ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Artificial Intelligent Techniques for Bio Medical Signal Processing: Edition-I
Clinical pharmacokinetics of parecoxib via intravenous and intramuscular injections in Chinese populations
Chenghai Wang1,2, Shaoyan Huang3, Jianzhong Zhang2, Jie Li2, Wei Shao2, Kaoxiang Sun4 and Mengyuan Zhang1*
1Department of Anesthesiology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, PR China
2Department of Anesthesiology, Yantaishan Hospital, Yantai, Shandong, PR China
3Department of Oncology, Yantaishan Hospital, Yantai, Shandong, PR China
4School of Pharmacy, Yantai University, Yantai, Shandong, PR China
- *Corresponding Author:
- Mengyuan Zhang
Department of Anesthesiology
Shandong Provincial Hospital Affiliated to Shandong University, PR China
Accepted on March 8, 2017
Objective: To study the clinical pharmacokinetics of parecoxib sodium via intravenous and intramuscular injections in Chinese populations.
Methods: A total of 20 patients undergoing unilateral lower limb surgery were selected, and their related parameters were as follows: ASA: Grade I to II, age: 20 to 60 years, and weight: 50 to 80 kg. They were randomly assigned to two groups: the intravenous injection group (IV group) and the intramuscular injection group (IM group), with 10 patients in each group (N=10). Then, they were subjected to intravenous or intramuscular injection of parecoxib at 40 mg (diluted with 2 ml of normal saline) and venous blood sampling (2 ml) at 0, 10, 20, 30, 40, 50, and 60 min, and at 1.5, 2, 4, 6, 8, 12, 16, 20 and 24 h after the injection. After that, the venous blood was added with heparin for anti-coagulation and centrifuged at 3000 rpm for 10min. The plasma was separated and placed in a refrigerator at -20°C. The plasma concentration of levobupivacaine was detected by means of High Performance Liquid Chromatography (HPLC). All statistical analysis was performed using SPSS17.0 statistical software. Measurement data were expressed as mean ± standard deviation (x? ± s), and the t test and repeated measurement analysis of variance were adopted for inter- and intra-group comparisons. The count data were compared using the χ2 test, and P<0.05 was considered statistically significant.
Results: After the intravenous and intramuscular injections of parecoxib, its plasma concentration was increased quickly, with its Cmax at 31.578 ± 13.407 and 21.311 ± 10.160 mg/L, respectively. Then its plasma concentration was decreased rapidly, with no parecoxib detected in the plasma of all patients at 4 h after its administration. Accordingly, the Cmax of valdecoxib in patients from the two groups was reached at 20 min and 1.5 h, respectively, with the values at 0.751 ± 0.290 and 0.543 ± 0.162 mg/L, respectively, and no parecoxib was detected in the plasma of all patients at 24 h after its administration. Processing by the pharmacokinetic software showed that the AUC of parecoxib of patients in Group II was higher than that in Group I (P<0.05) and that CL was lower than that in Group I (P<0.05), whereas the differences in the pharmacokinetic parameters of valdecoxib in patients between the two groups were not statistically significant (P>0.05).
Conclusion: The routes of administration can affect the clinical pharmacokinetic parameters of parecoxib, but not those of valdecoxib.
Keywords
Parecoxib, High performance liquid chromatography, Cmax, Cyclooxyenase-2 (COX-2) inhibitor.
Introduction
Parecoxib sodium is a specific Cyclooxyenase-2 (COX-2) inhibitor, and its inhibitory effect on COX-2 is 28,000-fold that on COX-1 [1,2]. It does not affect the function of the gastrointestinal tract, the platelets and the kidney, etc. while it exerts the analgesic and anti-inflammatory effects, and thus, it has fewer clinical side effects and adverse reactions [3-6] and is especially suitable for the treatment of perioperative pain in patients. It demonstrates better application prospects.
Parecoxib is a prodrug, and it is converted into valdecoxib by hydrolysis of liver enzymes in vivo, with the latter continuing to play anti-inflammatory and analgesic effects [1,2]. Currently, most of domestic clinical studies focus on pharmacodynamics of parecoxib [7-10] and there are few studies on its pharmacokinetics. Therefore, its data of pharmacokinetic parameters can only be obtained from foreign studies [11-13]. This study aimed to investigate the clinical pharmacokinetics of parecoxib via intravenous and intramuscular injections in Chinese populations, thereby providing a theoretical basis for clinical medication.
