ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 1
Cellular pathways affected by indoxyl sulfate in human proximal tubular cells
Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- *Corresponding Author:
- Su Hyun Kim
Department of Internal Medicine
Chung-Ang University Medical Center
102, Heukseok-ro, Dongjak-gu
Seoul 156-755, Korea
Accepted date: May 20, 2016
Indoxyl sulfate (IS) plays a substantial role in chronic kidney disease (CKD) progression, but there is insufficient understanding of the complex cellular pathways in the kidney. This study investigated the cellular pathways affected by IS in human proximal tubular cells (HK-2 cells). Cell viability, reactive oxygen species (ROS) production and protein expression were analyzed in HK-2 cells exposed to IS. In addition, the effects of catalase and Irbesartan were evaluated. Cell viability of HK-2 cells was decreased following exposure to IS (P=0.001). IS did not significantly increase ROS production compared to cells without IS (P=0.664). Among cellular proteins including ERK, PI3K, Nrf2 and JNK, the expression levels of PI3K and JNK were decreased in IS-exposed cells (P=0.045 and 0.001). In contrast, the expression of HO-1 and NOS3 was increased in proportion to the concentration of IS (P=0.034 and 0.046). In HK-2 cells exposed to 100 μM IS, treatment with catalase and irbesartan increased cell viability, but it was not significant (P=0.190). In addition, use of the drugs did not reverse IS-mediated changes in protein expression for ERK, PI3K, Nrf2 and JNK. In conclusion, cell viability was decreased in HK-2 cells according to IS exposure. This toxicity could involve the activation of ERK, PI3K-AKT, Nrf2-keap1 and JNK pathways as well as NOS3. However, this study could not show the ROS production and the favorable effects of antioxidants and angiotensin-receptor blockers in IS-induced cell injury.
Keywords
Chronic kidney disease, Intracellular signaling peptides and proteins, Reactive oxygen species, Uremia middle molecule toxins.
Introduction
Chronic kidney disease (CKD) is a debilitating chronic disease, which may increase the risk for cardiovascular events and allcause mortality [1,2]. Thus, various efforts have been studied to prevent the deteriorating renal function around the world [3,4]. Uremic toxins are waste products and they accumulate in patients with CKD [5]. They are classified according to molecular size and water solubility. Although water-soluble low-molecular-weight uremic toxins can be eliminated via hemodialysis, protein-bound uremic toxins cannot be efficiently removed [6,7]. Indoxyl sulfate (IS) is a major protein-bound uremic toxin and it is markedly increased in CKD patients [8]. Besides serving as a marker of renal dysfunction, IS may have a substantial role in the progression of CKD [9,10]. A previous study with uremic rats showed that administration of IS increased serum creatinine and decreased inulin clearance [8]. Moreover, patients with high levels of IS also showed more rapid CKD progression compared to those with low IS levels [11], and lowering IS by use of an oral adsorbent delayed the initiation of dialysis and also improved renal function [12]. Therefore, understanding the precise cellular mechanism of IS-induced CKD progression is necessary for the identification of a therapeutic target for treatment of this condition.
IS increases the expression of transforming growth factor-β1 and α-smooth muscle actin [13,14], which results in an epithelial-to-mesenchymal transition, known as the critical course of CKD [15,16]. In addition, IS also suppresses proliferation and induces senescence in proximal tubular cells by activation of nuclear factor-κB [17]. To address the above processes, production of reactive oxygen species (ROS) has been suggested to be a major mediator [17,18]. Several previous studies demonstrated that ROS production and the expression of related proteins were induced by exposure to IS [17-19]. Nevertheless, the findings were insufficient to explain the complex cellular pathways involved in IS-induced cell injury. Furthermore, despite the neutralization of a few ISinduced pathways by medications such as antioxidants and angiotensin receptor blockers [17,20], these groups found no improvement in cell viability.
In order to confirm the cellular pathways affected by IS, we investigated protein expression in human proximal tubular cells (HK-2 cells) exposed to IS. Furthermore, we evaluated whether antioxidants and angiotensin receptor blockers have favorable effects on IS-induced cell damage.
Materials and Methods
Reagents
Reagents and antibodies were purchased from the following companies: keratinocyte-serum-free medium (K-SFM), fetal bovine serum (FBS) and penicillin from Gibco (Grand Island, NY, USA); IS from Alfa Aesar (Lancashire, UK); polyethylene glycol-catalase, irbesartan, probenecid and anti-actin antibody produced in rabbit from Sigma-Aldrich (Saint Louis, MO, USA); antibodies to extracellular signal-regulated kinase (ERK) 1/2 (H-72) and nitric oxide synthase 3 (NOS3) (H-159) from Santa Cruz Biotechnology (Santa Cruz, Ca, USA); phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) p110r (D55d5) rabbit monoclonal antibody from Cell Signalling Technology (Beverly, MA, USA); and rabbit anti-nuclear factor-like 2 (Nrf2) polyclonal antibody, rabbit antihemeoxygenase 1 (HO-1) polyclonal antibody, rabbit anti-c- Jun N-terminal kinase (JNK) 1/3 polyclonal antibody and antirabbit IgG horseradish peroxidase (HRP)-linked antibody from Bioss (Boston, MA, USA).
Cell culture
HK-2 cells were obtained from American Type Culture Collection (Manassas, VA, USA). HK-2 cells were cultured in K-SFM supplemented with 10% FBS and penicillin of 60 μg/ml, and were maintained at 37 in a humidified 5% CO2/95% air atmosphere incubator. Fresh growth medium was added to the cells every 2 days until cells reached an adequate level of confluency for each experiment.
