ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2014) Volume 25, Issue 1
Comparison of FSH Receptor Polymorphisms Between Infertile and Fertile Women
1Department of Obstetrics and Gynecology, Susehri Government Hospital, Sivas, Turkey
2Department of Obstetrics and Gynecology, Akdeniz University, Antalya, Turkey
3Department of Medical Genetics, Akdeniz University, Antalya, Turkey
4Department of General Surgery, Susehri Government Hospital, Sivas, Turkey
Accepted date: November 04 2013
Many mutations change the function of FSHR (follicle-stimulating hormone receptor) at the plasma membrane. Mutations in FSHR can lead to ovarian dysgenesis and high gonadotropin levels. We aimed to investigate and compare the frequency of FSHR polymorphism (Ala307Thr and Ser680Asn) in fertile and infertile women. Our study enrolled all of the patients between 21 and 39 years of age who were admitted to the Department of Obstetrics and Gynecology at Akdeniz University between May 2009 and November 2010. The patients were divided into two groups corresponding to fertile and infertile. The infertile patients were divided into two subgroups corresponding to good and poor responders based on their response to fertility treatment. The genomic DNA was analyzed, and the Ala307Thr and Ser680Asn polymorphisms were screened in each group. We determined that in the good responder group, homozygosity for the polymorphisms was significantly less frequent (p=0.013). We also found that in the good responder group, the homozygous genotype (A/A) for the Ser680Asn polymorphism was significantly less frequent (p=0.013). In the poor responder group, heterozygosity for the polymorphism Ser680Asn was significantly more frequent (p=0.031). Because a high frequency of the homozygous genotype was found in the control group, we are unable to conclude that a homozygous genotype for these polymorphisms leads to a poor FSH receptor response. However, the frequency of the homozygous genotype was high for both Ser680Asn and Ala307Thr in the poor responder group. Doubleblinded, randomized, controlled, and prospective studies should be conducted with larger samples to reach a definite conclusion.
Keywords
FSHR polymorphism; Ala307Thr; Ser680Asn; fertility; ovarian response
Introduction
The FSH receptor (FSHR) is composed of 678 amino acids [1-4]. Recent studies have provided a better understanding of FSHR, including its crystalline structure, which contains a substantial extracellular domain (ECD), and the three dimensional configuration of the receptor and its involvement in binding to the ligand [5]. FSH (follicle-stimulating hormone) binds tightly to the concave surface of the ECD of FSHR. The receptor wraps itself around the central zone of the hormone. The contact area between the hormone and the receptor is large and highly electrically charged. Many coincidental hormone receptor mutations have been reported [6-10].
Many mutations reduce the localization of FSHR at the plasma membrane. Mutations in FSHR lead to ovarian dysgenesis and high gonadotropin levels. Moreover, defects of the FSHR gene in mice lead to a small uterus, small ovaries, and sterility [11].
Only five FSHR single nucleotide polymorphisms (SNPs) are exogenous, and they are all located on Exon 10. Four out of five of these SNPs lead to a code change (Ala307Thr, Arg524Ser, Ala665Thr and Ser680Asn). The most common and well-studied are Ala307Thr and Ser680Asn. These SNPs are not in a steady state and are known as allelic variants. Thr307/Asn680 and Ala307/Ser680 are equally distributed among white populations. Recent studies have shown that polymorphisms of the alpha gene of the estrogen receptor result in changes in the response to ovulation induction therapies [12].
This study aims to investigate and compare the frequency of FSH receptor polymorphisms (at positions 307 and 680) in fertile and infertile women.
Materials and Methods
The current study enrolled female patients between 21 and 39 years of age who were admitted to the Gynecology and Obstetrics Department at the Medical Faculty of Akdeniz University between May 2009 and November 2010. IRB (institutional review board) approval was obtained for this study.
For patients in the control group, the inclusion criterion was pregnancy achieved within one year after first intercourse without any fertility therapy. The patients who achieved an oocyte count higher than three after controlled ovarian hyper-stimulation for the treatment of infertility were included in the good responder group. Patients who could not achieve a sufficient E2 level (E2 ≤ 660 pg/mL) following infertility treatment were excluded [13-15]. For the group of poor responders, the inclusion criteria were: oocyte count ≤ 3 following controlled ovarian hyper-stimulation therapy and treatment-emergent E2 level < 660 pg/mL [13-15].