Parecoxib is a new molecular entity which represents the first parenteral “COX-2 selective” agent. For orally available compound valdecoxib, parecoxib sodium is a parenteral prodrug is considered in a current review. For safety and efficacy related with parecoxib sodium is due to dual development that has impacted, and confounded. This parecoxib sodium is supplied as a sterile, preservative-free, lyophilized powder which is equivalent to 20 mg or 40 mg parecoxib in single-use vials. It is designed to be make again with 1 ml (20 mg vials) or 2 ml (40 mg vials) sterile saline for injection (0.9% sodium chloride).
Side effects of parecoxib are ulcer and gastrointestinal bleeding, jaundice and abnormal liver function, heart failure, heart attack, slow heart rate, high/low blood pressure and abnormal heart rhythm, swelling, rash, itching and difficulty in breathing and back pain, low platelet counts, agitation, disturbed sleeping and decreased urination.
Materials and Methods
This study was approved by the Medical Ethics Committee. A total of 20 patients undergoing selective surgery on unilateral femur, tibia and fibula, ankle or toe were enrolled, and their related parameters were as follows: ASA: Grade I to II, age: 20 to 60 years, and weight: 50 to 80 kg. They all signed the informed consent before the surgery. The patients were randomly divided into two groups (N=10): the intravenous injection group (Group I) and the intramuscular injection group (Group II). Exclusion criteria: 1) patients aged<20 years or>60 years; 2) pregnant women and women in lactation; 3) patients with a history of drug allergy to NSAIDs; 4) patients with obvious abnormal function of the heart, the lung, the liver and the kidney; 5) patients administered with such hepatic microsomal enzyme inducers as carbamazepine and rifampin, or such hepatic microsomal enzyme inhibitors as cimetidine and isoniazid; 6) patients with a history of drinking and drug addiction; 7) patients with a history of mental illness; 8) patients with intraspinal anesthesia contraindications, such as scoliosis and coagulation abnormalities; 9) patients whose surgery time exceeded 3 h; 10) patients whose intraoperative blood loss exceeded 500 ml; and 11) patients whose intraoperative infusion volume exceeded 1000 ml or who needed blood transfusions, etc.
Anesthesia and monitoring Patients lied on their left or right side and experienced L3.4 gap positioning. After disinfection and local anesthesia, they underwent the epidural puncture, and the compression test with brine turned negative. The spinal anesthesia needle was inserted, and 1.5-2.0 ml of 0.5% ropivacaine injection was slowly injected into the subarachnoid space. Then, they switched to the supine position, and the anesthesia level was measured by means of needle punching. Intraoperative care is intensified: ECG, Bp, SpO2 and urine volume. Infusion was performed to supplement circulating blood volume. After the surgery, the patients were sent back to the ward for continued observation and treatment. Perioperative adverse reactions in patients were observed and recorded, and symptomatic treatment was given.
Administration of parecoxib and blood sampling Patients were intravenously or intramuscularly injected with 40 mg of parecoxib (diluted with 2 ml of normal saline) 30 min before the intraspinal anesthesia, and venous blood sampling (2 ml) was performed at 0, 10, 20, 30, 40, 50, and 60 min, and at 1.5, 2, 4, 6, 8, 12, 16, 20 and 24 h after the injection. After that, the venous blood was added with heparin for anti-coagulation and centrifuged at 3000 rpm for 10 min. The plasma was separated and placed in a refrigerator at -20°C.
HPLC was performed to detect the concentrations of parecoxib and valdecoxib in the plasma.
Chromatographic column: Diamonsil C18 column (5 μm, 150 mm × 4.6 mm i.d); precolumn: DIKMA C18 column (10mm, 4.6 μm); column temperature: 30°C; mobile phase: acetonitrile-water-phosphoric acid (57: 43: 0.01, v/v); flow rate: 1.0 ml/min; detection wavelength: 240 nm; internal standard: celecoxib; injection volume: 10 μl.
