ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 7
Clinical comparison of hand-assisted laparoscopic surgery and traditional laparotomy in radical resection of colorectal cancer
Department of Anorectal Surgery, Lishui People's Hospital, Lishui, PR China
- *Corresponding Author:
- Jingquan Guo
Department of Anorectal Surgery
Lishui People's Hospital
The Sixth Affiliated Hospital of Wenzhou Medical University, PR China
Accepted on December 27, 2016
Background: Colorectal cancer is a common malignant tumor of the digestive system. Minimally invasive surgeries, with Hand-Assisted Laparoscopic Surgery (HALS) as a representative technology, have challenged Traditional Laparotomy (TL). Some studies indicated that HALS has an apparent advantage in terms of intestinal function recovery, complications, and other parameters.
Objective: In this study, we aimed to compare the clinical effects of HALS and TL in radical resection of colorectal cancer, in order to investigate the feasibility of HALS in colorectal cancer resection.
Methods: We retrospectively analysed the clinical data on HALS and TL at our hospital from 2009 to 2011 (28 rectal carcinoma patients underwent laparoscopic surgery and 32 rectal cancer patients underwent traditional open surgery), and compared the differences in operative time, blood loss, number of dissected mesenteric lymph nodes, intestinal function recovery time, postoperative hospital stay, and postoperative complication incidence.
Results: The HALS group exhibited less blood loss, shorter intestinal function recovery time, and shorter postoperative hospital stay than the TL group (intraoperative blood loss: 80.5 ± 13.3 ml vs. 170.5 ± 35.5 ml, t=12.650, p-value<0.05; intestinal function recovery time: 45.4 ± 15.8 h vs. 89.5 ± 13.3 h, t=11.750, p-value<0.05; postoperative hospitalization stay: 8.2 ± 2.0 days vs. 11.6 ± 2.0 days, t=6.518, pvalue< 0.05). However, the difference in operative time, number of intraoperative lymph nodes dissected, and postoperative complication incidence between the two groups was not significant (p-value>0.05).
Conclusions: HALS showed the advantages of less bleeding, quicker recovery, and minimal invasiveness over TL. Moreover, it did not increase the operative time or postoperative complications. HALS could achieve the same results as those obtained with TL in radical resection.
Keywords
Hand-assisted laparoscopy, Colorectal cancer, Rectal resection.
Introduction
Laparoscopic surgery has a long history, and its application has matured in multiple areas of surgery. In colorectal cancer surgery, especially in Total Mesorectal Excision (TME) for low colorectal cancer, laparoscopy has not been popular because of the difficulty of the procedure [1-3]. However, this surgery has evolved rapidly in recent years, and has been included in the Non-Small Cell Lung Cancer guidelines. Laparoscopic colorectal cancer resection has a long learning curve; however, hand-Assisted Laparoscopic Surgery (HALS) could overcome some of the challenges and achieve the same results with minimal invasiveness [4-6]. HALS is a minimally invasive surgical approach developed in the 1990s, in which surgeons use hand-assisted instruments through small abdominal wall incisions to maintain pneumoperitoneum, and place their hands into the abdominal cavity of patients to manipulate laparoscopic instruments, enabling the completion of complex surgical procedures. The learning curve is shorter because tactile sensation is involved. Although HALS has the specific advantage of being minimally invasive [7-9], it is not clear whether it could also achieve the same radical resection results as those obtained with Traditional Laparotomy (TL) [10]. In this study, we compared and analysed the clinical data for HALS and TL in colorectal cancer resection, through a comparison of the differences between the two methods in operative time, blood loss, number of dissected mesenteric lymph nodes, intestinal function recovery time, postoperative hospital stay, and the incidence of postoperative complications, with the aim of determining the advantages and investigating the feasibility of HALS in colorectal cancer resection [11-14]. We retrospectively analysed the clinical surgical data for 60 cases of colorectal cancer resection performed during the same period (pathological staging included Dukes’ A or B without lymph node metastasis), to compare the clinical effects of HALS and TL in radical resection of colorectal cancer.