Treatment
HK-2 cells were seeded by 1 × 104 cells per well in tissue culture plates before treatment. Cells were treated with a serial concentration of IS in each well, and were incubated for 24 hours in K-SFM containing 2% FBS, followed by analysis. Next, the effects of medications such as catalase, irbesartan and probenecid on IS-induced cell injury were evaluated. For these experiments, cells were treated with 100 μM IS and then either catalase of 100 unit/ml, irbesartan at 20 μM, or probenecid at 1 mM was added. Cells were then incubated for 24 hours in KSFM containing 2% FBS before analysis.
Cell viability
Cell viability was determined using 3-(4,5-cimethylthiazol-2- yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma-Aldrich, Saint Louis, MO, USA) assay. After treatment with IS or IS and drug, MTT was added to each well at a concentration of 100 μg/ml. Two hours later, the medium was aspirated and cells were washed in phosphate-buffered saline (PBS, Sigma- Aldrich, St. Louis, MO, USA). To dissolve formazan crystals, cells were exposed to 500 μl isopropyl alcohol (Sigma-Aldrich, Saint Louis, MO, USA), then incubated for 10 minutes at room temperature. Finally, the absorbance was measured at a wavelength of 570 nm using an enzyme-linked immunosorbent assay (ELISA) reader.
Cell viability was assessed both in HK-2 cells exposed to serial concentrations of IS and in those treated with catalase, irbesartan, or probenecid in addition to 100 μM IS.
ROS production
HK-2 cells were seeded at 1 × 105 cells per well in tissue culture plates. Then, cells were exposed to IS at concentrations between 5 μM and 100 μM. After 24 hours of incubation, cells were detached with 0.05% trypsin-EDTA (Gibco, Grand Island, NY, USA), and were washed twice with PBS. The cells were then incubated for 30 minutes at 37°C in Hank’s Balanced Salt Solution (HBSS, Sigma-Aldrich, St. Louis, MO, USA) with 10 μM 2’,7’-dichlorodihydrofluorescein diacetate (DCF-DA, Sigma-Aldrich, Saint Louis, MO, USA). Cells were washed again by HBSS, and were incubated for ten minutes at 37 in 5% FBS. The ROS production was measured by FACS Calibur flow cytometry (BD Biosciences, San Jose, CA, USA).
Western blot analysis
Treated cells were lysed with lysis buffer (Gibco, Grand Island, NY, USA) and boiled for ten minutes, separated by SDS-PAGE with 12% resolving and 5% stacking gels, and then transferred to nitrocellulose membranes. The nitrocellulose membranes were blocked for one hour with 5% skim milk (Sigma-Aldrich, Saint Louis, USA), and were then incubated in a 1:1,000 dilution of a primary antibody to ERK, PI3K, Nrf2, HO-1, JNK or NOS3 for 2 hours at room temperature. After incubation, membranes were washed with a mixture of Tris-buffered saline with Tween 20 (TBST, Cell Signaling Technology, Beverly, MA, USA) twice for ten minutes and were then exposed to secondary antibodies for 30 minutes in 5% skim milk. Finally, nitrocellulose membranes were washed thrice for 10 minutes with TBST. By analyzing signals captured on the nitrocellulose membranes using a Chemi-doc image analyzer (Bio-Rad, Hercules, CA, USA), the levels of protein expression were determined.
To investigate whether certain cellular pathways were activated by exposure to IS at serial concentrations, the expression levels of several proteins including ERK, PI3K, Nrf2, HO-1, JNK and NOS3 were measured using Western blot analysis. Next, the expression levels of ERK, PI3K, Nrf2 and JNK were evaluated in the same way after treatment with catalase, irbesartan or probenecid in addition to 100 μM IS.
Statistical analysis
The data were expressed as mean ± standard deviation. Intergroup differences were analyzed using ANOVA with the Tukey-Kramer method for multiple comparisons. Multiple comparisons between HK-2 cells at serial concentrations of IS were conducted by using cells without IS as a control. To evaluate the therapeutic effects of drugs, trend analysis was performed excluding cells without IS treatment, and cells exposed to 100 μM IS without medications were used as a control in multiple comparisons. All statistical analyses were performed using SPSS Statistics version 18.0 (IBM corp., Armonk, NY, USA). A two-sided P value <0.05 was considered significant.
Results
Cell viability according to IS exposure in HK-2 cells
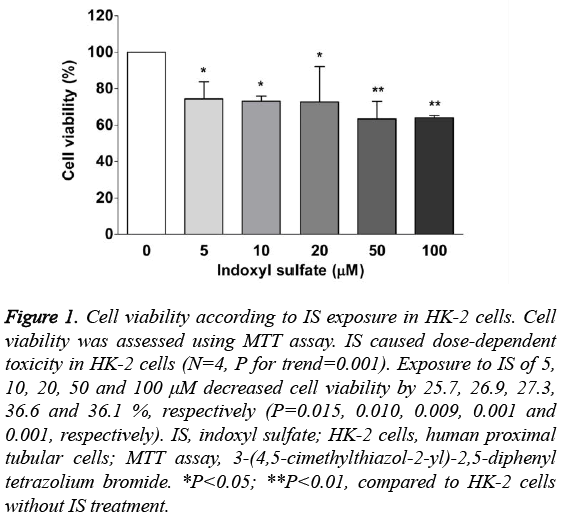
IS-induced cell death was assessed using the MTT assay. As Figure 1 shows, we found that IS had a dose-dependent cytotoxicity in HK-2 cells (N=4, P for trend=0.001). In multiple comparisons, the viability of cells exposed to IS at 5 μM, 10 μM, 20 μM, 50 μM and 100 μM was decreased by 25.7%, 26.9%, 27.3%, 36.6% and 36.1%, compared to cells without IS (all P<0.05).
