ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 15
Comparison and choice of different treatment modalities for cesarean scar pregnancy
Fenghua Zhang1#, Hongyan Gao1#, Shan Meng2, Yi Ding3, Xingguang Lin4, Qing Wang1, Jiming Chen5* and Zhi Liao6*
1Department of Obstetrics and Gynaecology, the Third Affiliated Hospital of Soochow University, Changzhou Jiangsu Province, PR China
2Department of Obstetrics and Gynaecology, the First Affiliated Hospital of Third Military Medical University, Chongqing, PR China
3Department of Obstetrics and Gynaecology, Jintan Hospital Affiliated to Jiangsu University, Jintan Jiangsu, PR China
4Department of Obstetrics and Gynaecology, Tongji Hospital, Tongji medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, PR China
5Department of Obstetrics and Gynaecology, the Second Affiliated Hospital of Chongqing Medical University, Chongqing, PR China
6Department of Obstetrics and Gynaecology, Hospital of the University of Electronic Science and Technology of China, Sichuan Provincial People’s Hospital, Chengdu, Sichuan Province, PR China
#These authors have equally contributed for this work
- *Corresponding Author:
- Jiming Chen
Department of Obstetrics and Gynaecology
The Second Affiliated Hospital of Chongqing Medical University
Chongqing, PR China
Zhi Liao
Department of Obstetrics and Gynaecology
Hospital of the University of Electronic Science and Technology of China
Sichuan Provincial People’s Hospital
Chengdu, PR China
Accepted on June 07, 2017
Background: Cesarean Scar Pregnancy (CSP) is caused by implantation of embryonic tissues into the left on uterine tissues left by previous Cesarean section. Severe consequences including uterine rupture and haemorrhage shock is highly likely without timely termination of pregnancy. Various surgical methods have been proposed but lacking a systemic guideline for choice of treatment. This study thus investigated the treatment efficacy and safety of various methods targeting CSP in a retrospective case study manner.
Methods: A total of 21 CSP patients who were admitted from Jun 2010 to Dec 2014 in our hospital were recruited, including 5 cases receiving Methotrexate (MTX) followed by ultrasound assisted uterine curettage, 10 patients undergoing MTX with ultrasound assisted uterine curettage plus uterine artery embolization (UAE), and 5 patients having MTX+UAE+hysteroscopy assisted lesion resection. Using retrospective manner, we revised clinical parameters including pregnancy mass size, bleeding volume, pre/post-op HCG level and general conditions of patients. Both efficacy and safety of different treatment modalities were analysed and compared.
Results: All 21 patients (including one patient refusing UAE or total hysterectomy) had successful treatment without infection, haemorrhage or liver/renal dysfunction. By choosing different methods according to pre-op conditions of patients, all those 20 patients keeping uterus intact had normal menstrual cycles.
Conclusion: Different approaches should be selected based on individual conditions of CSP patients to both minimize trauma and to maximize efficacy.
Keywords
Cesarean scar pregnancy, Methotrexate, Uterine artery embolization, Uterine curettage, Total hysterectomy.
Introduction
Cesarean Scar Pregnancy (CSP) is one ectopic pregnancy when the embryo implants into the micro-cleft at scar tissues after cesarean section. In recent decades, with increasing rate of cesarean section, incidence of CSP is also gradually increasing [1]. Previous studies showed that overall incidence of CSP were around 1:1800 to 1:2216 [2,3]. There were 21 CSP cases out of 920 ectopic pregnancy patients (2.28%) in one single hospital from Jun 2010 to Dec 2014. The diagnosis of CSP mainly depends on ultrasound examination and MRI, although the golden standard is the biopsy for embryonic tissues from scar site under assisted by ultrasound or hysteroscopy.