Standard curve: Parecoxib and valdecoxib standard solutions at a series of concentrations were precisely measured and added to centrifuge tubes containing 500 μL blank plasma, respectively. 50 μL of celecoxib internal standard solutions (25 mg/L) were added for mixing. Drug-containing plasma with the concentration of parecoxib at 100, 50, 20, 10, 5, 2 and 1 mg/L and with the concentration of valdecoxib at 5, 2, 1, 0.5, 0.1 and 0.05 mg/L were prepared, respectively. The ratios of the peak areas of parecoxib and valdecoxib to the peak areas of the internal standard (Y) and their mass concentrations (X) were subjected to linear regression, respectively, to yield the linear equation of parecoxib: Y=0.0178X-0.0229, the correlation coefficient: R2=0.9999, and linear range: 1~100 mg/L, and the linear equation of valdecoxib: Y=0.3152X-0.0051, the correlation coefficient: R2=0.9998, and linear range: 0.05~5 mg/L.
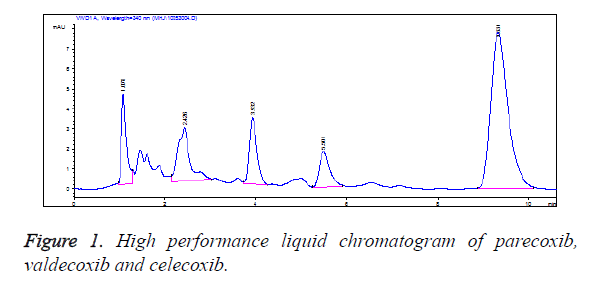
Specificity: The blank plasma was free from interference of endogenous impurities at the peak of the samples, and the baseline was stable. The retention times of valdecoxib, parecoxib and celecoxib were approximately 3.9 min, 5.5 min and 9.4 min, respectively. All peaks were well separated (Figure 1).
Precision and recovery: The recoveries of valdecoxib and parecoxib solutions at 3 different concentrations were all greater than 85%, and the inter- and intra-day variations were all smaller than 15%, meeting the requirements on extraction and measurement of biological samples and ensuring the accuracy and precision of the measurement results. This indicated that the precision of the method was in line with the requirements on the analysis of biological samples. Besides, it showed good repeatability and small errors, and thus it could meet the needs for pharmacokinetic studies (Tables 1 and 2).
| Conc. (mg/L) | Inter-day | Intra-day | Recovery RSD/% (%) | ||
|---|---|---|---|---|---|
| ?x ± s | RSD/% | ?x ± s | RSD/% | ||
| 0.05 | 0.048 ± 0.0044 | 9.17 | 0.048 ± 0.004 | 8.33 | 96.75 ± 0.0032 6.64 |
| 0.5 | 0.498 ± 0.0010 | 0.2 | 0.4681 ± 0.007 | 1.5 | 98.54 ± 0.0043 8.83 |
| 5 | 5.029 ± 0.0456 | 0.91 | 4.326 ± 0.320 | 7.4 | 99.30 ± 0.0037 7.45 |
Table 1. Precision and recovery of parecoxib.
| Conc. (mg/L) | Inter-day | Intra-day | Recovery RSD/% (%) | ||
|---|---|---|---|---|---|
| ?x ± s | RSD/% | ?x ± s | RSD/% | ||
| 0.05 | 0.053 ± 0.0058 | 10.9 | 0.044 ± 0.002 | 9.5 | 103.80 ± 0.0015 2.917 |
| 0.5 | 0.478 ± 0.0065 | 1.36 | 0.381 ± 0.019 | 5.29 | 95.64 ± 0.0065 1.353 |
| 5 | 5.013 ± 0.0.56 | 0.71 | 4.216 ± 0.258 | 6.14 | 100.30 ± 0.0035 0.711 |
Table 2. Precision and recovery of valdecoxib.
Processing of pharmacokinetic parameters: The plasma concentration-time data of patients from the two group were processed using the WonNonlin6.4 pharmacokinetic software, and the pharmacokinetic parameters for calculation included the elimination half-life (t1/2β), Area Under the Curve (AUC), apparent volume of distribution (Vd) and Clearance rate (CL). The actually measured values of peak plasma concentration (Cmax) and time to peak (Tmax) were used.
Statistical processing
All statistical analysis was performed using SPSS17.0 statistical software. Measurement data were expressed as mean ± standard deviation (?x ± s), and the t test and repeated measurement analysis of variance were adopted for inter- and intra-group comparisons. The count data were compared using the χ2 test, and P<0.05 was considered statistically significant.
