ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Review Article - Biomedical Research (2017) Volume 28, Issue 13
Comparison of subthalamic deep brain stimulation with pedunculopontine nucleus stimulation in the treatment of Parkinsonian motor and axial symptoms with and without subthalamic stimulation
Chenyu Ding, Lianghong Yu, Hongliang Ge, Yuangxiang Lin, Xiaobin Zheng, Zhangya Lin and Dezhi Kang*
Department of Neurosurgery, the First Affiliated Hospital of Fujian Medical University, Fuzhou, PR China
- *Corresponding Author:
- Dezhi Kang
Department of Neurosurgery
The First Affiliated Hospital of Fujian Medical University, PR China
Accepted date: May 24, 2017
Aims: The study compares efficacy of Pedunculopontine Nucleus (PPN) Deep Brain Stimulation (DBS), subthalamic DBS and combined PPN-subthalamic stimulation in the treatment of advanced Parkinson’s Disease (PD).
Methods: MEDLINE/PubMed, EMBASE, and Cochrane Library were searched for studies published before September 1, 2015. Search terms included various combinations of “subthalamic”, “pedunculopontine”, “deep brain stimulation” and “Parkinson disease”. Two investigators independently examined titles, abstracts, and references. Results of studies on clinical outcomes of PPN DBS and subthalamic DBS for PD were analysed. Therapeutic outcomes of included studies were evaluated using Unified Parkinson’s Disease Rating Scale III (UPDRSIII). Meta-analysis was performed using Review Manager Software version 5.3.
Results: Three studies were included in the meta-analysis. Subthalamic DBS reduced UPDRSIII scores (off-medication phases) to levels lower than PPN DBS. However, improvement in UPDRSIII axial subscores (on-medication phases) after PPN DBS was greater than that after subthalamic DBS. UPDRSIII scores for combined PPN-subthalamic DBS were not significantly different from subthalamic DBS (on- and off-medication phases). Improvements in UPDRSIII axial subscores for combined PPNsubthalamic DBS were significantly greater than subthalamic DBS (on- and off-medication phases).
Conclusions: The study demonstrates differences in therapeutic efficacy for Parkinsonian motor and axial symptoms between PPN DBS and subthalamic DBS based on UPDRSIII scores and axial UPDRSIII subscores. PPN is a better target for PD gait disorders than subthalamic nucleus in onmedication phases. Subthalamic DBS has similar effect on postoperative motor features with combined PPN-subthalamic stimulation. Combined PPN-subthalamic DBS is more effective than subthalamic DBS in treating axial symptoms of PD.
Keywords
Parkinson’s disease, Deep brain stimulation, Subthalamic nucleus, Pedunculopontine nucleus, Axial symptom
Introduction
Axial signs, including gait impairment, postural abnormalities and postural instability, are the most severe problems in patients with advanced Parkinson’s disease (PD) [1]. Axial signs usually occur when a patient’s attention is shifted or during a directional change, and they are responsible for falls as the upper body continues moving forward while the feet remain glued to the ground [2]. Axial signs are disabling phenomena that affect the autonomy and life quality of patients [3]. However, the efficacy of dopaminergic regulations and the standard Deep Brain Stimulation (DBS) on axial signs are not satisfactory and still remain controversial [4].
Due to the role in gait initiation, the modulation of Pedunculopontine Nucleus (PPN) activity by DBS has attracted a lot of concerns [5]. According to experiments on nonhuman primate model of PD, low-frequency PPN stimulation has improved movement counts, posture, and balance [6-8]. Other studies show that low-frequency PPN stimulation selectively improves gait dysfunction in patients with PD [9-11]. Bilateral stimulation of mid-lower PPN without co-stimulation of Subthalamic Nucleus (STN) is beneficial for PD patients who still have severe freezing of gait, postural instability, and falls even on medication [12].