The pathogenesis mechanism of CSP has not been fully illustrated yet. Most scholars agreed that unsatisfactory healing of cesarean section wounds, leaving cleft or local injury sites are major risk factors causing CSP. On classical opinion believed that CSP was caused by the implantation of embryo into uterine scar, which caused by the cleft after incomplete healing of cesarean section wounds [4]. Therefore, those factors affecting wound healing may also be related with CSP occurrence. Such factors include cesarean section on uterus with under-developed cervical, single-layer suture of cesarean section wounds, and double/multiple cesarean sections. Other factors such as wide distance between sutures, crossed layers of uterine muscles, or micro-cleft at scar sites by inflammation, might cause embryo implantation and hence force CSP.
Currently treatment methods for CSP have not been unified, as different reports obtained diversified treatment efficacy under specific scenarios [2-4]. CSP without timely management frequently causes uterus rupture, causing major haemorrhage, shock or even death. The pregnancy should be terminated once having confirmed diagnosis. It is critical to establish appropriate treatment method in order to maximally maintain patient’s fertility and to avoid severe complication. We thus performed a retrospective study covering 21 CSP patients who were admitted in our hospital from Jun 2010 to Dec 2014, in order to investigate the treatment efficacy and safety of different methods targeting CSP.
Information and Methods
Patient information
A total of 21 CSP patients who were admitted in Third Affiliated Hospital of Suzhou University from Jun 2010 to Dec 2014 were recruited in this study. Patients aged between 25-43 y (average age=35.5 ± 4.3 y).
Diagnostic criteria of CSP
The diagnosis of CSP followed standards stipulated by Godin et al. [1]. All patients in this study were recruited based on the following criteria: (1) No evidence of pregnancy in uterus; (2) No pregnant indication in cervical canal; (3) Gestational sac, which was measured as long and short diameters under computer-assisted ultrasound on anterior wall of uterus isthmus; (4) Uterus muscular defects between pregnant sac and bladder.
Choice of clinical treatment methods
Specific treatment method was chosen based on patient’s conditions, body signs and lab results. In specific: (1) Methotrexate (MTS) injection plus ultrasound assisted uterine curettage was adopted in those with small pregnant mass (<1.0 × 1.0 cm), lower HCG level (<3000 IU/L), less vaginal bleeding and stable vital signs. MTX (1 mg/kg) was applied daily for two days by intramuscular injection, plus local MTX application (25 mg into pregnant mass and 25 mg into uterus muscular layer, total dosage<200 mg). 24 h later, 0.1 mg/kg Calcium Folinate (CF) was applied daily in two days via intramuscular injection. When ultrasound showed dead embryos at the scar site with worsening blood supply, curettage was performed under ultrasound assistance. (2) Uterine arterial embolization (UAE) plus ultrasound assisted uterine curettage was performed on those patients with less than 2.0 × 2.0 cm pregnant mass, which had insufficient blood supply at uterine scar site as shown by colored ultrasound. UAE was performed using embolization using MTX and gelatin sponge via intra arterial injection from uterine artery, after focal anaesthesia and puncture of right femoral artery by Seldinger approach. (3) UAE plus resection of scar pregnant mass under hysteroscopy was adopted when pregnant mass size was between 2.0 × 2.0 cm ~3.0 × 3.0 cm, less vaginal bleeding, stable vital signs and insufficient blood supply at uterine scar sites. UAE was performed as above mentioned. 48~72 h after UAE, diathermy loop was used to resect pregnant mass under hysteroscopy. (4) Total hysterectomy was adopted in one 39 y old patient with more vaginal bleeding and refusing UAE.
Results
General information of patients
The time intervals between post-cesarean section and CSP occurrence were between 9 m to 18 y (average age=4.4 ± 3.6 y), with 14 patients had 2~5 y intervals. A total of 4 patients had twice cesarean sections before whilst the remaining 17 ones had one time of cesarean section. Numbers of miscarriage ranged from 0-5 (average=2.0 ± 1.2), with two patients had no history of miscarriage.