Results
All patients completed the entire study successfully. Patients in the two groups were not significantly different in terms of such indicators as gender, age, weight, preoperative liver function and kidney function, operation time, infusion volume and blood loss (P>0.05) (Table 3). Perioperative SBP, DBP, HR and SPO2 in patients from the two groups were stable, with no violent fluctuations, and the difference between the two groups was not statistically significant (P>0.05).
| Group | Gender (Male/Female) | Age (Years) | Weight (kg) | HGB (g/L) | Total protein albumin | ALT (IU/L) | AST (IU/L) | BUN (mmol/L) | Cr (umol/L) | UA (umol/L) | Operation time (min) | Infusion volume (ml) | Blood loss (ml) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5/5 | 41.20 ± 9.10 | 65.00 ± 4.16 | 124.80 ± 7.19 | 76.00 ± 5.98 | 13.67 ± 5.10 | 25.96 ± 6.79 | 4.57 ± 1.36 | 58.67 ± 15.64 | 224.51 ± 49.80 | 92.50 ± 17.83 | 148.00 ± 38.53 | 705.00 ± 76.19 |
| 2 | 6/4 | 38.50 ± 7.44 | 63.60 ± 4.77 | 126.60 ± 8.97 | 74.90 ± 3.78 | 19.21 ± 5.51 | 27.99 ± 4.76 | 3.56 ± 1.61 | 56.87 ± 16.07 | 255.16 ± 62.64 | 85.50 ± 17.39 | 140.00 ± 51.63 | 675.00 ± 67.70 |
Table 3. General data of patients in the two groups (?x ± s, n=10).
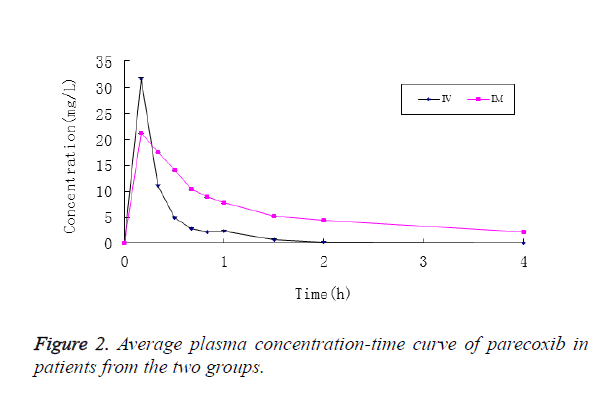
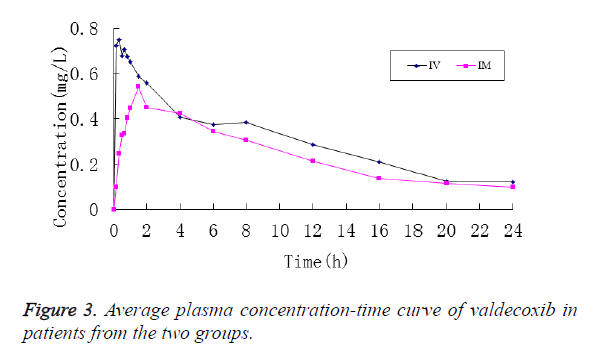
After patients in Group I were intravenously injected with parecoxib, its mean plasma concentration reached Cmax in 10 min, which stood at 31.578 ± 13.407 mg/L, and then it was decreased rapidly. At 2 h after administration, parecoxib was detected in the plasma of only 2 patients, and no parecoxib was detected in the plasma of all patients at 4 h. Accordingly, the plasma concentration of valdecoxib reached Cmax in 20 min, which stood at 0.751 ± 0.290 mg/L, and then it was decreased rapidly. At 24 h, valdecoxib at the concentration of 0.133 ± 0.0551 mg/L could still be detected in plasma (Figure 2).
In contrast, the mean plasma concentration of parecoxib in patients from Group II after the intramuscular injection reached Cmax in 10 min, which stood at 21.311 ± 10.160 mg/L, and then it was decreased slowly. At 4 h, parecoxib could be detected in the plasma of all patients, and its concentration was 2.226 ± 0.997 mg/L, while no parecoxib was not detected in the plasma of all patients at 6 h. Accordingly, the mean plasma concentration of valdecoxib was slowly increased and peaked at 1.5 h, standing at 0.543 ± 0.162 mg/L. After that it was slowly decreased. And valdecoxib at the concentration of 0.098 ± 0.039 mg/L could still be detected in the plasma (Figure 3).