Methods
Patient information
We preoperatively diagnosed all patients as having rectal adenocarcinoma by using electronic colonoscopy and biopsy. During preoperative evaluation, we allocated the patients to either of the two groups, according to their preference. The HALS group included 28 cases and the TL group included 32 cases (Table 1). There was no significant difference in sex, age, Dukes’ stage, tumor size, tumor-anus distance, or comorbidities (cardiac functional grade III or above or severe respiratory dysfunction), etc., between the two groups (p>0.05), and they were considered comparable. This study was conducted in accordance with the declaration of Helsinki. Our study was conducted with approval from the Ethics Committee of Lishui People's Hospital. Written informed consent was obtained from all participants.
| Group | Gender | Age (years) | Dukes staging | Tumor size (cm) | Tumor-anus distance (cm) | Complications | ||
|---|---|---|---|---|---|---|---|---|
| M | F | A | B | |||||
| HALS (n=28) | 42.86 (12) | 57.14 (16) | 59.7 ± 11.4 | 17.86% (5) | 82.14% (23) | 4.48 ± 1.24 | 9.25 ± 1.62 | 10.7% (3) |
| TL (n=32) | 50% (16) | 50% (16) | 55.5 ± 12.6 | 21.88% (7) | 78.12% (25) | 5.12 ± 1.28 | 8.36 ± 1.81 | 9.4% (3) |
| T (χ2) | χ2=0.306 | t=1.404 | χ2=0.151 | t=-1.960 | t=1.995 | χ2=0.000 | ||
| P | 0.58 | 0.166 | 0.698 | 0.055 | 0.051 | 1 | ||
Table 1. General information of the two groups (counted data were compared with the χ2 test).
Operation
The same physicians operated on all patients from the two groups under general anesthesia. The patients were in the head low-foot high lithotomy position; however, those in the HALS group were slightly tilted toward the right.
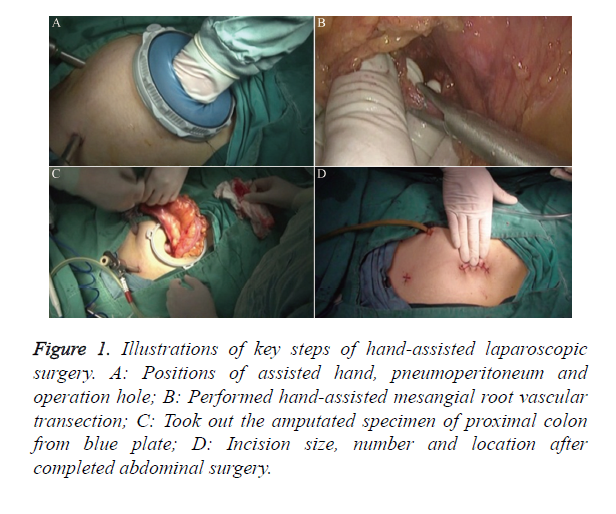
Operative method for the HALS group: Position the hand-assisted device before establishing pneumoperitoneum; perform a 5-7 cm median periumbilical incision; enter the abdomen by layers; position the hand-assisted device (blue plate); place a 10 mm trocar into the hand-assisted device looper as the exploration mouth; tighten the looper; establish pneumoperitoneum with a pressure of 14 mmHg (1 mmHg=0.133 kPa); conventionally explore the abdominal cavity to determine the tumor size, location, presence or absence of multiple foci and invasion degree, intra-abdominal seeding or organ metastasis, presence or absence of enlarged lymph nodes at the mesenteric vascular roots, and other parameters. After exploration, reverse the pneumoperitoneum and place a piece of gauze inside the abdominal cavity; insert the nondominant surgical hand into the abdominal cavity through the blue plate; prepare the laparoscopic port with a 10 mm trocar above the pubis; re-establish the pneumoperitoneum; place a 10 mm trocar at the corresponding position on the lower right abdominal wall; insert the operating forceps or ultrasonic knife through the operating port (Figure 1A); place a 5 mm trocar in the left lower abdomen as a secondary operating port if needed. Under monitored guidance, use the hand-assisted ultrasonic knife for dissection, with attention to the correctness of the operative plane; small blood vessels can be cut directly with the ultrasonic knife, whereas great vessels should be clamped first, then dissociated (Figure 1B); perform sigmoid and rectal resection; dissect lymph nodes from submesenteric vascular roots; isolate the inferior mesenteric artery and vein, with care to protect the ureters; ligate and cut free from the vascular roots; free the sigmoid mesentery, with the freed proximal end about 10 cm distant from the tumor; the distal end should be freed up to the rear part and bilateral mesentery of the rectum; flatly expose the mesorectum from approximately 5 cm to the lower edge of the tumor; follow the