Summary of different treatment methods
In all 21 CSP patients, 5 of them received MTX+ultrasound assisted uterine curettage, 10 patients received MTX+UAE +ultrasound assisted uterine curettage, 5 patients had MTX +UAE+resection of scar pregnant mass under hysteroscopy, whilst the remaining one patient without pregnant requirement had total hysterectomy. All 21 patients had successful surgeries without severe complications including infection, major haemorrhage, and liver/renal dysfunction. 20 patients who retained uterus had normal menstrual cycles after surgery. A brief summary of clinical parameters among various treatment methods was shown in Table 1.
| Surgical approach | N | Mass size (cm) | Pre-op HCG (IU/L) |
3d post-op HCG (IU/L) | Pre-op hemorrhage (ml) | Hospitalized period (d) |
|---|---|---|---|---|---|---|
| MTX+curettage | 5 | 0.8 ± 0.4 | 2867 ± 3956 | 2167 ± 64 | 125 ± 35 | 6.5 ± 3.5 |
| MTX+UAE+curettage | 10 | 1.4 ± 1.6 | 105892 ± 133088 | 2389 ± 3160 | 1 350 ± 1626 | 10.5 ± 2.1 |
| MTX+UAE+hysteroscopy | 5 | 2.3 ± 1.6 | 31317 ± 24 941 | 1607 ± 2238 | 80 ± 28 | 10.5 ± 2.1 |
| Total hysterectomy | 1 | 6.7 ± 6.4 | 815 | 116 | 2000 | 11 |
Table 1. Comparison of clinical parameters among all treatment.
MTX plus ultrasound assisted uterine curettage
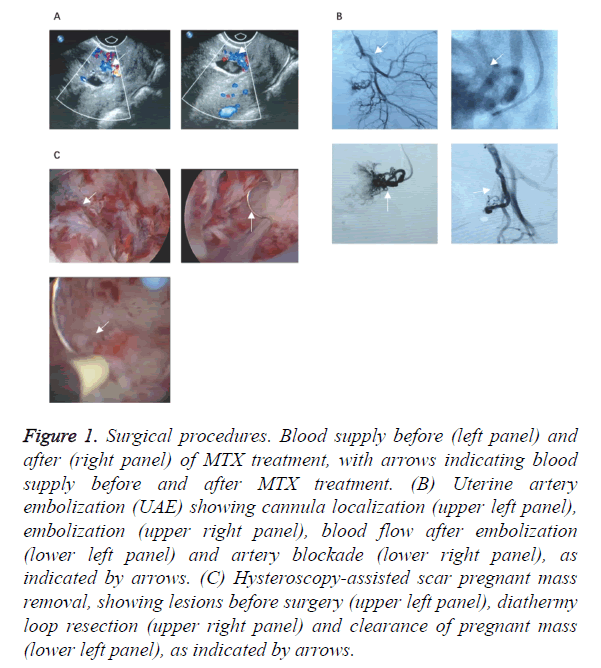
Five patients received MTX treatment plus ultrasound assisted uterine curettage. Four of them were diagnosed as CSP at early stage. Clinical manifestations included minor bleeding from vagina, without lower abdominal pain. Average size of mass was 0.75 ± 0.4 cm, with HCG at 2867 ± 3956 IU/L, plus 125 ± 35.4 ml pre-op bleeding volume. After surgery, HCG level was significantly decreased (2167 ± 64 IU/L, p<0.05 by student t-test). The hospitalization period of all patients ranged from 4 to 19 d (average=6.5 ± 3.5 d). Blood supply conditions at uterine scar sites before and after MTX application were shown in Figure 1A.
Figure 1. Surgical procedures. Blood supply before (left panel) and after (right panel) of MTX treatment, with arrows indicating blood supply before and after MTX treatment. (B) Uterine artery embolization (UAE) showing cannula localization (upper left panel), embolization (upper right panel), blood flow after embolization (lower left panel) and artery blockade (lower right panel), as indicated by arrows. (C) Hysteroscopy-assisted scar pregnant mass removal, showing lesions before surgery (upper left panel), diathermy loop resection (upper right panel) and clearance of pregnant mass (lower left panel), as indicated by arrows.