Subthalamic DBS is a potential treatment of axial Parkinsonian symptoms [13-17]. It improves not only limb motor symptoms but also gait dysfunction in PD [18]. Hamani et al. have reported an improvement of 64% for gait and 69% for postural stability one year after STN DBS surgery [19]. Bejjani et al. also conclude that axial Parkinsonian symptoms can be improved by subthalamic stimulation at 6 months after surgery [20]. Subthalamic DBS improves posture and gait dysfunction by directly affecting PPN [18,21]. In addition, Khan et al. report that PPN and STN DBS synergistically alleviate axial signs of advanced PD patients [22]. The authors refer to the subthalamic target of stimulation as caudal Zona Incerta (cZI), which is the area immediately dorsal to STN [22]. Lower frequency cZI DBS leads to motor improvement with a similar magnitude as that by conventional high-frequency STN DBS [23]. Of note, the combination of PPN and subthalamic DBS seems to be superior to subthalamic stimulation alone when tested on medication [22]. However, some noteworthy exceptions still exist [11,24].
There have been some reports on partial improvements in gait dysfunction and postural instability with PPN stimulation [11,22,24-28], but the number of cases is still small. A recent study on ten PD patients shows that STN DBS improves axial symptoms by modulating PPN/mesencephalic locomotor region activity [29]. It is still controversial whether the optimal site for stimulation is situated in PPN or STN. In addition, it is also unclear whether the combination of PPN and subthalamic stimulation has superior effects than individual stimulation of either region. In the present study, we perform a meta-analysis to assess the overall efficacy of PPN DBS, subthalamic DBS and the combination of both in PD patients with motor and axial symptoms.
Materials and Methods
Literature search
Literatures were carefully searched in databases including MEDLINE, PubMed, EMBASE, and Cochrane Library from the construction date of the databases to June 1, 2016, without any publication language limitation. The search terms included various combinations of “subthalamic”, “pedunculopontine”, “deep brain stimulation” and “Parkinson disease”. Additional studies were identified from reference lists in the studies identified by searches. Only published manuscripts were ultimately included in the analyses.
Inclusion and exclusion criteria
The following inclusion criteria were used: i) controlled clinical trials comparing subthalamic DBS with PPN DBS to treat idiopathic PD; ii) studies describing patients with severe response fluctuations or symptoms of falling, freezing of gait, or postural instability despite of optimal pharmacological treatments; iii) studies that used the Unified Parkinson’s Disease Rating Scale (UPDRS) to measure post-treatment results; iv) reports in which outcomes were measurable using continuous variables. Studies and patients were excluded according to the following exclusion criteria: i) the study was based on only a single DBS target; ii) DBS was performed in pathologies other than PD; iii) patients who had severe gait disorders despite of subthalamic DBS were implanted with PPN DBS electrodes; or iv) data could not be extracted.
Efficacy measures
Therapeutic outcomes of the included studies were evaluated using Unified Parkinson’s Disease Rating Scale III (UPDRSIII), which is a widely used clinical tool that assesses motor performance [30,31]. UPDRSIII has been demonstrated to be reliable and valid [32], and examines speech, facial expression, rigidity, finger taps, hand movements, ability to rise from a sitting position, gait, posture and postural stability, bradykinesia of the body, and action or postural tremors [30-32]. Axial UPDRSIII subscores computed in the present study included gait-related symptoms such as rising from chair, posture, gait and postural stability. Particular care was given when assessing motor and axial subscores, as well as the specific benefits of PPN versus subthalamic mediation.
Data extraction
Two investigators (D.C. and G.H.) independently examined the titles, abstracts, and references of all identified articles. In case of any disagreement between the two investigators, the decision was made after thorough discussion with another investigator (K.D.). The two investigators (D.C. and G.H.) independently applied the inclusion and exclusion criteria, selected the studies, and extracted data and outcomes. The following data were extracted from each article: i) the number of patients in the study; ii) details of treatment regimens; iii) patient characteristics; and iv) outcome measures. Most studies provided means and Standard Deviations (SDs) of pre- and postoperative results, and reported differences between the values. If these values were not explicitly reported, we determined them by extracting baseline means and subtracting them from outcome means. To obtain SD changes from baseline, we used the equation in the Chinese translation of Cochrane Handbook for Systematic Reviews of Interventions (version 3.0.2, chapter 7.7.3.3 and 16.1.3.2).