Figure 1: Cell viability according to IS exposure in HK-2 cells. Cell viability was assessed using MTT assay. IS caused dose-dependent toxicity in HK-2 cells (N=4, P for trend=0.001). Exposure to IS of 5, 10, 20, 50 and 100 μM decreased cell viability by 25.7, 26.9, 27.3, 36.6 and 36.1 %, respectively (P=0.015, 0.010, 0.009, 0.001 and 0.001, respectively). IS, indoxyl sulfate; HK-2 cells, human proximal tubular cells; MTT assay, 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide. *P<0.05; **P<0.01, compared to HK-2 cells without IS treatment.
ROS production after IS exposure in HK-2 cells
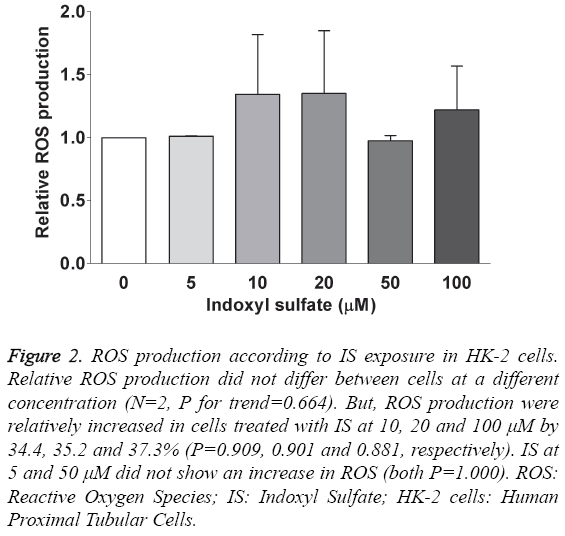
ROS production was measured after 24 hours of exposure at the various concentrations of IS. Figure 2 shows the relative ROS production compared to those without IS exposure. The differences between the groups were not significant (N=2, P for trend=0.664). Exposure to IS at 10 μM, 20 μM and 100 μM insignificantly increased ROS production by 34.4%, 35.2% and 37.3%, respectively (P=0.909, 0.901 and 0.881). We did not see an increase in ROS production at IS concentrations of 5 μM and 50 μM.
Figure 2: ROS production according to IS exposure in HK-2 cells. Relative ROS production did not differ between cells at a different concentration (N=2, P for trend=0.664). But, ROS production were relatively increased in cells treated with IS at 10, 20 and 100 μM by 34.4, 35.2 and 37.3% (P=0.909, 0.901 and 0.881, respectively). IS at 5 and 50 μM did not show an increase in ROS (both P=1.000). ROS: Reactive Oxygen Species; IS: Indoxyl Sulfate; HK-2 cells: Human Proximal Tubular Cells.
Protein expression by exposing to IS in HK-2 cells
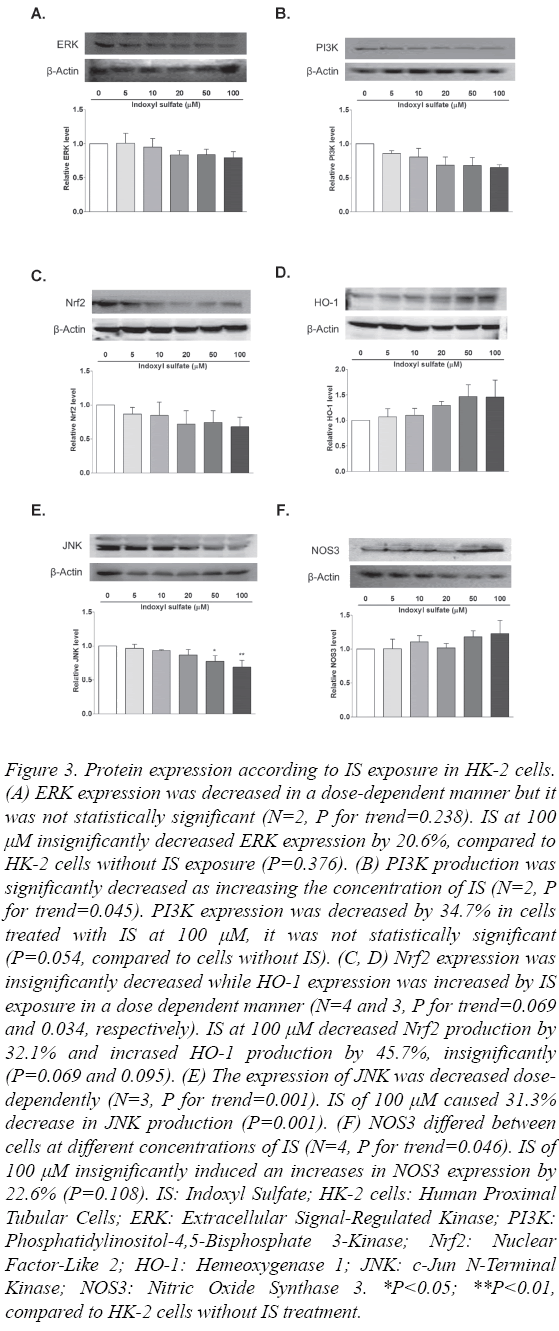
To determine whether the cellular pathways were activated by exposing to IS at the serial concentration, the levels of expression of several proteins including ERK, PI3K, Nrf2, HO-1, JNK and NOS3 were measured using Western blot analysis. The level of ERK expression tended to decrease in a dose-dependent manner, but the difference was not statistically significant (N=2, P for trend=0.238, Figure 3A).