Inter-group comparisons showed that the Cmax of parecoxib in patients from Group II after the intramuscular injection, 21.597 ± 9.861 mg/L, and that after the intravenous injection, 31.578 ± 13.407 mg/L, was not statistically significant (P>0.05), while the Tmax, which stood at 0.233 ± 0.086 h was significantly longer than that in patients from Group I, which was 0.167 ± 0.000 h (P<0.05). After patients in Group II were injected intramuscularly with parecoxib, the peak concentration of valdecoxib was 0.583 ± 0.144 mg/L, which was significantly lower than 0.939 ± 0.233 mg/L, Cmax of valdecoxib of patients in Group I (P<0.05), while the Tmax (1.767 ± 0.864 h) was significantly longer than that of patients in Group I (0.452 ± 0.272 h) (P<0.05) (Table 4).
| Cmax (mg/L) | Tmax (h) | t1/2β (h) | AUC (h.mg.L-1) | Vd (L) | CL (L/h) | ||
|---|---|---|---|---|---|---|---|
| Group I | Parecoxib | 31.578 ± 13.407 | 0.167 ± 0.000 | 0.556 ± 0.456 | 19.015 ± 7.985 | 1.406 ± 0.669 | 2.349 ± 1.058 |
| Valdecoxib | 0.939 ± 0.233 | 0.452 ± 0.272 | 8.009 ± 2.707 | 9.107 ± 2.337 | 51.952 ± 16.329 | 4.660 ± 1.195 | |
| Group II | Parecoxib | 21.597 ± 9.861 | 0.233 ± 0.086* | 0.810 ± 0.506 | 27.409 ± 5.890* | 1.727 ± 1.118 | 1.520 ± 0.320* |
| Valdecoxib | 0.583 ± 0.144* | 1.767 ± 0.864* | 10.454 ± 7.073 | 7.360 ± 1.490 | 78.380 ± 38.999 | 5.617 ± 1.021 | |
| *Compared to Group I, P<0.05. | |||||||
Table 4. The main pharmacokinetic parameters of parecoxib and valdecoxib in patients from the two groups (?x ± s, n=10).
The WonNonlin6.4 pharmacokinetic software was used to calculate the main pharmacokinetic parameters of parecoxib and valdecoxib in patients form the two groups. After patients from Group II were intramuscularly injected with parecoxib, its AUC, the main pharmacokinetic parameters, was 27.409 ± 5.890 h.mg.L-1, which was significantly higher than that of patients from Group I, which stood at 19.015 ± 7.985 h.mg.L-1 (P<0.05). However, there were no statistically significant differences between the two groups in terms of such parameters as t1/2β, Vd and CL (P>0.05). In contrast, the differences in the above pharmacokinetic parameters of valdecoxib between patients from the two groups were not statistically significant (P>0.05) (Table 4).
Adverse reactions
The adverse reactions that occurred during the entire study are shown in Table 5, and they were mild and could be tolerated by the patients. They were not subjected to special treatment, and did not result in serious consequences to the patients.
| Group I | Group II | Total | |
|---|---|---|---|
| Injection site pain | 1 | 1 | 2 |
| Nausea | 1 | 0 | 1 |
| Drowsiness | 1 | 0 | 1 |
Table 5. Perioperative adverse reactions in patients from the two groups (n).
Discussion
HPLC is a common method for studying and detecting the plasma concentration of drugs in pharmacokinetics [10]. In this study, Diamonsil C18 column (5 μm, 150 mm × 4.6 mm i.d.) was adopted, with acetonitrile-phosphoric acid aqueous solution (57:43, V/V) as mobile phase and celecoxib as internal standard. Quantification was achieved by means of peak areas, and valdecoxib within 0.05~5 mg/L (R2=0.9998) and parecoxib within 1~100 mg/L (R2=0.9999) were in a good linear relationship to ensure the detectability of various blood sampling time point specimens. The intra- and inter-day differences between parecoxib and valdecoxib at low, medium and high concentrations were less than 15%, suggesting that the precision of such a method was in line with the requirements on the analysis of biological samples. The recovery rates of three concentrations were all greater than 85%, indicating that such a method showed good accuracy. In the chromatogram, the retention time of valdecoxib was about 3.9 min, that of parecoxib was 5.5 min, and that of the internal standard celecoxib was about 9.4 min. All peaks were well separated, and there were no interference from other drugs and endogenous impurities to the test substances, indicating that the specificity of such a method conformed to the requirements on the detection of the biological samples and the measured data were accurate and reliable.