Quality assessment
The quality of each study was independently assessed by the two investigators mentioned above (D.C. and G.H.). Controlled clinical trials that compared subthalamic DBS with PPN DBS in the treatment of idiopathic PD, excluding self-controlled pilot studies, were assessed using the “assessing risk of bias” tables provided in the Chinese translation of Cochrane Handbook for Systematic Reviews of Interventions (version 3.0.2). Disagreements between the two investigators were resolved by another investigator (K.D.).
Statistical analysis
Meta-analysis was performed using Review Manager Software version 5.3 (http://www.cochrane.org/). Statistical analyses for continuous variables were performed, and heterogeneity was measured using I-square and Chi-square tests. Probability values<0.05 were considered statistically significant. In cases where significant heterogeneity existed, a random-effect model was used for analysis. Otherwise, a fixed-effect model was used. In the present study, all outcomes were continuous data. The data were pooled using Mean Differences (MDs) of changes from baseline (change scores) to compare PPN DBS, subthalamic DBS and combined PPN-subthalamic stimulation. Outcomes were expressed as MD with 95% confidence intervals.
Results
Characteristics of the included studies
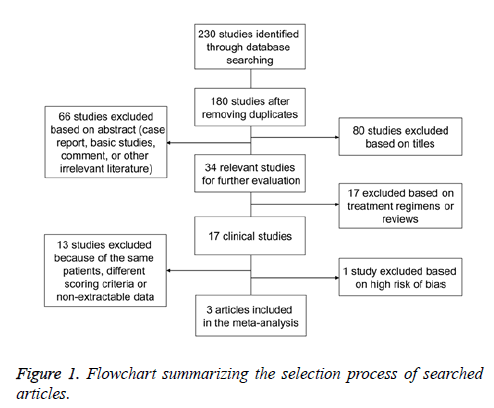
A total of 230 articles were initially searched. After excluding duplications, case reports, editorials, comments, laboratory studies, and other irrelevant literatures, 34 studies were preliminarily chosen. According to the inclusion and exclusion criteria, another 17 studies were excluded. Then, the remaining 17 studies were subjected to detailed evaluation, and 13 studies were excluded because of the same group of patients, inappropriate scoring criteria or non-extractable data. In addition, controlled clinical trial [33] had a high risk of bias (patients were not randomized in retrospective study) based on the “assessing risk of bias” table in the Cochrane Handbook for Systematic Reviews of Interventions, and was excluded because patients in the PPN DBS group exhibited significantly more DOPA-resistant gait disorders compared to STN DBS group (Figure 1). Finally, our meta-analysis included 3 articles, which reported a total of 15 patients suffering from gait dysfunction in spite of receiving of pharmacotherapy [11,22,24]. The studies did not specify experimental and control groups. Subthalamic DBS was more widely used than PPN DBS or combined PPN-subthalamic DBS. Therefore, we considered subthalamic DBS as control group and PPN DBS and combined PPN-subthalamic stimulation as experimental groups. DBS surgery was performed according to standard protocols, in which the final electrode position was verified by brain magnetic resonance imaging or computed tomography. Therapeutic outcomes were evaluated by using various scales, including the total scores of UPDRSIII and the axial subscores derived from items 27 to 30. MDs were determined by subtracting baseline means from outcome means. Higher UPDRSIII scores corresponded to more severe PD. Demographic characteristics of participants were not significantly different among studies (Table 1).