There were 100 patients in the control group, 42 good responders and 40 poor responders. Venous blood samples (5 ml/patient) were drawn into K3EDTA sterile blood tubes. The samples were stored at +4oC until DNA isolation. The genomic DNA was extracted from the venous blood using a modified non-enzymatic method. The data were analyzed using SPSS (version 18.0 statistics software pack) (SPSS Inc., Chicago, USA). Descriptive statistics were used, including the distribution of the frequency, the mean, and the standard deviation. Kruskal Wallis analysis was used to compare the Ala307 and Ser680 polymorphisms. In the case of any difference, the Mann Witney U test was used to identify the difference after the Bonferroni correction was performed. Moreover, the Mann-Whitney U test or the Student’s ttest was used when the demographics of the A/A+G/A genotype and the G/G genotype were compared. The Chisquared test was used in the inter-group analysis of the genotypes. The significance level was 95% (or alpha = 0.05 margin of error) to determine the analytical differences. Molecular genetic studies were conducted on the FSHR gene in the genomic samples from the study participants. The FSHR gene region in the good responders, poor-responders, and control subjects was amplified using the PCR technique. The resultant amplifications products were analyzed on a 1% agarose gel.
Results
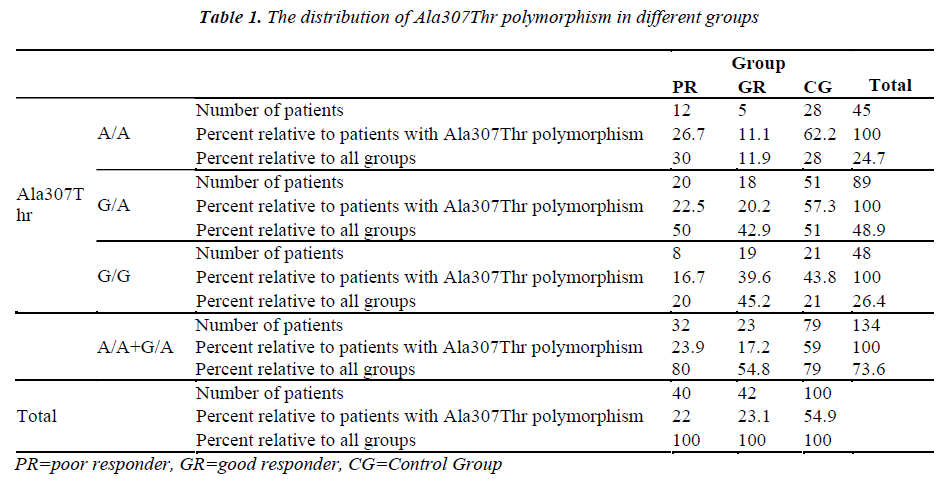
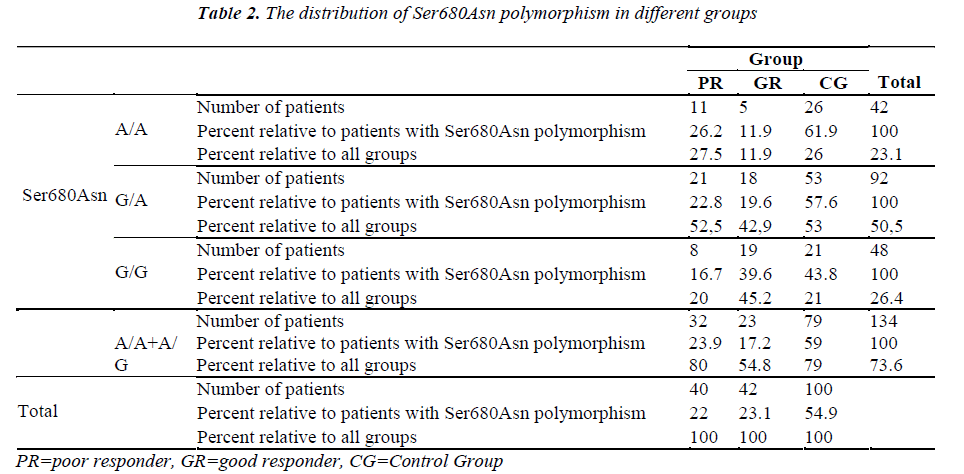
The Ala307Thr and Ser680Asn polymorphisms were screened in the genomic DNA samples of the control and study groups. The identified polymorphisms were separately analyzed for two regions. Homozygous (A/A) and heterozygous (G/A) genotyping were performed. The homozygous G/G genotype was reported if no polymorphism was found in the region analyzed.