principles of TME; cut and close the distal rectum with endoscopic linear cutter-stapler and staple cartridge (Echelon 60, USA Ethicon Endo-Surgery). Remove the proximal bowel through the blue plate; resect the specimens in vitro (Figure 1C); place the nail set of the end-to-end Anastomat into the proximal sigmoid (Covidien EEA Autosuture Circular Stapler, Tyco International Ltd., USA); insert the Anastomat through the anus; complete the anastomosis intraperitoneally; reestablish pneumoperitoneum after completing the anastomosis; observe whether the mesentery is rotated; perform haemostasis and rinse the abdominal cavity; place the drainage tube; and close the abdomen by layers (Figure 1D).
Figure 1. Illustrations of key steps of hand-assisted laparoscopic surgery. A: Positions of assisted hand, pneumoperitoneum and operation hole; B: Performed hand-assisted mesangial root vascular transection; C: Took out the amputated specimen of proximal colon from blue plate; D: Incision size, number and location after completed abdominal surgery.
Operative method for the TL group: Perform conventional colorectal cancer radical surgery, following the principles of TME and tumor radical resection, and use the end-to-end Anastomat.
Observation indicators
We estimated the intraoperative blood loss from the amount in the blood drainage bottles and blood absorbed by gauze. We recorded the operative time, number of dissected lymph nodes, intestinal function recovery time (time to evacuation), and postoperative hospital stay. We considered the patients eligible for discharge when normal flatus and defecation had returned, a normal diet was resumed, and symptoms had improved. We also recorded the occurrence of postoperative ileus during hospitalization, incidence of anastomotic leakage, abdominal and wound infections, and urinary retention.
Statistical analysis
We used SPSS 17.0 software for statistical analysis. We expressed the measured data as ͞x ± s, and performed intergroup comparison by using the t-test. We compared the counted data with the χ2 test, and considered p<0.05 as statistically significant.
Results
We successfully performed HALS surgery in all patients (n=28) of the HALS group. The operative time, postoperative complication rate, and number of lymph nodes dissected for the two groups showed no significant difference (p>0.05). However, the HALS group exhibited obvious advantages with regard to the amount of intraoperative bleeding, intestinal function recovery time, and postoperative hospital stay (p<0.05). The postoperative pathological TNM staging of the two groups was T1-4N0M0 (Dukes’ stage A or B). One case of intestinal obstruction in each group improved with conservative treatment. Anastomotic leakage did not occur in either group. The TL group had two cases of wound infection, which healed after the wound was opened and drained. One patient in the TL group had urinary retention after catheter removal; this patient was discharged after being recatheterized and returned to our hospital 1 month later for catheter removal (Table 2).
| Group | Intraoperative bleeding (ml) | Operative time (min) | Intestinal function recovery time (h) | Postoperative hospital stay (d) | Postoperative complication rate (%) | Number of lymph node dissection |
|---|---|---|---|---|---|---|
| HALS (n=28) | 80.5 ± 13.3 | 165.5 ± 40.9 | 45.4 ± 15.8 | 8.2 ± 2.0 | 3.6 (1/28) | 9.0 ± 4.5 |
| TL (n=32) | 170.5 ± 35.5 | 152.7 ± 30.5 | 89.5 ± 13.3 | 11.6 ± 2.0 | 12.5 (4/32) | 11.0 ± 4.3 |
| t (χ2) | t=-12.650 | t=1.388 | t=-11.750 | t=-6.518 | χ2=0.609 | t=-1.760 |
| P | <0.05 | 0.17 | <0.05 | <0.05 | 0.435 | 0.084 |
Table 2. Comparison of intraoperative and postoperative indexes between the 2 groups (counted data were compared with the χ2 test).