Pharmacokinetics of drugs reflects the absorption, distribution, metabolism and excretion of drugs in vivo, and it is associated with such factors as patients’ age, routes of administration, liver and kidney functions. Intraoperative factors, such as operation time, adjuvants, the amount of blood loss and infusion volume, will also affect drug metabolism. Therefore, it is necessary to study the clinical pharmacokinetics, thus guiding the rational and safe clinical medication and improving the effectiveness of clinical medication.
Mutations of one or more alleles occur in the long evolutionary process of mankind, which alter the genes of drug metabolizing enzymes and corresponding enzyme proteins are generated to enable mankind to adapt to environmental changes. Such a mutation of genes can be passed onto the next generations through inheritance, and therefore, the genetic polymorphism of human drug metabolizing enzymes is gradually formed. The cytochrome oxidases P45O (CYP) that participate in the metabolism of parecoxib in vivo are mainly CYP3A4 and CYP2C9. Basic and clinical studies have shown that CYP3A4 and CYP2C9 demonstrate racial differences [14-16]. Therefore, clinical medication cannot simply rely on foreign parameter data and it is necessary to study the clinical pharmacokinetics of Chinese population, thereby guiding clinical medication, avoiding adverse drug reactions as far as possible, and enhancing the safety of clinical medication.
There are few studies on the clinical pharmacokinetics of parecoxib in China, and therefore, clinical medication can only rely on foreign data [11-13]. To minimize the impact of intraoperative medication, blood loss and infusion volume, etc. on the plasma concentrations of parecoxib and valdecoxib, the subjects in this study were patients undergoing unilateral lower limb surgery and the operation time was controlled within 3 h, to ensure the accuracy of blood samples as far as possible.
Laine et al. [11] reported on the clinical pharmacokinetics of parecoxib injected intramuscularly in adult patients at different doses, and the results showed that after the intramuscular injection of parecoxib (40 mg), its Cmax, Tmax and t1/2β were 1.681 mg/L, 0.25 h and 0.87 h, respectively, and that those of valdecoxib were 0.498 mg/L, 2.59 h and 7.35 h, respectively accordingly. After the intramuscular injection of parecoxib (40 mg) in this study, it’s Cmax, Tmax and t1/2β were 21.597 mg/L, 0.233 h and 0.810 h, respectively, and those of valdecoxib were 0.583 mg/L, 1.767 h and 10.454 h, respectively. There were differences between domestic and foreign data, and here was the analysis of its reasons: 1) The main reason might be the genetic polymorphism of drug metabolizing enzymes, indicating that the clinical medication of Chinese people cannot rely solely on foreign data; 2) Laine et al. [11] performed blood sampling at 1, 2, 5, 15 min until 96 h after the intramuscular injection of parecoxib, but the sampling was done at 10, 20, 30 min until 24 h after the intramuscular administration in this study. The setting of time points for sampling had a huge impact on the pharmacokinetic parameters, especially on Cmax and Tmax, suggesting that the clinical pharmacokinetics of parecoxib in Chinese people demand further studies; 3) in this study, patients with unilateral lower limb surgery were enrolled. They had a short operation time, less blood loss, and the corresponding infusion volume was not large, so that the impact of bleeding, infusion and hemodilution on the plasma concentration could be avoided as far as possible.
In this study, the plasma concentration of parecoxib was quickly increased after its intravenous and intramuscular injections, with the peak concentration reached both at 10 min, the first blood sampling time point. If blood sampling time points were set within 10 min in this study, its Cmax might be higher, and Tmax would be shorter. The comparison of pharmacokinetic parameters showed that the Tmax was longer while CL was lower in Group II, as compared with Group I (P<0.05), but it was of little clinical significance. Although the difference in Cmax of parecoxib of patients between the two groups was not statistically significant (P>0.05), the difference of nearly 10 mg/L between the two was of clinical significance, suggesting that the route of administration can affect the plasma concentrations and pharmacokinetic parameters of parecoxib.
