-167
- Naidoo U., Goff DC, Klibanski A. Hyperprolactinemia and bone mineral density: the potential impact of antipsychotic agents” Psychoneuroendocrinology 2003; 28: 97-108.
- O'Keane V, Meaney AM. Antipsychotic Drugs. A new risk factor for osteoporosis in young women with schizophrenia? J Clin Psychopharmacol 2005; 25: 26- 31.
- Compton MT, Miller AH. Antipsychotic-induced hyperprolactinemia and sexual dysfunction Psychopharmacology Bullettin 2002 ; 36(1): 143-164.
- Montgomery J., Winterbottom E, Jessani M, Kohegyi E, Fulmer J, Seamonds B, Josiassen RC. Prevalence of hyperprolactinemia in schizophrenia: Association with typical and atypical antipsychotic treatment” 2004 J Clin Psychiatry 65: 1491-1498.
- Melkersson K. Differences in prolactin elevation and related symptoms of atypical antipsychotics in schizophrenic patients J Clin Psychiatry 2005; 66 761-767.
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia” Scizophr Bull 1987; 13: 261-276.
- National Institute of National Health “Clinical global impression scale”. Psychopharmacol Bull 1985; 4: 839- 841.
- Burris KD. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist ay human dopamine D2 receptor J Pharmacol Exp Ther 2002; 302: 381-389
- McGavin JK, Goa KL. Aripiprazole. CNS 2002; 16: 779-786.