| Author, year (Country) | No. of patients | Age (year) | Sex (No. M/F) | Duration of PD (year) | Type of intervention | Outcome measure | Duration of intervention (months) | L-Dopa equivalent before surgery (mg) | L-Dopa equivalent after surgery in “off” stimulation state (mg) |
|---|---|---|---|---|---|---|---|---|---|
| Khan et al. [22] | 4 | 63.3 ± 1.7 | 4/0 | 14.5 ± 4.0 | Bilateral PPN and subthalamic DBS for all patients | UPDRSIII, UPDRSIII axial subscore | 23.8 ± 22.3 | 1272.80 ± 372.1 | 1070.8 ± 222.7 |
| Peppe et al. [24] | 5 | 57.8 ± 8.8 | 5/0 | 16.0 ± 10.0 | Bilateral PPN and subthalamic DBS for all patients | UPDRSIII, UPDRSIII axial subscore, walking trials | 12 | No data | No data |
| Stefani et al. [11] | 6 | 64.5 ± 3.2 | NA | 12.1 ± 3.0 | Bilateral PPN and subthalamic DBS for all patients | UPDRSIII axial subscore, UPDRSII (ADLs), S&E | 6 | 1091.6 ± 227.3 | About 780 |
Table 1. Details of studies included in the meta-analysis.
Comparison of changes in UPDRSIII scores between PPN DBS and subthalamic DBS
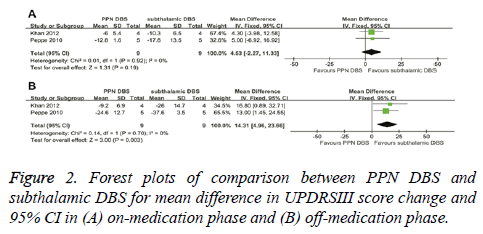
UPDRSIII score for PPN DBS was not significantly different from the score for subthalamic DBS in the on-medication phase (4.53; 95% CI, -2.27 to 11.33; P=0.19). Based on Chisquare and I-square analyses, no significant heterogeneity was observed between therapeutic regimens (χ2=0.01; df=1; P=0.92; I2=0%) (Figure 2A). In the off-medication phase, the overall pooled MD outcome value was 14.31 (95% CI, 4.96 to 23.66; P=0.003) (Figure 2B). Significant differences were observed between PPN DBS and subthalamic DBS. Based on Chi-square and I-square analyses, no significant heterogeneity was observed between therapeutic regimens (χ2=0.14; df=1; P=0.70; I2=0%).
Comparison of changes in UPDRSIII axial subscores between PPN DBS and subthalamic DBS
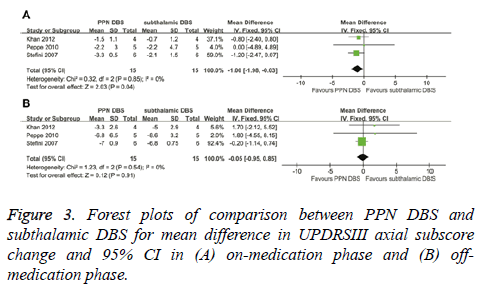
In the on-medication phase, the overall pooled MD outcome value was -1.00 (95% CI, -1.98 to -0.03; P=0.04) (Figure 3A). PPN DBS had a greater reduction in UPDRSIII axial subscores compared with subthalamic DBS. Based on Chi-square and Isquare analyses, significant differences in heterogeneity were not observed between therapeutic regimens (χ2=0.32; df=2; P=0.85; I2=0%). In the off-medication phase, no significant difference in UPDRSIII axial subscores was observed between PPN DBS and subthalamic DBS (-0.05; 95% CI, -0.95 to 0.85; P=0.91) (Figure 3B). Significant differences in heterogeneity were not observed between therapeutic regimens (χ2=1.23; df=2; P=0.54; I2=0%).