The genetic analysis of the control patients indicated that 28 were homozygous A/A and 51 were heterozygous G/A in the Ala307Thr (GCT >ACT) region, and 26 were homozygous A/A and 53 were heterozygous G/A in the Ser680Asn (AGT >AAT) region.
Seven patients had positive b-hCG levels on the 14th day after embryo transfer. Genetic analysis of the genomic DNA samples obtained from 42 good responders revealed homozygous A/A polymorphism in 5 patients and heterozygous G/A polymorphism in 18 patients for both the Ala307Thr and Ser680Asn regions. No statistically significant difference was found between the A/A, G/A and G/G groups with respect to age, FSH on Day 3, the dose and duration of gonadotropin (p=0.669/ 0.818 / 0.057 / 0.058, respectively).
The treatment protocol of the poor responders was an antagon protocol. The FSH values on Day 3 ranged from 5 mIU/mL to 27 mIU/mL (mean: 11.82±4.12). The E2 values ranged between 4 pg/mL and 82 pg/mL (mean: 35.88±15.68). No follicle development was observed in 14 patients although high dose of gonadotropin was administered throughout the treatment period and the treatment cycle was cancelled. The mean follicle count was 1.13±0.966 for the poor responders. A total of 22 patients had the embryo transfer procedure, and the venous ß-hCG level was high in 3 patients only. Two of these patients delivered uneventfully. One of them aborted at 8 weeks of gestation. Based on the genetic analysis of 40 poor responders, there were 12 patients with A/A genotype and 20 patients with the G/A genotype for the Ala307Thr region. No statistically significant difference was found between the A/A, G/A and G/G groups with respect to age (p=0.131), FSH level on Day 3 (p=0.427), dose of gonadotropin (p=0.132), and treatment period (p=0.582). A genetic analysis was conducted to examine the Ser680Asn polymorphism in the poor responders, and it was observed that 11 patients were homozygous for the A/A genotype, and 21 patients were heterozygous for the G/A genotype. Significant difference was not found between the A/A, G/A and G/G groups with respect to age (p=0.145), level of FSH on Day 3 (p=0.367), dose of gonadotropin (p=0.226) and treatment period (p=0.841).
Results of Inter-group Comparisons
The number of patients and rates are given for the three groups in the Tables 1-2. Statistically significant difference was found between the poor and good responders with regard to the presence of polymorphisms and the types of polymorphisms (for Ala307Thr region: p=0.024) (for Ser680Asn region: p=0.031). There was no statistical significance in terms of the existence of polymorphisms between the poor responder group and the control group (for Ala307Thr region: p=0.971) (for Ser680Asn region: p=0.980). The good responder group was also examined in comparison with the control group. Statistically significant difference was found between the A/A, G/A and G/G genotypes (for the Ala307Thr region: p=0.007) (for the Ser680Asn region: p=0.009). The frequency of polymorphisms was higher in the control group.