UAE plus ultrasound assisted uterine curettage
A total of 10 CSP patients received ultrasound assisted uterine curettage, which was performed at 48~72 h after UAE. Before surgery, averaged mass size was 1.6 ± 1.4 cm, haemorrhage volume was 1350 ± 1626 ml, and HCG level was 105892 ± 133088 IU/L. Minor bleeding occurred during the surgery. Post-up HCG level was rapidly decreased to 2389 ± 3160 IU/L (p<0.05 by student t-test), with complete clearance of mass. The average hospitalized period was 10.5 ± 21 days. UAE procedure was illustrated in Figure 1B.
UAE plus resection of scar pregnant mass under hysteroscopy
Five patients received resection of scar pregnant mass under hysteroscopy at 48~72 h after UAE surgery. Before surgery, averaged mass size was 2.3 ± 1.6 cm, haemorrhage volume was 80 ± 28 ml, and HCG level was 31371 ± 24941 IU/L. No major bleeding occurred during the surgery. HCG level was rapidly decreased to 1607 ± 2238 IU/L (p<0.05 by student t-test), with complete clearance of mass. The average hospitalized period was 10.5 ± 2.1 d. Surgical procedure was shown in Figure 1C.
Total hysterectomy
One 39 y old patient received Cesarean section 4 y ago, followed by three artificial miscarriages. The patient was misdiagnosed as early intra-uterus pregnancy and received drug abortion plus uterine curettage. 24 d afterwards, 3 h of major bleeding (~2000 ml) occurred. Ultrasound examination showed a 6.7 × 6.4 cm mass with mixed echo at lower cervical region, with sufficient blood flow and thinning of scar tissue at lower uterus. Intra-uterus cavity was packed before transferring to our hospital. HCG level before surgery was 815.9 IU/L. Total hysterectomy was performed UAE under patient’s willingness. HCG level dropped to 116.8 IU/L at 3 d after surgery (p<0.05 by student t-test). Patient was hospitalized for 11 d.
Discussion
Due to the relatively lower incidence, there has been no unified treatment plan for CSP [5,6]. It is necessary to terminate pregnancy once being diagnosed with CSP, as it can cause uncontrollable bleeding by uterus rupture. The maintenance of fertility of young patients is one major concern. In past decades, total hysterectomy is the most popular surgical plan to avoid severe bleeding [7]. With advancement of ultrasound diagnosis, CSP now has multiple treatment choices including medicine or surgery, the latter of which includes MTX +ultrasound assisted uterine curettage, UAE+ultrasound assisted uterine curettage, UAE+resection of scar pregnant mass under hysteroscopy and total hysterectomy. This study thus compared and contrasted these four surgical approaches by analysing clinical indexes in a retrospective manner.
MTX is the most common drug for ectopic pregnancy with definitive efficacy as it can inhibit proliferation of trophoblast and inactivate embryos, thus invading bleeding during uterine curettage. MTX+ultrasound assisted uterine curettage can be applied in those CSP patients with minor conditions. Previous studies suggested MTX treatment followed by ultrasound assisted uterine curettage could be applied to those CSP patients with stable vital signs, less virginal bleeding, no abdominal haemorrhage and more than 2 mm sero-muscular layer [8,9]. In this study, 5 out of 21 patients received MTX +ultrasound assisted uterine curettage and obtained satisfactory results with shorter hospitalization period. Therefore, we believed that MTX+ultrasound assisted uterine curettage could be applied to CSP patients with less than 1.0 × 1.0 cm mass, lower than 3000 IU/L pre-op HCG level, less bleeding and stable vital signs.