Comparison of changes in UPDRSIII scores between combined PPN-subthalamic DBS and subthalamic DBS
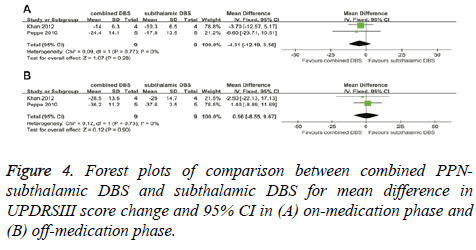
In the on-medication phase, the improvement in UPDRSIII scores of combined PPN-subthalamic DBS was not significantly different from that of subthalamic DBS with a score change of -4.31 (95% CI, -12.19 to 3.56; P=0.28) (Figure 4A). There was no significant heterogeneity between therapeutic regimens (χ²=0.09; df=1; P=0.77; I2=0%). In the off-medication phase, the overall pooled MD outcome value was 0.56 (95% CI, -8.55 to 9.67; P=0.90) (Figure 4B). No significant difference was observed between combined PPNsubthalamic DBS and subthalamic DBS. Based on Chi-square and I-square analyses, significant differences in heterogeneity were not observed between therapeutic regimens (χ2=0.12; df=1; P=0.73; I2=0%).
Comparison of changes in UPDRSIII axial subscores between combined PPN-subthalamic DBS and subthalamic DBS
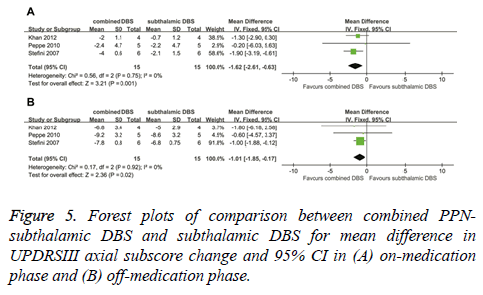
In the on-medication phase, the improvement in UPDRSIII axial subscores of combined PPN-subthalamic DBS was significantly greater that of subthalamic DBS, with an overall pooled MD of -1.62 (95% CI, -2.61 to -0.63; P = 0.001) (Figure 5A). Based on Chi-square and I-square analyses, significant differences in heterogeneity were not observed between therapeutic regimens (χ2=0.56; df=2; P=0.75; I2=0%). In the off-medication phase, the reduction in UPDRSIII axial subscores of combined PPN-subthalamic DBS was significantly greater than that of subthalamic DBS (-1.01; 95% CI, -1.85 to -0.17; P=0.02) (Figure 5B). Based on Chi-square and I-square analyses, significant differences in heterogeneity were not observed between therapeutic regimens (χ2=0.17; df=2; P=0.92; I2=0%).
Discussion
Treatments for advanced PD patients with axial symptoms are mainly focused on PPN and subthalamic DBS. To identify the optimal site of stimulation, outcomes after DBS have been studied by many researchers. The present meta-analysis has included three self-controlled studies [11,22,24] that compare PPN DBS or combined PPN-subthalamic DBS with subthalamic DBS for the treatment of advanced PD. Changes in UPDRSIII and UPDRSIII axial subscores from baseline values after bilateral subthalamic and PPN DBS placement are used to evaluate improvements in motor and axial functions. Subthalamic DBS reduces UPDRSIII scores (off-medication phases) to levels lower than PPN DBS. PPN DBS has greater improvements in UPDRSIII axial subscores (on-medication phase) compared with subthalamic DBS. UPDRSIII scores (on- and off-medication phases) have shown no significant difference between combined PPN-subthalamic DBS and subthalamic DBS, suggesting that combined DBS and subthalamic DBS improve motor symptoms of PD with similar efficacy. However, combined PPN-subthalamic DBS has significantly greater improvements in UPDRSIII axial subscores (on- and off-medication phases) compared with subthalamic DBS.
Symptoms of PD include the classic Parkinsonian triad (tremor, bradykinesia, and rigidity associated with dopaminergic denervation), axial Parkinsonian symptoms associated with nondopaminergic transmission (postural instability, impairments of gait, and posture), and nonmotor symptoms [34,35]. Motor control is the major goal of treatment for PD patients. Patients in on-medication state show similar improvements in UPDRSIII after PPN or STN DBS (n=9; overall pooled MD outcome value, 4.53; P=0.19). A potential explanation may be that a small sample size has been used. In off-medication phase, improvement of UPDRSIII score after subthalamic DBS is greater than that after PPN DBS. Being consistent with previous studies [9,10,36], PPN DBS does not lead to significant motor improvement when used alone. The effect of PPN DBS on Parkinsonian symptoms is much less clear.