Figure 3: Protein expression according to IS exposure in HK-2 cells. (A) ERK expression was decreased in a dose-dependent manner but it was not statistically significant (N=2, P for trend=0.238). IS at 100 μM insignificantly decreased ERK expression by 20.6%, compared to HK-2 cells without IS exposure (P=0.376). (B) PI3K production was significantly decreased as increasing the concentration of IS (N=2, P for trend=0.045). PI3K expression was decreased by 34.7% in cells treated with IS at 100 μM, it was not statistically significant (P=0.054, compared to cells without IS). (C, D) Nrf2 expression was insignificantly decreased while HO-1 expression was increased by IS exposure in a dose dependent manner (N=4 and 3, P for trend=0.069 and 0.034, respectively). IS at 100 μM decreased Nrf2 production by 32.1% and incrased HO-1 production by 45.7%, insignificantly (P=0.069 and 0.095). (E) The expression of JNK was decreased dosedependently (N=3, P for trend=0.001). IS of 100 μM caused 31.3% decrease in JNK production (P=0.001). (F) NOS3 differed between cells at different concentrations of IS (N=4, P for trend=0.046). IS of 100 μM insignificantly induced an increases in NOS3 expression by 22.6% (P=0.108). IS: Indoxyl Sulfate; HK-2 cells: Human Proximal Tubular Cells; ERK: Extracellular Signal-Regulated Kinase; PI3K: Phosphatidylinositol-4,5-Bisphosphate 3-Kinase; Nrf2: Nuclear Factor-Like 2; HO-1: Hemeoxygenase 1; JNK: c-Jun N-Terminal Kinase; NOS3: Nitric Oxide Synthase 3. *P<0.05; **P<0.01, compared to HK-2 cells without IS treatment.
The level of PI3K expression was measured, and it was found to be significantly decreased in HK-2 cells with exposure to increasing concentrations of IS (N=2, P for trend=0.045, Figure 3B). This result showed that the PI3K-AKT pathway was activated by exposure to IS. IS at 100 μM decreased the PI3K expression by 34.7% but it was not statistically significant (P=0.054).
The Nrf2-keap1 pathway was evaluated by measuring the levels of Nrf2 and HO-1 (Fig. 3-C and 3-D). Exposure of HK-2 cells to IS caused the expression of Nrf2 to be decreased although that was not statistically significant (N=4, P for trend=0.069), while that of HO-1 was increased dosedependently (N=3, P for trend=0.034). However, multiple comparisons analysis did not show significant differences in the expression levels of Nrf2 and HO-1 (67.9% and 145.7% at IS at 100 μM, P=0.069 and 0.095, respectively).
The activation of the JNK pathway was also evaluated. The expression level of JNK in HK-2 cells was decreased in a dosedependent manner (N=3, P for trend=0.001, Figure 3E). Exposing cells to IS at 50 μM and 100 μM resulted in 22.9% and 31.3% decreases in the JNK level (P=0.015 and 0.001, respectively).
We measured the level of NOS3 to determine the impact of IS in HK-2 cells. As Figure 3F shows, we found that the expression of NOS3 differed between the concentrations of IS used (N=4, P for trend=0.046). We found that NOS3 expression increased following IS treatment at 10 μM, 50 μM and 100 μM, but multiple comparisons did not differ significantly (22.6% increase at IS at 100 μM, P=0.108).
Effect of medication on viability in HK-2 cells exposed to IS
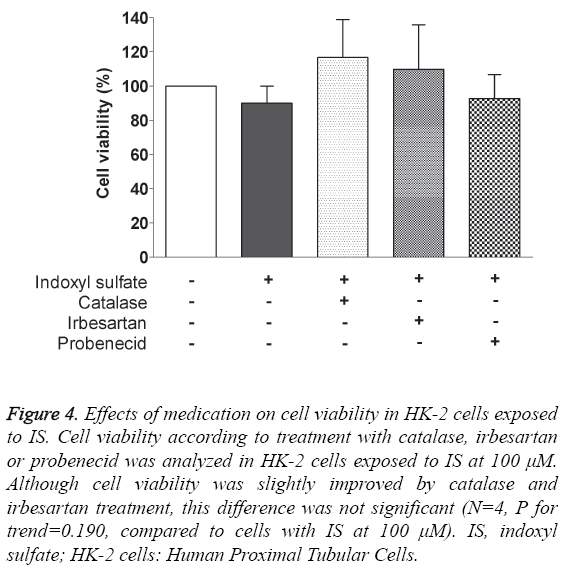
Treatment with catalase, irbesartan and probenecid was performed to determine their influences on IS-induced cell toxicity. As Figure 4 shows, we did not find any statistically significant differences in cell viability (N=4, P for trend=0.190). Although viability was increased by 26.7% and 19.8% in cells treated with catalase and irbesartan, compared to cells treated with 100 μM IS only, those were not statistically significant (P=0.249 and 0.484, respectively).
Figure 4: Effects of medication on cell viability in HK-2 cells exposed to IS. Cell viability according to treatment with catalase, irbesartan or probenecid was analyzed in HK-2 cells exposed to IS at 100 μM. Although cell viability was slightly improved by catalase and irbesartan treatment, this difference was not significant (N=4, P for trend=0.190, compared to cells with IS at 100 μM). IS, indoxyl sulfate; HK-2 cells: Human Proximal Tubular Cells.