Discussion
Laparoscopic-Assisted Colectomy (LAC) has been used in the treatment of colorectal cancer for many years, and is now a mature procedure. Compared with laparotomy, LAC has the advantage of a shorter hospital stay and faster postoperative recovery, and is minimally invasive. A recent consensus revealed that LAC even had a better cosmetic result. However, the operative techniques are complex and the surgical time is longer. LAC has the added disadvantages of lacking tactile sensation and a long learning curve. HALS compensated for the above deficiencies, and achieved similar results with minimal invasiveness [15].
HALS has shown advantages in radical resection of colorectal cancer. Because of the involvement of the surgeon’s hand, the tactile sensation inside the abdominal cavity is similar to that in conventional laparotomy, thus reducing the difficulty of complex laparoscopic procedures [16,17]. The mean operative time of this study was 165.5 min, with intraoperative blood loss of 80.5 ml. HALS also maintains the advantages of laparoscopic surgery; by amplifying the surgical field and providing better exposure of blood vessels and nerves, it achieves minimal invasion. HALS had resulted in complete tumor resection and normative lymph node dissection, with results much closer to those seen in TL. The largest number of lymph nodes dissected in this study was 23 in one patient, with an average of 9, which is fewer than the average number (n=15) reported in the literature [18]. HALS can significantly improve lymph node dissection in the enterocelia of patients with gastric cancer and in the pelvic cavity of patients with gynaecologic tumors. A possible reason might be the limited surgeon experience with HALS. We sampled the pathological specimens, and the postoperative staging was Dukes’ A or B in all cases. Concerning lymph node dissection, some authors believe that the number of nodes obtained is the same between rectal laparoscopic surgery and HALS [5]. Targarona performed statistical analysis on the oncology-related indices of these two procedures, and found no significant difference [19]. HALS reduces the rate of intraoperative conversion to laparotomy. When inadvertent surgical injuries occur, timely and accurate management is possible. The surgeon can easily control a major bleeding point by using the hand, thus avoiding emergent conversion to laparotomy [19,20]. HALS can take full advantage of the blue plate to place the incision. Performing mesenteric root lymph node dissection is sometimes difficult. If necessary, an incision can be directly performed from the blue plate for open mesenteric lymph node dissection, in order to simplify the surgery [21-24].
Certain considerations are necessary when HALS is used for colorectal cancer resection. To avoid seeding at the abdominal incision, suture the puncture cannula onto the abdominal wall; do not directly clamp the tumor; remove the specimens from the center hole of the blue plate; first reverse the pneumoperitoneum, then disconnect the cannula; after removing the specimens, use saline to rinse the abdominal cavity and the incision; perform repeat rinsing after removing the blue plate. Use the unipolar electric shear to dissect tissues quickly and accurately. This is much closer to the traditional electric knife, and does not result in injuries under the guidance of hand assistance, thus shortening the operation time. The ultrasonic knife can be used to dissect thicker tissues, as well as manage small blood vessels, with good haemostatic effects. In this study, no ureter or bowel injury or haemorrhage occurred. If haemorrhage occurs, the surgeon should calmly use the assisting hand to control the bleeding and then perform haemostasis. In most situations, this should achieve satisfactory results. The trocar position and the HALS incision are not static, and require continuous experimentation and practice. The best approach is to make decisions according to the actual clinical situation. HALS takes full advantage of the multiple uses of hand-assisted devices, which can be utilized through the initial abdominal exploration port. This can assist anatomic dissection and vascular ligation under direct vision, as well as assist the dissection of regional lymph nodes, removal of resected specimens, and completion of the anastomosis [25-27].