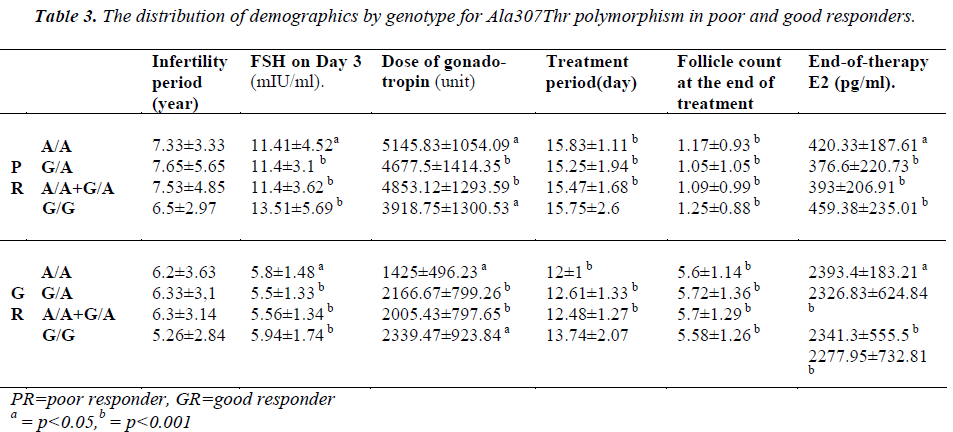
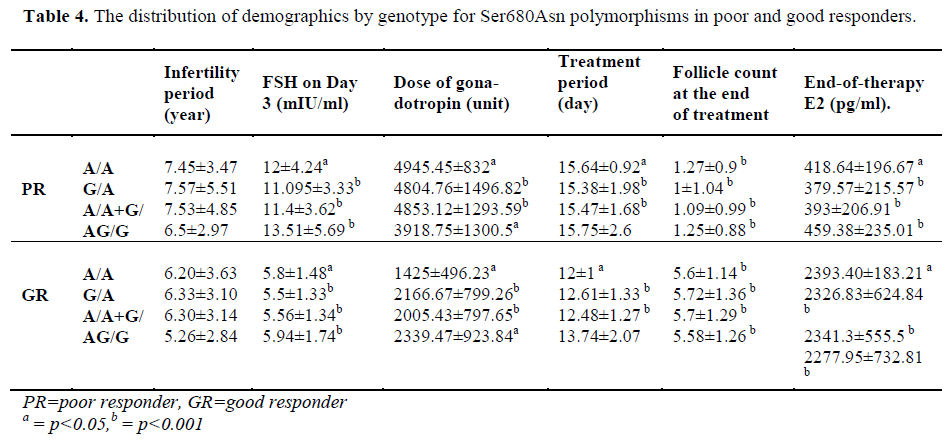
The patients of the poor responder, good responder, and control groups were compared with respect to demographics and the presence of polymorphisms (Table 3-4).
Discussion
The gene encoding the FSH receptor is found on chromosome 2 [16]. Structural changes in this region will lead to changes in the amino acid configuration of the FSH receptor gene, resulting in functional changes in the gene. Some of these changes lead to enhanced functioning of the receptor, while some reduce functionality.
Inactivating mutations of FSHR may result in primary and secondary amenorrhea, infertility and premature ovarian syndrome (POS). In contrast, activating mutations may lead to ovarian hyper-stimulation syndrome (OHSS), irrespective of exogenous FSH replacement. In addition to point mutations, a response to the exogenous FSH may be seen in the case of FSHR gene polymorphisms (particularly, codon 307 and 680) [16]. In such cases, the changes will be observed in response to therapy when assisted reproductive techniques are practiced.
Loutradis et al. [13] examined Ser680Asn polymorphisms of the FSH receptor in a study conducted in 2006. The patients were examined only with respect to the Ser680Asn polymorphism, and the patients were classified according to their Ser/Ser (G/G), Ser/Asn (G/A) and Asn/Asn (A/A) genotypes. We used a similar classification for Ser680Asn, but we investigated another polymorphism of the FSH receptor, namely Ala307Thr. Loutradis et al. [13] observed that the distribution of three genotypes was similar in the poor-responders. However, the Ser/Asn polymorphism was more frequently observed in the good-responders (p=0.005). In the current study, the rate of homozygous Ala307Thr polymorphism appears significantly lower in the good responder group (p=0.013). Similarly, we found that homozygous genotype (A/A) of the Ser680Asn polymorphism was significantly lower in the good responder group (p=0.013). We performed a statistical comparison between the patients in the poor responders. When the group of poor responder was examined with respect to the Ser680Asn polymorphism, we found a significantly higher frequency of heterozygous polymorphism (p=0.031). In our control group, we found that a heterozygous genotype was more frequent for both the Ser680Asn and Ala307Thr polymorphisms in comparison with the good responders (the frequency of the Ser680Asn G/A and the Ala307Thr G/A polymorphism was significantly high (p=0.001).
Loutradis et al. [13] found that at the end of therapy, E2 levels were higher in patients with G/A genotype in the good responders. However, in the current study, no significant difference was found between the genotypes in good responders with respect to both Ser680Asn and Ala307Thr (p=0.779). Treatment periods were compared, but no difference was evident. However, when all patients with polymorphism were examined, we found a significant increase in treatment period in comparison with patients with the (A/A+G/A) genotype (for both Ala307Thr and Ser680Asn, p=0.024).