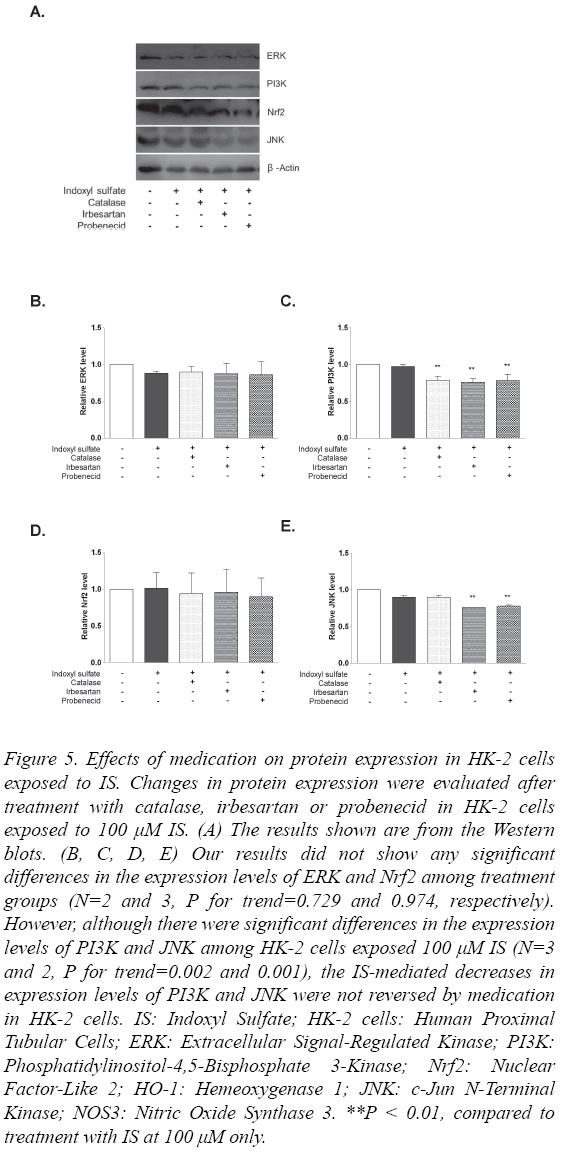
Effect of medication on protein expression in HK-2 cells exposed to IS
To determine changes in protein expression according to medications including catalase, irbesartan and probenecid, the expression levels of ERK, PI3K, Nrf2 and JNK were quantified from lysates of cells that were exposed to these drugs following treatment with 100 μM IS. Figure 5A shows the results of Western blot analysis. Although the expression of ERK was decreased by 100 μM IS treatment, none of the medications reversed this change (N=2, P for trend=0.988, Figure 5B).
Figure 5: Effects of medication on protein expression in HK-2 cells exposed to IS. Changes in protein expression were evaluated after treatment with catalase, irbesartan or probenecid in HK-2 cells exposed to 100 μM IS. (A) The results shown are from the Western blots. (B, C, D, E) Our results did not show any significant differences in the expression levels of ERK and Nrf2 among treatment groups (N=2 and 3, P for trend=0.729 and 0.974, respectively). However, although there were significant differences in the expression levels of PI3K and JNK among HK-2 cells exposed 100 μM IS (N=3 and 2, P for trend=0.002 and 0.001), the IS-mediated decreases in expression levels of PI3K and JNK were not reversed by medication in HK-2 cells. IS: Indoxyl Sulfate; HK-2 cells: Human Proximal Tubular Cells; ERK: Extracellular Signal-Regulated Kinase; PI3K: Phosphatidylinositol-4,5-Bisphosphate 3-Kinase; Nrf2: Nuclear Factor-Like 2; HO-1: Hemeoxygenase 1; JNK: c-Jun N-Terminal Kinase; NOS3: Nitric Oxide Synthase 3. **P < 0.01, compared to treatment with IS at 100 μM only.
Despite the significant difference between cells with IS treatment (N=3, P for trend=0.002, Figure 5C), none of the catalase, irbesartan or probenecid treatments inhibited the decrease in PI3K expression. The Nrf2 levels also did not differ among treatment groups (N=3, P for trend=0.736, Figure 5D). JNK expression differed between cells with IS (N=2, P for trend=0.001). However, as Figure 5E shows, treatment of catalase did not induce the decrease in JNK level although it did not show significance because JNK level in cells with IS only did not decrease.
Discussion
In this study, we found that IS induced dose-dependent cell toxicity in HK-2 cells, which might be related to increased production of ROS. We investigated the relevant signalling pathways and our results showed decreases in the expression levels of ERK, PI3K, Nrf2 and JNK, and an increase in the expression of HO-1 following IS treatment. We further analysed the effects of medications, such as catalase and irbesartan, on cells exposed to IS. Despite slight improvements in cell viability by catalase and irbesartan, we could not show any significant changes in protein expression.
IS is a protein-bound uremic toxin derived from dietary proteins. Tryptophan is metabolized into indole by tryptophanase in intestinal bacteria. Then, indole is absorbed into the blood and metabolized into IS by sulfate conjugation in the liver [21]. Circulating IS is excreted via organic anion transporters in proximal tubular cells and therefore, IS accumulates in CKD patients as renal function decreases [22,23]. Marked increases in IS in uremic rats and uremic patients were previously demonstrated [8,24]. Moreover IS has a substantial role in CKD progression. Niwa et al. [11] evaluated the association of IS levels with the progression of kidney disease and showed that patients with high levels of IS experienced more rapid progression of CKD than did those with low levels of IS. Despite the close relationship between IS and CKD progression, our understanding of IS-related molecular pathways in kidney cells remains insufficient. Furthermore, current therapeutic options regarding IS include only disturbance of its intestinal absorption, but do not target specific molecular pathways.
IS-induced oxidative stress may be a major mediator of CKD progression [18,25-27]. A study by Palm et al. [26] showed that the abnormal oxygen metabolism mediated by IS-induced oxidative stress could lead to renal deterioration, and that the reduction in serum IS level related to treatment with AST-120 improved oxygenation of the kidney. Previous studies with Nacetylcysteine as an antioxidant demonstrated a reversal of the protein expression induced by IS [17,28]. Unfortunately, our results could not show direct impact of IS exposure on ROS production. Although we used 100 μM IS as the maximum concentration because a previous study reported that the mean concentration of IS in uremic patients is 23.1 mg/L (~100 μM) [29], a concentration of IS might be insufficient to induce ROS in HK-2 cells. A previous study used higher concentration of IS to show ROS production in HK-2 cells [18]. In addition, a small number of experiments or inadequate experimental settings might be limited to find differences.