In the present meta-analysis, we have observed significant differences between PPN and subthalamic DBS regarding improvements in UPDRSIII axial subscores in the onmedication phase. PPN has been a DBS target due to failure of globus pallidus internus [37], ventral intermediate thalamic nucleus (Vim) [38] and subthalamic DBS [39] in improving freezing of gait and gait disorders. Interestingly, dopamineresistant gait disorders can be mildly improved by STN DBS at low frequency, whereas reduced STN DBS frequency can worsen dopamine-sensitive motor symptoms in certain patients [23]. From a clinical point of view, low-frequency STN DBS cannot be used as an alternative to high-frequency STN DBS. Several reports [9-11] have shown that low-frequency PPN stimulation in patients with PD appears to selectively improve freezing of gait, postural instability and falls. Indications for PPN stimulation are severe FOG, postural instability, and falls that persist even when the patient is on medication [12]. However, these results have not been reproduced in three double-blind randomized controlled studies [9,10,33]. One of the significant differences in surgical techniques between these two groups of studies is the use of bilateral PPN stimulation in the studies included in our meta-analysis and unilateral PPN stimulation in one of the control studies [10]. In one of the double-blind randomized controlled studies [9], PPN DBS electrodes are implanted into six patients who have severe gait disorders after subthalamic DBS surgery. One study [33] shows that patients in PPN DBS group have exhibited significantly more DOPA-resistant gait disorders than STN DBS group. However, patients with bilateral STN and PPN DBS placement in different surgeries and owing to different criteria have been excluded from the present meta-analysis because of high risk of selection bias.
STN improves the triad of dopaminergic symptoms, and PPN improves gait disorders in PD [40]. In addition, our data suggest that the combination of PPN and subthalamic stimulations leads to further reduction in UPDRSIII axial subscores (on- and off-medication phases) compared with subthalamic stimulation alone. Unlike motor symptoms, these axial symptoms are often resistant to treatments, and are important for the quality of life [41]. Combined PPN and subthalamic DBS are superior to subthalamic DBS alone, allowing for greater improvement in axial Parkinsonian symptoms. Several studies [11,22,24] have demonstrated significant improvements in motor and axial functions after treatment with combined PPN and subthalamic DBS. Two studies [11,22] included in our meta-analysis show that combined stimulation of both targets provides more benefits than either location alone in the on-medication state. However, in the off-medication state, adding PPN to STN fails to result in a benefit that is better than STN alone. The present study confirms that improvements in axial Parkinsonian symptoms after treatment with combined PPN and subthalamic DBS are greater than that after treatment with subthalamic DBS in both on- and off-medication phases. We observe no difference between subthalamic DBS and the combined PPN and subthalamic DBS regarding improvements in motor UPDRS. However, it seems that PPN stimulation has no antagonism towards subthalamic DBS in motor symptoms. Khan et al. [22] conclude that bilateral subthalamic stimulation improves motor UPDRS by 30.5%, and combined bilateral PPN and subthalamic stimulation improve motor UPDRS by 41.8%. Moreover, combined DBS of both targets promotes substantial amelioration in the performance of daily living activities [11]. A synergistic effect has been observed when bilateral PPN is stimulated in conjunction with bilateral STN.
The present meta-analysis still has some limitations. One study lacks detailed data on UPDRSIII score changes [11]. UPDRS “gait” item is not the main outcome indicator. Particular inclusion and exclusion criteria can be limiting factors, and it will be more convincing if studies included in our metaanalysis are randomized controlled trials. There are also potential issues in using UPDRSIII scores and axial UPDRSIII subscores as primary measures to compare PPN DBS, subthalamic DBS and combined DBS. UPDRS motor function scores may have several PD-related problems [31]. One study [11] included in the present meta-analysis contains much smaller standard deviations than the other two studies, and may lead to bias.