Our results showed that HALS has obvious advantages over TL in the treatment of colorectal cancer, mainly in four aspects: amount of bleeding, operation time, recovery of postoperative intestinal function, and hospital stay after the operation [4,8]. HALS cannot increase the numbers of dissected lymph nodes or improve the incidence rate of postoperative complications; however, it could significantly improve lymph node dissection in the enterocelia of patients with gastric cancer and pelvic cavity of patients with gynaecologic tumors [28,29]. The main reasons maybe as follows: (i) the colorectum have a full set of intestinal mesangial colorectal in dissection, and lymphatic metastasis is always detected in the mesentery when tumors are found within the lumen. TME, TL, HALS, and total laparoscopic surgery are mature procedures, and therefore these methods show no significant difference in the numbers of dissected lymph nodes. (ii) The number of surgeries with HALS is limited in our laboratory, which may be a reason for the differences with previous studies. Generally, the incidence of complications has close relationships with the preoperative neoplasm staging, tumor location, metastasis, nutrient status, postoperative preventive colostomy, neoadjuvant chemoradiotherapy, chemoradiotherapy, and economic conditions [30-32]. Moreover, we did not divide the difference of these factors before the operation, which is also limitation of this study and needs further investigation.
HALS also has some disadvantages. With a pneumoperitoneum established, the surgeon might partly fill the abdominal cavity by using the hand, and the field of vision might not be as wide as with traditional laparoscopy. Moreover, the hand of the surgeon might interfere with the laparoscope and the operating clamp, and this requires a period of adjustment. HALS requires a period for learning, and the mastery of flexible and delicate finger movements requires experience. HALS necessitates the use of the blue plate, ultrasonic knife, and other equipment; thus, the treatment costs are higher than for TL [33,34].
In contrast to full laparoscopic colorectal cancer radical resection, an incision is made in the beginning of HALS. Therefore, many experts believe that this is not in keeping with the principles of laparoscopy or the concept of minimal invasion; however, as complete laparoscopic rectal tumor specimens could only be removed after extending the trocar penetration, the incision sizes then become comparable. Therefore, establishing a small incision in the initial stage of surgery should not increase the wound size. The surgery can still be assisted by hand, thus reducing the incidence of conversion resulting from the use of laparoscopic instruments. HALS could be particularly useful for laparoscopy novices. Compared with laparotomy, HALS has the advantages of less bleeding, faster intestinal function recovery, shorter hospital stay, and minimal invasion. HALS can achieve the same result in tumor radical resection as TL [35,36]. HALS has become a useful technique with broader applications and prospects in minimally invasive surgery for colorectal cancer [37-40].
Conclusion
With the development of minimally invasive surgery, HALS will be more and more popular in colorectal cancer surgery. Our experience and research suggest that HALS has advantages over TL in terms of surgical trauma, intraoperative bleeding, and postoperative rehabilitation, which the hospital has, no total laparoscopic operation. Concerning the learning curve, however, the laparoscopic surgical field and equipment costs still need to be further improved. We believe that with further research and improvement, gasless laparoscopic technology will have an important role in the treatment of colorectal tumors in the future.
Conflicts of Interest
Disclosure of conflict of interest we, the authors, declare that we have no commercial association that might pose a conflict of interest in connection with the submitted article. We hereby disclose any financial and personal relationship with other people and organizations that could have influenced our work.
References
- Sheng QS, Lin JJ, Chen WB, Liu FL, Xu XM, Hua HJ, Lin CZ, Wang JH. Comparison of hand-assisted laparoscopy with open total colectomy for slow transit constipation: a retrospective study. J Dig Dis 2014; 15: 419-424.
- Liu FL, Sheng QS, Chen WB, Wang JH, Hua HJ. Hand-assisted laparoscopic surgery in radical resection of rectal cancer. Zhonghua Wai Ke Za Zhi 2012; 50: 622-624.
- Liu T, Guo P, Leng X. Comparison of effects of hand-assisted laparoscopic and traditional laparoscopic right hemicolectomy on immune function. Zhonghua Wei Chang Wai Ke Za Zhi 2015; 18: 817-820.
- Tajima T, Mukai M, Noguchi W, Higami S, Uda S, Yamamoto S, Hasegawa S, Nomura E, Sadahiro S, Yasuda S, Makuuchi H. Comparison of hand-assisted laparoscopic surgery and conventional laparotomy for rectal cancer: Interim results from a single center. Mol Clin Oncol 2015; 3: 533-538.