We investigated the expression of relevant proteins to understand the cellular pathways induced by IS. This study confirmed that IS treatment decreased Nrf2 expression and increased HO-1 expression, indicating that the Nrf2-keap1 pathway was activated. Until recently, it was uncertain whether the Nrf2-keap1 system, which plays a crucial role in cellular defense against oxidative stress, was included in uremic toxinsinduced renal dysfunction [30]. A recent report by Bolati et al. [31] found that IS downregulated Nrf2 expression in HK-2 cells and decreased expression of Nrf2 and HO-1 in CKD rats. Our results supported this earlier finding. To explain the differences between our results and those by Bolati et al. regarding expression of HO-1, we suggest that the decreased expression of HO-1 in CKD rats might have resulted from increased consumption of this protein to defend against oxidative stress.
Our findings regarding the activation of the ERK and JNK pathways are in parallel with those of previous reports [15,19]. In contrast, our results on the activation of the PI3K-AKT pathway differed with those of previous reports [32,33]. A connection between ROS and PI3K-AKT activation has been described previously [34-36]. However, how this pathway acts and its cross-talk with other pathways, such as the ERK pathway, in renal cells exposed to IS remaining unclear.
We also found that the expression of NOS3, also known as endothelial NOS, was increased by IS. While excessive production of NO by inducible NOS is associated with increased glomerular injury, NO derived from endothelial NOS preserves endothelial integrity and may limit glomerular disease [37]. In CKD patients, the capacity of NO production is impaired because of reduced expression of NOS3 [38]. Although the role of NOS3 in proximal tubular cell is still not elucidated [39], this study showed that IS might activate the NOS3 system in HK-2 cells. We assumed that the downregulation of NOS3 is caused by increasing consumption of this protein to protect against IS, according to our results. In addition, a previous report found that AST-120 increased NO production by increasing the expression of glomerular endothelial NOS and tubulointerstitial neuronal NOS in uremic rats [40].
AST-120, which is an oral adsorbent for removing uremic toxins, is currently the most investigated treatment and has been demonstrated to significantly retard CKD progression [24,41,42]. Moreover, AST-120 reduced oxidative stress in experimental studies [43,44], which could be a result of lowered circulating IS levels. Besides AST-120, a few drugs have shown some potential to prevent IS-induced cell injury [32,45]. However, most studies, excluding some with AST-120, have provided only preliminary data, and more studies are needed in order to find novel therapeutic options. Therefore, we evaluated the therapeutic effects of antioxidants and angiotensin receptor blockers in IS-induced cell damage. In cells exposed to 100 μM IS, which is the mean concentration of IS in uremic patients [29], catalase and irbesartan were added.
Despite the important role of oxidative stress, there are few data regarding the improvement of viability using antioxidants. Thus, this study evaluated the therapeutic effect of catalase, an antioxidant, to determine its effect on cell viability in HK-2 cells exposed to IS. Because catalase cannot enter the cell, this study used catalase conjugated to polyethylene glycol, which enhances cell binding of normally membrane-impermeable enzymes [46]. However, we did not find any significant changes with this drug, even though there was a trend of a slight increase in cell viability. Several studies have suggested that a reduction in oxidative stress can be helpful to suppress IS-induced renal cell injury [17,28,47].
Previous clinical studies have proposed that there may be pleiotropic effects of angiotensin II receptor blockers beyond the blood pressure-lowering effect [48,49]. Moreover, some in vitro studies have shown that those drugs might have protective effects for renal cell injury which were induced by toxins such as transforming growth factor-β1 and advanced glycation end product [50,51]. We evaluated the therapeutic effect of irbesartan, an angiotensin II receptor blocker, but we did not find any significance for this drug in IS-induced cell injury. Although we evaluated a pleiotropic effect, treating with irbesartan alone could be another problem without the evidence that IS independently activates the antiotensin II receptor. On the other hand, Sun et al. [20] found that activating the renal renin-angiotensin system might have an important role in ISinduced kidney injury, and that losartan could attenuate the renal fibrosis. Thus, treatment with an angiotensin receptor blocker may be a candidate for retarding CKD progression induced by IS.
The interpretation of our results regarding medication were limited because we could not show significant effects with probenecid, which interrupts IS entry into cells by blocking an organic anion transporter [15,22]. However, blocking IS transport may be an issue to be investigated. Since the entry of IS via an organic anion transporter demands energy and makes decrease in the energy level, the capacity of IS transport may be decreased in HK-2 cells. There should be another in vitro research to explain whether the capacity of an organic anion transporter changes according to IS treatment, by comparing IS-treated cells with untreated cells.
This study has several limitations. First, the power is limited because the experiment could not be repeated enough times. Nevertheless, our results, especially regarding cell viability and protein expression according to dose of IS, showed consistent trends. Despite the limited power, those findings might still be noteworthy. Second, because we did not assess the time dependence of viability and protein expression, changes according to IS exposure by time could not be analyzed. However, this study focused on investigating the IS-related signaling mechanisms and the therapeutic effects of drugs on cell viability, so the time from a pilot study was selected to be used in all of the experiments. Third, this study was performed entirely in vitro. We were not able to expand our findings to an in vivo study.