UAE can be applied in CSP patients, with MTX infusion during the embolization to further decrease blood HCG level for decreasing blood supply at uterine scar tissues, thus preparing for further uterine curettage. The blind uterine curettage in CSP patients often causes uncontrollable haemorrhage and should be avoided. The application of uterine curettage after UAE surgery is still debatable at current stage. Some scholars believed that uterine curettage could be applied to those with minor bleeding, lower HCG (<1000 IU/L), smaller pregnant mass (≤ 3 cm) and far away from the seromuscular layer (≥ 2 mm) with insufficient blood supply at scar site [10]. We performed uterine curettage on 10 patients within 48~72 h after UAE and obtained satisfactory results as complete removal of mass without major haemorrhage, and rapid decrease of post-op HCG. Our results showed that uterine curettage could be performed after UAE surgery in CSP patients with small mass (<2.0 × 2.0 cm) and insufficient blood supply at scar tissues+ultrasound assisted uterine curettage.
UAE+resection of scar pregnant mass under hysteroscopy is recognize as one treatment method for CSP [11]. It has advantages as visual guidance during removal of pregnant mass, plus clotting haemostasis. It is thus applicable for those patients with inward growing pregnant mass. However, this approach has not been widely promoted, and has potential risks as the require for open surgery once encountered major bleeding under hysteroscopy, thus causing major trauma wounds for patients, affecting its promotion. Recently, the intravaginal incision of uterus has been suggested in CSP radical surgery, as it has relatively shorter operation time, less trauma or bleeding, rapidly decreased HCG level and faster recovery of menstrual cycle, and is thus one alternative treatment plan for CSP [12]. We performed 5 cases of UAE +resection of scar pregnant mass under hysteroscopy. Patients had relatively larger mass (2.3 × 1.55 cm on average) and higher HCG level (31371 ± 24941 IU/L) compared to other two groups. However, no major bleeding occurred during the surgery. Patients had satisfactory recovery and rapidly decreased HCG. In sum, UAE+resection of scar pregnant mass under hysteroscopy may be applied to those with larger mass size (2.0 × 2.0 cm to 3.0 × 3.0 cm), stable vital signs and insufficient blood supply at scar tissues.
Total hysterectomy is the last resort method when major haemorrhage threatens patient’s life and no other choices are available in CSP patients. The primary principle in CSP treatment is to retain fertile function to maximal level. Therefore, total hysterectomy may be only available for those patients with abundant haemorrhage or even shock, and occurrence of severe complications such as DIC. In this study, only one patient chose this approach due to relatively larger vaginal bleeding, plus no willingness of pregnancy in future. Satisfactory results were obtained by hysterectomy as bleeding was effectively managed and lab results rapidly restored to normal levels.
In summary, this study indicates the necessary for individualized treatment on CSP patients. Major consideration should be made according to the size of pregnant mass, pre-op HCG level and vaginal bleeding volume [13]. Three approaches, including MTX+ultrasound assisted uterine curettage, MTX+UAE+ultrasound assisted uterine curettage, and MTX+UAE+resection of scar pregnant mass under hysteroscopy can all achieve satisfactory efficacy once being chosen and applied reasonably and appropriately. All these methods had minor surgical trauma, less post-op complication, plus the ability to retain of uterine and fertile functions. The selection among these methods should be made based on mass size, pre-op haemorrhage volume and pre-op HCG level. In general, we believe that MTX+curettage may be applied to patients with smaller lesion size (<1 cm), relatively lower pre-op HCG level (~3000 IU/L) and relatively less haemorrhage (~100 ml). MTX+UAE+curettage can be employed for those patients with medium mass size (~1.5 cm), higher pre-op HCG level (>100000 IU/L), and more haemorrhage (>1000ml). MTX+UAE+resection of scar pregnant mass under hysteroscopy can be applied in those with larger mass size (>2 cm), relatively higher pre-op HCG (around 30000 IU/L), and fewer haemorrhage (~80 ml). In one word, MTX+UAE +curettage can be applied in the most severe CSP cases, whereas MTX+curettage can be adopted to patients with smaller mass size, and MTX+UAE+hysteroscopy is appropriate for patients with larger mass size but fewer bleeding. As an alternative choice, total hysterectomy can be applied to patients with severe bleeding and under life threatening critical conditions. CSP mainly occurs in scar tissues after caesarean section, therefore, caesarean section should be strictly controlled under normal circumstance. During caesarean section, the uterus wound should be carefully sutured to accelerate wound healing. Once having found CSP, early diagnosis and treatment should be performed immediately to stipulate individualized treatment plan, to achieve the best prognosis for patients.