- Jadlowiec CC, Mannion EM, Thielman MJ, Bartus CM, Johnson KH, Sardella WV, Vignati PV, Cohen JL. Evolution of technique in performance of minimally invasive colectomies. Dis Colon Rectum 2014; 57: 1090-1097.
- Heneghan HM, Martin ST, Kiran RP, Khoury W, Stocchi L, Remzi FH, Vogel JD. Laparoscopic colorectal surgery for obese patients: decreased conversions with the hand-assisted technique. J Gastrointest Surg 2013; 17: 548-554.
- Li Z, Li D, Jie Z, Zhang G, Liu Y. Comparative study on therapeutic efficacy between hand-assisted laparoscopic surgery and conventional laparotomy for acute obstructive right-sided colon cancer. J Laparoendosc Adv Surg Tech A 2015; 25: 548-554.
- Wang DY, Lin JJ, Xu XM, Liu FL. The role of hand-assisted laparoscopic surgery in total colectomy for colonic inertia: a retrospective study. J Korean Surg Soc 2013; 85: 123-127.
- Schiphorst AH, Verweij NM, Pronk A, Borel Rinkes IH, Hamaker ME. Non-surgical complications after laparoscopic and open surgery for colorectal cancer-A systematic review of randomised controlled trials. Eur J Surg Oncol 2015; 41: 1118-1127.
- Bishawi M, Fakhoury M, Denoya PI, Stein S, Bergamaschi R. Surgical site infection rates: open versus hand-assisted colorectal resections. Tech Coloproctol 2014; 18: 381-386.
- Pendlimari R, Holubar SD, Dozois EJ, Larson DW, Pemberton JH, Cima RR. Technical proficiency in hand-assisted laparoscopic colon and rectal surgery: determining how many cases are required to achieve mastery. Arch Surg 2012; 147: 317-322.
- Zhang H, Li M, Zhan TC, Yao YF, Peng YF, Gu J. Comparison of short-term postoperative outcomes between hand-assisted laparoscopic and conventional sigmoidectomy. Zhonghua Wei Chang Wai Ke Za Zhi 2011; 14: 462-464.
- Qu H, Liu Y, Bi DS. Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: a systematic review and meta-analysis. Surg Endosc 2015; 29: 3608-3617.
- Ju H, Huang X, Zhu Y, Feng H, Li D. Comparison of short-term outcomes of hand-assisted laparoscopic, laparoscopic, and open surgery in the treatment of rectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2014; 17: 574-577.
- Vogel JD, Lian L, Kalady MF, de Campos-Lobato LF, Alves-Ferreira PC, Remzi FH. Hand-assisted laparoscopic right colectomy: how does it compare to conventional laparoscopy? J Am Coll Surg 2011; 212: 367-372.
- Jiang HY, Zhang XF, Wang XZ, Li J, Zhang C. Short-term outcomes of radical resection of rectal carcinoma: hand-assisted laparoscopy versus traditional laparoscopic approach. Zhonghua Wei Chang Wai Ke Za Zhi 2012; 15: 517-519.
- Wu J, Shao Y, Rong W, Wang X, Zhao D, Wang J, Bi J, Gao J, Zhang H, Liu Q, Zhang J. Hand-assisted laparoscopic surgery in colorectal carcinoma resection: a report of 14 cases. Zhonghua Zhong Liu Za Zhi 2002; 24: 599-601.
- Zhou ZX, Zhao LY, Lin T, Liu H, Deng HJ. Long-term oncologic outcomes of laparoscopic vs open surgery for stages II and III rectal cancer: A retrospective cohort study. World J Gastroenterol 2015; 21: 5505-5512.
- Targarona EM, Gracia E, Garriga J, Martinez-Bru C, Cortes M, Boluda R, Lerma L, Trias M. Prospective randomized trial comparing conventional laparoscopic colectomy with hand-assisted laparoscopic colectomy: applicability, immediate clinical outcome, inflammatory response, and cost. Surg Endosc 2002; 16: 234-239.