Conclusion
This study showed that IS had a dose-dependent cytotoxicity in HK-2 cells, which might be related to increased ROS production. The mechanism for this cytotoxicity might include activation of the ERK, PI3K-AKT, Nrf2-keap1 and JNK pathways, in addition to activation of NOS3. We did not find any significant effects of medication on viability and protein expression in cells treated with IS. Despite these negative results, we suggest that cell viability could be improved by treatment with antioxidants and angiotensin-receptor blockers. We expect that more therapeutic options for IS-induced CKD progression will be developed, and our findings can form the basis for further in vitro and in vivo research into IS-related renal damage.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (No. NRF - 2012R1A1A1011816).
References
- Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004; 351: 1285-1295.
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004; 351: 1296-1305.
- Karayakali M, Ozbek K, Altunkas A, Karaman K, Altunkas F, Celik A. Contrast-induced nephropathy: focused on risk factors. Acta Med Anatol. 2014; 2: 34-36.
- Kurt H, Yavuz T, Toprak Ö, Demirkıran D. The relationship of ABO Blood groups with chronic renal failure. Eur J Health Sci. 2015; 1: 109-113.
- Vanholder R, De Smet R, Glorieux G, Argiles A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jorres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W, European Uremic Toxin Work Group. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003; 63: 1934-1943.
- Liabeuf S, Drueke TB, Massy ZA. Protein-bound uremic toxins: new insight from clinical studies. Toxins (Basel). 2011; 3: 911-919.
- Meijers BK, Evenepoel P. The gut-kidney axis: indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrol Dial Transplant. 2011; 26: 759-761.
- Niwa T, Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J Lab Clin Med. 1994; 124: 96-104.
- Miyazaki T, Ise M, Seo H, Niwa T. Indoxyl sulfate increases the gene expressions of TGF-beta 1, TIMP-1 and pro-alpha 1(I) collagen in uremic rat kidneys. Kidney Int Suppl. 1997; 62: S15-22.
- Niwa T. Organic acids and the uremic syndrome: protein metabolite hypothesis in the progression of chronic renal failure. Semin Nephrol. 1996; 16: 167-182.
- Niwa T, Aoyama I, Takayama F, Tsukushi S, Miyazaki T, Owada A, Shiigai T. Urinary indoxyl sulfate is a clinical factor that affects the progression of renal failure. Miner Electrolyte Metab. 1999; 25: 118-122.
- Nakamura T, Kawagoe Y, Ueda Y, Ebihara I, Koide H. Effects of oral adsorbent AST-120 in patients with chronic renal failure with or without diabetes. Ren Fail. 2004; 26: 99-101.
- Miyazaki T, Ise M, Hirata M, Endo K, Ito Y, Seo H, Niwa T. Indoxyl sulfate stimulates renal synthesis of transforming growth factor-beta 1 and progression of renal failure. Kidney Int Suppl. 1997; 63: S211-214.
- Shimizu H, Yisireyili M, Nishijima F, Niwa T. Stat3 contributes to indoxyl sulfate-induced inflammatory and fibrotic gene expression and cellular senescence. Am J Nephrol. 2012; 36: 184-189.
- Kim SH, Yu MA, Ryu ES, Jang YH, Kang DH. Indoxyl sulfate-induced epithelial-to-mesenchymal transition and apoptosis of renal tubular cells as novel mechanisms of progression of renal disease. Lab Invest. 2012; 92: 488-498.
- Bolati D, Shimizu H, Higashiyama Y, Nishijima F, Niwa T. Indoxyl sulfate induces epithelial-to-mesenchymal transition in rat kidneys and human proximal tubular cells. Am J Nephrol. 2011; 34: 318-323.
- Shimizu H, Bolati D, Adijiang A, Muteliefu G, Enomoto A, Nishijima F, Dateki M, Niwa T. NF-kappaB plays an important role in indoxyl sulfate-induced cellular senescence, fibrotic gene expression, and inhibition of proliferation in proximal tubular cells. Am J Physiol Cell Physiol. 2011; 301: C1201-1212.
- Motojima M, Hosokawa A, Yamato H, Muraki T, Yoshioka T. Uremic toxins of organic anions up-regulate PAI-1 expression by induction of NF-kappaB and free radical in proximal tubular cells. Kidney Int. 2003; 63: 1671-1680.
- Shimizu H, Bolati D, Higashiyama Y, Nishijima F, Shimizu K, Niwa T. Indoxyl sulfate upregulates renal expression of MCP-1 via production of ROS and activation of NF-kappaB, p53, ERK, and JNK in proximal tubular cells. Life Sci. 2012; 90: 525-530.
- Sun CY, Chang SC, Wu MS. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS One. 2012; 7: e34026.
- Vanholder R, De Smet R. Pathophysiologic effects of uremic retention solutes. J Am Soc Nephrol. 1999; 10: 1815-1823.
- Deguchi T, Ohtsuki S, Otagiri M, Takanaga H, Asaba H, Mori S, Terasaki T. Major role of organic anion transporter 3 in the transport of indoxyl sulfate in the kidney. Kidney Int. 2002; 61: 1760-1768.
- Enomoto A, Takeda M, Tojo A, Sekine T, Cha SH, Khamdang S, Takayama F, Aoyama I, Nakamura S, Endou H, Niwa T. Role of organic anion transporters in the tubular transport of indoxyl sulfate and the induction of its nephrotoxicity. J Am Soc Nephrol. 2002; 13: 1711-1720.
- Niwa T, Nomura T, Sugiyama S, Miyazaki T, Tsukushi S, Tsutsui S. The protein metabolite hypothesis, a model for the progression of renal failure: an oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int Suppl. 1997; 62: S23-28.
- Gelasco AK, Raymond JR. Indoxyl sulfate induces complex redox alterations in mesangial cells. Am J Physiol Renal Physiol. 2006; 290: F1551-1558.