Castro et al. studied the effects of Ser680Asn polymorphism [14]. The authors observed that the Ser/Ser (G/G) genotype was more common in the poor responders. We, however, found that the number of patients with the G/G genotype was significantly lower in the group of poor responder in comparison with the group of good responder (for both Ser/Ser and Ala/Ala; p = 0.024). Moreover, we compared all patients with genotype (A/A+G/A) with patients with normal genotype and observed that the number of patients with G/G genotype (Ser/Ser and Ala/Ala) was significantly higher in the good responders [Table 2]. In addition, Castro et al. [14] reported that although it was not statistically significant, patients with the Ser/Ser genotype could be induced with a higher dose of gonadotropin. We did not find any significant difference between the genotypes in good responders as well as in poor responders with respect to gonadotropin (for the poor responders, p=0.226; for the good responders, p=0.057). Castro et al. [14] tried to find out a correlation between the age and genotype, but could not find any significant difference. We made intra-group comparisons, including the control group, to investigate the relationship between polymorphhism and age, but did not find any difference for the controls (p=0.447), for the good responders (p=0.669), or for the poor responders (p=0.13). In conclusion, Although Castro et al. concluded that polymorphism could correlate with age and ovarian reserve, yet they failed to show statistically significant results [14].
In a study conducted in 2005, Koning et al. [15] compared 38 poor responders with 50 good responders with respect to the Ser680Asn polymorphism. The authors found that the number of patients with polymophism was significantly higher among the poor responders (p<0.05). We made comparisons between the two groups, and we found a statistically significant difference for both the Ala307Thr and Ser680Asn polymorphism [the number of patients with the polymerphism was higher in the poor responders (p=0.015 for Ala307Thr; p=0,031 for Ser680Asn)] (Table 1, 3). Again, two groups were compared with respect to the treatment period, and similar to our study, a significantly longer period was found in the poor responders. Koning et al. [15] examined the relationship between age and genotype, but they could not find any significant difference (p=0.8). Similarly, Van Montfrans et al. [17] and Van Rooij et al. [18] could not find significant differences with respect to FSH polymorphism.
Hasbargen et al. [19] examined the distribution of polymorphisms in the European population and obtained the rates of 22.3%, 55.5%, and 22.3% for the G/G, G/A and A/A genotypes, respectively. Conversely, Perez et al. [20] reported the rates of 29%, 45%, and 26% for these three genotypes, respectively. Sudo et al. [21] reported rates of 43.5%, 43.5%, and 12.1%, respectively, for the Japanese population. In the current study, the rates were found to be 20%, 52.5%, and 27.5%, respectively for ser680Asn, and 20%, 50%, and 30%, respectively, for Ala307Thr.
Achrekar et al. [22] conducted a study in India in 2009 and reported on 50 normogonadotropic infertile patients. Similar to the current study, they investigated both the Ala307Thr and Ser680Asn polymorphisms. The rate of heterozygosity for the polymorphisms was found to be above 50% in both the poor and good responder groups. Similarly, the frequency of heterozygous (G/A) genotype was high (in the poor responders: Ala307Thr 50%, Ser680Asn 52.5%; in the good responders: Ala307Thr 42.9%, Ser680Asn 42.9%).
In conclusion, a high frequency of the homozygous genotype was found in the control group prevents us from making a generalized conclusion that a homozygous polymorphism leads to a poor FSH receptor response, although the frequency of homozygous genotype was found to be high for both Ser680Asn and Ala307Thr in the group of poor responder. Double-blinded, randomized, controlled, and prospective studies should be conducted with larger samples to reach a definite conclusion.
References
- Minegishi T, Nakamura K, Takakura Y. Cloning and sequencing of human LH/hCG receptor cDNA. Biochem Biophys Res Commun 1990; 172: 1049-1054.
- Jia C, Oikawa M, Bo M. Expression of human luteinizing hormone (LH) receptor: interaction with LH and chorionic gonadotropin from human but not equine, rat, and ovine species. Mol Endocrinol 1991; 5: 759-768.
- Minegishi T, Nakamura K, Takakura Y. Cloning and sequencing of human FSH receptor cDNA. Biochem Biophys Res Commun 1991; 175: 1125-1130.