After the intravenous and intramuscular injections of parecoxib, the plasma concentration of valdecoxib was also rapidly increased, with its Cmax reached at 20 min and 1.5 h, respectively. The comparison of pharmacokinetic parameters showed that the Cmax in Group II was lower than that in Group I (P<0.05), while it was of little clinical significance. Besides, the differences in other pharmacokinetic parameters between the two groups were not statistically significant (P>0.05), indicating that route of administration of parecoxib had little effect on the plasma concentrations and pharmacokinetic parameters of valdecoxib.
During the entire study, injection site pain occurred in two patients, nausea and drowsiness occurred in 1 patient, respectively. Such events were not subjected to special treatment and the patients could tolerate them. The plasma concentrations showed that the Cmax of parecoxib and valdecoxib in all patients were 51.82 mg/L and 1.21 mg/L, respectively, indicating that parecoxib demonstrate good safety profiles, and thus it is suitable for clinical applications.
To sum up, the clinical pharmacokinetics of parecoxib via intravenous and intramuscular injections in Chinese populations were investigated in this study, providing a theoretical basis for its clinical medication. The route of administration of parecoxib can affect its plasma concentrations and pharmacokinetic parameters, whereas the impact on the plasma concentration and pharmacokinetic parameters of its metabolite valdecoxib is of no clinical significance. Furthermore, there are still shortcomings in terms of setting of sampling time points, and we will carry out further studies to provide more accurate parameters on the clinical pharmacokinetics of parecoxib in Chinese populations, thereby guiding the rational and safe clinical medication.
References
- Habibi AH, Puig D, Solanas A. A probabilistic approach for breast boundary extraction in mammograms. Comp Math Methods Med 2013.
- Shi G, Liu W, Zhang L, Li F. An efficient folded architecture for lifting-based discrete wavelet transform. IEEE Trans Circ Sys II Express Briefs 2009; 56: 290-294.
- Wu BF, Lin CF. A high-performance and memory-efficient pipeline architecture for the 5/3 and 9/7 discrete wavelet transform of JPEG2000 codec. IEEE Trans Circ Sys Video Technol 2005; 15: 1615-1628.
- Zhang W, Jiang Z, Gao Z, Liu Y. An efficient VLSI architecture for lifting-based discrete wavelet transform. IEEE Trans Circ Sys II Express Briefs 2012; 59: 158-162.
- Mohanty BK, Meher PK. Memory efficient modular VLSI architecture for highthroughput and low-latency implementation of multilevel lifting 2-D DWT. IEEE Trans Sign Proc 2011; 59: 2072-2084.
- Huang CT, Tseng PC, Chen LG. Generic RAM-based architectures for two-dimensional discrete wavelet transform with line-based method. IEEE Trans Circ Sys Video Technol 2005; 15: 910-920.
- Tian X, Wu L, Tan YH, Tian JW. Efficient multi-input/multi-output VLSI architecture for two-dimensional lifting-based discrete wavelet transform. IEEE Trans Comp 2011; 8: 1207-1211.
- Ching CJ, Yusong H. A memory efficient scalable architecture for 2D discrete wavelet transform. IEEE Trans Image Process 2013; 16: 607-614.
- Ezhilarasi P, Nirmalkumar P. Algorithmic based VLSI architecture of integrated image compression for CMOS image sensor. Nat Acad Sci Lett 2015; 38: 49-59.
- Karobari FM, Bharathi SH. VLSI architectures for 3D discrete wavelet transform and applications of wavelet transform-a comprehensive study 2015.
- Laine A, Fan J, Yang W. Wavelets for contrast enhancement of digital mammography. IEEE Eng Med Biol Magaz 1995; 14: 536-550.
- Martinez-Peiro M, Boemo EI, Wanhammar L. Design of high-speed multiplierless filters using a nonrecursive signed common subexpression algorithm. IEEE Trans Circ Sys II Analog Dig Sign Proc 2002; 49: 196-203.
- Ni SH, Huang YH, Lo KF, Lin DC. Buried pipe detection by ground penetrating radar using the discrete wavelet transform. Comp Geotech 2010; 37: 440-448.
- Daubechies I, Sweldens W. Factoring wavelet transforms into lifting steps. J Fourier Anal Appl 1998; 4: 247-269.
- Parhi KK. VLSI digital signal processing systems: design and implementation. John Wiley Sons 2007.
- Mohanty BK, Mahajan A, Meher PK. Area-and power-efficient architecture for high-throughput implementation of lifting 2-D DWT. IEEE Trans Circ Sys II Exp Briefs 2012; 59: 434-438.