- Palm F, Nangaku M, Fasching A, Tanaka T, Nordquist L, Hansell P, Kawakami T, Nishijima F, Fujita T. Uremia induces abnormal oxygen consumption in tubules and aggravates chronic hypoxia of the kidney via oxidative stress. Am J Physiol Renal Physiol. 2010; 299: F380-386.
- Shimizu H, Bolati D, Adijiang A, Enomoto A, Nishijima F, Dateki M, Niwa T. Senescence and dysfunction of proximal tubular cells are associated with activated p53 expression by indoxyl sulfate. Am J Physiol Cell Physiol. 2010; 299: C1110-1117.
- Saito S, Yisireyili M, Shimizu H, Ng HY, Niwa T. Indoxyl sulfate upregulates prorenin expression via nuclear factor-kappaB p65, signal transducer and activator of transcription 3, and reactive oxygen species in proximal tubular cells. J Ren Nutr. 2015; 25: 145-148.
- Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A, European Uremic Toxin Work G. Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012; 23: 1258-1270.
- Stockler-Pinto MB, Fouque D, Soulage CO, Croze M, Mafra D. Indoxyl sulfate and p-cresyl sulfate in chronic kidney disease. Could these toxins modulate the antioxidant Nrf2-Keap1 pathway? J Ren Nutr. 2014; 24: 286-291.
- Bolati D, Shimizu H, Yisireyili M, Nishijima F, Niwa T. Indoxyl sulfate, a uremic toxin, downregulates renal expression of Nrf2 through activation of NF-kappaB. BMC Nephrol. 2013; 14: 56.
- Wang WJ, Chang CH, Sun MF, Hsu SF, Weng CS. DPP-4 inhibitor attenuates toxic effects of indoxyl sulfate on kidney tubular cells. PLoS One. 2014; 9: e93447.
- Adelibieke Y, Shimizu H, Saito S, Mironova R, Niwa T. Indoxyl sulfate counteracts endothelial effects of erythropoietin through suppression of Akt phosphorylation. Circ J. 2013; 77: 1326-1336.
- Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000; 275: 14624-14631.
- Kwon SH, Pimentel DR, Remondino A, Sawyer DB, Colucci WS. H(2)O(2) regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. J Mol Cell Cardiol. 2003; 35: 615-621.
- Silva A, Girio A, Cebola I, Santos CI, Antunes F, Barata JT. Intracellular reactive oxygen species are essential for PI3K/Akt/mTOR-dependent IL-7-mediated viability of T-cell acute lymphoblastic leukemia cells. Leukemia. 2011; 25: 960-967.
- Trachtman H. Nitric oxide and glomerulonephritis. Semin Nephrol. 2004; 24: 324-332.
- Vaziri ND, Ni Z, Wang XQ, Oveisi F, Zhou XJ. Downregulation of nitric oxide synthase in chronic renal insufficiency: role of excess PTH. Am J Physiol. 1998; 274: F642-649.
- Wang T. Role of iNOS and eNOS in modulating proximal tubule transport and acid-base balance. Am J Physiol Renal Physiol. 2002; 283: F658-662.
- Tumur Z, Niwa T. Oral sorbent AST-120 increases renal NO synthesis in uremic rats. J Ren Nutr. 2008; 18: 60-64.
- Schulman G, Agarwal R, Acharya M, Berl T, Blumenthal S, Kopyt N. A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study of AST-120 (Kremezin) in patients with moderate to severe CKD. Am J Kidney Dis. 2006; 47: 565-577.
- Shoji T, Wada A, Inoue K, Hayashi D, Tomida K, Furumatsu Y, Kaneko T, Okada N, Fukuhara Y, Imai E, Tsubakihara Y. Prospective randomized study evaluating the efficacy of the spherical adsorptive carbon AST-120 in chronic kidney disease patients with moderate decrease in renal function. Nephron Clin Pract. 2007; 105: c99-107.
- Nakagawa N, Hasebe N, Sumitomo K, Fujino T, Fukuzawa J, Hirayama T, Kikuchi K. An oral adsorbent, AST-120, suppresses oxidative stress in uremic rats. Am J Nephrol. 2006; 26: 455-461.
- Owada S, Maeba T, Sugano Y, Hirayama A, Ueda A, Nagase S, Goto S, Nishijima F, Bannai K, Yamato H. Spherical carbon adsorbent (AST-120) protects deterioration of renal function in chronic kidney disease rats through inhibition of reactive oxygen species production from mitochondria and reduction of serum lipid peroxidation. Nephron Exp Nephrol. 2010; 115: e101-111.
- Takayama F, Taki K, Niwa T. Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am J Kidney Dis. 2003; 41: S142-145.
- Beckman JS, Minor RL, Jr., White CW, Repine JE, Rosen GM, Freeman BA. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J Biol Chem. 1988: 263(14): 6884.
- Chao CT, Chiang CK. Uremic toxins, oxidative stress, and renal fibrosis: an interwined complex. J Ren Nutr. 2015; 25: 155-159.
- Lewis EJ, Hunsicker LG, Clarke WR. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001: 345: 851.
- Parving HH, Lehnert H, Brochner-Mortensen J. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001: 345: 870.
- Matsui T, Yamagishi S, Takeuchi M, Ueda S, Fukami K, Okuda S. Irbesartan inhibits advanced glycation end product (AGE)-induced proximal tubular cell injury in vitro by suppressing receptor for AGEs (RAGE) expression. Pharmacol Res. 2010: 61: 34.
- Chen Y, Luo Q, Xiong Z, Liang W, Chen L, Xiong Z. Telmisartan counteracts TGF-beta1 induced epithelial-to-mesenchymal transition via PPAR-gamma in human proximal tubule epithelial cells. Int J Clin Exp Pathol. 2012: 5: 522.