Treatment for CSP should be individualized based on size of pregnant mass, HCG level before treatment and haemorrhage volume of vagina: (1) For those lesions smaller than 1.0 × 1.0 cm and pre-op HCG level<3000 IU/L, minor vaginal bleeding and stable vital signs, MTX+curettage can be performed under ultrasound monitor. (2) For pregnant mass<2.0 × 2.0 cm, with insufficient blood supply at uterus scar, curettage can be performed at 48~72 h after UAE surgery. (3) Post-UAE hysteroscopy can be performed to clear pregnant mass with size between 2.0 × 2.0 cm ~3.0 × 3.0 cm, with minor virginal bleeding, stable vital signs and insufficient blood supply at scar site; (4) For those patients having no birth plan, major bleeding, unstable vital signs or even shock or DIC, hysterectomy can be performed. In summary, under optimized choice, all treatment measures as above-mentioned can achieve satisfactory efficacy.
Acknowledgment
None
Conflict of Interest
All authors declare that they have no conflict of interest.
Funding
This study is sponsored by Changzhou youth medical innovation talent project funding [NO (2010) 368] and Changzhou Youth Science and technology talent project [QN 201406 KY201445].
References
- Godin PA, Bassil S, Donnez J. An ectopic pregnancy developing in a previous caesarian section scar. Fertil Steril 1997; 67: 398-400.
- Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL. Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol 2004; 23: 247-253.
- Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol 2003; 21: 220-227.
- Fylstra DL, Pound-CT, Miller MG, Cooper A, Miller KM. Ectopic pregnancy within a cesarean delivery scar: a case report. Am J Obstet Gynecol 2002; 187: 302-304.
- Hsieh BC, Hwang JL, Pan HS, Huang SC, Chen CY, Chen PH. Heterotopic Caesarean scar pregnancy combined with intrauterine pregnancy successfully treated with embryo aspiration for selective embryo reduction: case report. Hum Reprod 2004; 19: 285-287.
- Wang CJ, Chao AS, Yuen LT, Wang CW, Soong YK, Lee CL. Endoscopic management of cesarean scar pregnancy. Fertil Steril 2006; 85: 494.
- Ghezzi F, Lagana D, Franchi M, Fugazzola C, Bolis P. Conservative treatment by chemotherapy and uterine arteries embolization of a cesarean scar pregnancy. Eur J Obstet Gynecol Reprod Biol 2002; 103: 88-91.
- Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol 2006; 107: 1373-1381.
- Rheinboldt M, Osborn D, Delproposto Z. Cesarean section scar ectopic pregnancy: a clinical case series. J Ultrasound 2015; 18: 191-195.
- Shu SR, Luo X, Wang ZX, Yao YH. Cesarean scar pregnancy treated by curettage and aspiration guided by laparoscopy. Ther Clin Risk Manag 2015; 11: 1139-1141.
- Deans R, Abbott J. Hysteroscopic management of cesarean scar ectopic pregnancy. Fertil Steril 2010; 93: 1735-1740.
- Yamaguchi M, Honda R, Uchino K, Tashiro H, Ohba T, Katabuchi H. Transvaginal methotrexate injection for the treatment of cesarean scar pregnancy: efficacy and subsequent fecundity. J Minim Invasive Gynecol 2014; 21: 877-883.
- Timor-Tritsch IE, Monteagudo A, Cali G, Vintzileos A, Viscarello R, Al-Khan A, Zamudio S, Mayberry P, Cordoba MM, Dar P. Cesarean scar pregnancy is a precursor of morbidly adherent placenta. Ultrasound Obstet Gynecol 2014; 44: 346-353.