- Kelton C, Cheng SVY, Nugent NP. The cloning of the human follicle stimulating hormone receptor and its expression in cos-7, cho, and y-1 cells. Mol Cell Endocrinol 1992; 89: 141-151.
- Sanders J, Chirgadze DY, Sanders P. Crystal structure of the TSH receptor in complex with a thyroidstimulating autoantibody. Thyroid 2007; 17: 395-410.
- Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor: a 2002 perspective. Endocr Rev 2002; 23: 141-174.
- Costagliola S, Urizar E, Mendive F. Specificity and promiscuity of gonadotropin receptors. Reproduction 2005; 130: 275-281.
- Latronico AC, Segaloff DL. Naturally occurring mutations of the luteinizing-hormone receptor: lessons learned about reproductive physiology and G proteincoupled receptors. Am J Hum Genet 1999; 65: 949-958.
- Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitarygonadal function. Endocr Rev 2000; 21: 55105-55183.
- Piersma D, Verhoef-Post M, Berns EMJJ. LH receptor gene mutations and polymorphisms: an overview. Mol Cell Endocrinol 2007; 260-2: 282-286.
- Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M. Impairing folliclestimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA 1998; 95: 13612–13617.
- Sundarrajan C, Liao W, Roy AC, Ng SC. Association of oestrogen receptor gene polymorphisms with outcome of ovarian stimulation in patients undergoing IVF. Mol Hum Reprod 1999; 5: 797–802.
- Loutradis D, Patsoula E, Minas V, Koussidis G, Antsaklis A, Michalas S, Makrigiannakis A. FSH receptor gene polymorphisms have a role for different ovarian responde to stimulation in patients entering IVF/ICSI-ET programs. J Assist Repro Gene 2006; 23: 177-184
- Castro F, Ruiz R, Montoro L, Perez D, Sanchez E, Real L, Ruiz A. Role of follicle-stimulating hormone receptor Ser680Asn polymorphisms in the efficacy of follicle-stimulating hormone. Fertil Steril 2003; 80: 571-576.
- Koning CH, Benjamins T, Harms P, Homburg R, Montfrans J, Gromoll J, Simoni M, Lambalk CB. The distribution of FSH receptor isoforms is related to basal FSH levels in subfertile women with normal menstrual cycles. Hum Repro 2006; 21: 443-446.
- Keuchler A, Hauffa B, Köninger A, Kleinau G, Albrecht B, Horsthemke B, Gromoll J. An unbalanced translocation unmasks a recessive mutation in the follicle-stimulating hormone receptor (FSHR) gene and causes FSH resistance. Eur J Hum Genet 2010; 74:656-661. doi:10.1038/ejhg.2009.24
- Van Montfrans JM, Hoek A, van Hooff MHA, de Koning CH, Tonch N, Lambalk CB. Predictive value of basal FSH concentrations in a general subfertility population. Fertil Steril 2000; 74: 97–103.
- Rooij IA, Bancsi LF, Broekmans FJ, Looman CW, Habbema JD, Velde ER. Women older than 40 years of age and those with elevated follicle-stimulating hormone levels differ in poor response rate and embryo quality in in vitro fertilization. Fertil Steril 2003; 79: 482–488.
- Hasbargen U, Thaler CJ, Ruebsamen H, Fuchshuber S, Lohse P. S680N substitution of the follicle-stimulating hormone receptor is a common polymorphism not associated with spontaneous human twinning. Eur J Med Rep 2001; 6: 315–316.
- Perez M, Gromoll J, Behre HM, Gassner C, Hieschlag E, Simoni M. Ovarian response to follicle-stimulating hormone (FSH) stimulation depends on the FSH receptor genotype. J Clin Endocrinol Metab 2000; 85: 3365–3369.
- Sudo S, Kudo M, Wada S. Genetic and functional analyses of polymorphisms in the human FSH receptor gene. Mol Hum Reprod 2002; 8: 893–899.
- Achrekar S, Modi D, Desai SK, Mangoli VS, Mangoli RV, Mahale SD. Follicle-stimulating hormone receptor polymorphism (Thr307Ala) is associated with variable ovarian response and ovarian hyperstimulation syndrome in Indian women. Fertil Steril 2009; 91: 432-439.