- Walsh TM, Sangi-Haghpeykar H, Ng V, Zurawin R, Guan X. Hand-assisted laparoscopic hysterectomy for large uteri. J Minim Invasive Gynecol 2015; 22: 1231-1236.
- Meshikhes AW. Controversy of hand-assisted laparoscopic colorectal surgery. World J Gastroenterol 2010; 16: 5662-5668.
- Orenstein SB, Elliott HL, Reines LA, Novitsky YW. Advantages of the hand-assisted versus the open approach to elective colectomies. Surg Endosc 2011; 25: 1364-1368.
- Hasegawa Y, Koffron AJ, Buell JF, Wakabayashi G. Approaches to laparoscopic liver resection: a meta-analysis of the role of hand-assisted laparoscopic surgery and the hybrid technique. J Hepatobiliary Pancreat Sci 2015; 22: 335-341.
- Rajab A, Pelletier RP. The safety of hand-assisted laparoscopic living donor nephrectomy: the Ohio State University experience with 1500 cases. Clin Transplant 2015; 29: 204-210.
- Wolf JS Jr. Selection of patients for hand-assisted laparoscopic surgery. J Endourol 2004; 18: 327-332.
- Aalbers AG, Doeksen A, Van Berge Henegouwen MI, Bemelman WA. Hand-assisted laparoscopic versus open approach in colorectal surgery: a systematic review. Colorectal Dis 2010; 12: 287-295.
- Posner MC, Alverdy J. Hand-assisted laparoscopic surgery for cancer. Cancer J 2002; 8: 144-153.
- Ferron G, Querleu D, Martel P, Chopin N, Soulie M. Laparoscopy-assisted vaginal pelvic exenteration. Gynecol Obstet Fertil 2006; 34: 1131-1136.
- Cao Y, Gong J, Zhou J, Liu L, Gan W, Huang L, Zhang G, Wang P, Luo G, Song Y. Efficacy comparison of lymph node dissection patterns of the reverse and the cabbage in hand-assisted laparoscopic D2 radical gastrectomy. Zhonghua Wei Chang Wai Ke Za Zhi 2016; 19: 200-203.
- Chan A, Wong A, Langevin J, Khoo R. Preoperative concurrent 5-fluorouracil infusion, mitomycin C and pelvic radiation therapy in tethered and fixed rectal carcinoma. Int J Radiat Oncol Biol Phys 1993; 25: 791-799.
- Hanna MH, Vinci A, Pigazzi A. Diverting ileostomy in colorectal surgery: when is it necessary? Langenbecks Arch Surg 2015; 400: 145-152.
- Kokelaar RF, Evans MD, Davies M, Harris DA, Beynon J. Locally advanced rectal cancer: management challenges. Onco Targets Ther 2016; 9: 6265-6272.
- Chao TE, Mandigo M, Opoku-Anane J, Maine R. Systematic review of laparoscopic surgery in low- and middle-income countries: benefits, challenges, and strategies. Surg Endosc 2016; 30: 1-10.
- Stifelman M, Nieder AM. Prospective comparison of hand-assisted laparoscopic devices. Urology 2002; 59: 668-672.
- Meshikhes AW, El Tair M, Al Ghazal T. Hand-assisted laparoscopic colorectal surgery: initial experience of a single surgeon. Saudi J Gastroenterol 2011; 17: 16-19.
- Khitaryan AG, Glumov EE, Veliev KS. Comparative research of traumatic injury of open hand-assisted laparoscopic anterior resection of the rectum. Vestn Khir Im II Grek 2015; 174: 52-58.
- Chan DK, Chong CS, Lieske B, Tan KK. Laparoscopic resection for rectal cancer: what is the evidence? Biomed Res Int 2014; 2014: 347810.
- Zhou X, Liu F, Lin C, You Q, Yang J, Chen W, Xu J, Lin J, Xu X. Hand-assisted laparoscopic surgery compared with open resection for mid and low rectal cancer: a case-matched study with long-term follow-up. World J Surg Oncol 2015; 13: 199.
- Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015; 372: 1324-1332.
- Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (corean trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncology 2010; 11: 637-645.