In conclusion, our meta-analysis shows differences between PPN and subthalamic DBS on outcomes of motor function based on UPDRSIII (off-medication phase) and axial motor features based on axial UPDRSIII subscores (on-medication phase). PPN is proven as a better treatment target for gait disorders in PD than STN in the on-medication phase. Subthalamic DBS has similar effect for postoperative motor features compared with combined PPN-subthalamic stimulation. Alternatively, combined DBS is more effective in treating axial symptoms of PD than STN DBS, based on axial UPDRSIII subscores (on- and off-medication phases). Considering the limitations described above, further evaluation and long-term observations in larger controlled trials are needed in order to determine the efficacy of PPN DBS and combined PPN-subthalamic stimulation. The present study provides a basis for selecting treatment methods for Parkinsonian motor and axial symptoms.
Acknowledgement
This study was supported by key Clinical Specialty Discipline Construction Program of Fujian, P.R. China.
Declaration of Conflict of Interest
None
References
- Fasano A, Aquino CC, Krauss JK, Honey CR, Bloem BR. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat Rev Neurol 2015; 11: 98-110
- Canning CG, Paul SS, Nieuwboer A. Prevention of falls in Parkinsons disease: a review of fall risk factors and the role of physical interventions. Neurodegener Dis Manag 2014; 4: 203-221.
- Walton CC, Shine JM, Hall JM, OCallaghan C, Mowszowski L, Gilat M, Szeto JY, Naismith SL, Lewis SJ. The major impact of freezing of gait on quality of life in Parkinsons disease. J Neurol 2015; 262: 108-115.
- Ferraye MU, Debu B, Pollak P. Deep brain stimulation effect on freezing of gait. Mov Disord 2008; 23: 489-494.
- Vingerhoets FJ, Tagliati M. Treating PD axial signs with DBS: is two better than one? Neurology 2012; 78: 1036-1037.
- Wen P, Li M, Xiao H, Ding R, Chen H, Chang J, Zhou M, Yang Y, Wang J, Zheng W, Zhang W. Low-frequency stimulation of the pedunculopontine nucleus affects gait and the neurotransmitter level in the ventrolateral thalamic nucleus in 6-OHDA Parkinsonian rats. Neurosci Lett 2015; 600: 62-68.
- Park E, Song I, Jang DP, Kim IY. The effect of low frequency stimulation of the pedunculopontine tegmental nucleus on basal ganglia in a rat model of Parkinsons disease. Neurosci Lett 2014; 577: 16-21.
- Jenkinson N, Nandi D, Oram R, Stein JF, Aziz TZ. Pedunculopontine nucleus electric stimulation alleviates akinesia independently of dopaminergic mechanisms. Neuroreport 2006; 17: 639-641.
- Ferraye MU, Debû B, Fraix V, Goetz L, Ardouin C, Yelnik J, Henry-Lagrange C, Seigneuret E, Piallat B, Krack P, Le Bas JF, Benabid AL, Chabardès S, Pollak P. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinsons disease. Brain2010; 133: 205-214.
- Moro E, Hamani C, Poon YY, Al-Khairallah T, Dostrovsky JO, Hutchison WD, Lozano AM. Unilateral pedunculopontine stimulation improves falls in Parkinsons disease. Brain 2010; 133: 215-224.
- Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinsons disease. Brain 2007; 130:1596-1607.
- Thevathasan W, Coyne TJ, Hyam JA, Kerr G, Jenkinson N, Aziz TZ, Silburn PA. Pedunculopontine nucleus stimulation improves gait freezing in Parkinson disease. Neurosurgery2011; 69: 1248-1254.
- Sidiropoulos C, Xie T, Vigil J, MacCracken E, Warnke P, Kang UJ. Low-frequency stimulation of STN-DBS reduces aspiration and freezing of gait in patients with PD. Neurology 2015; 85: 557.
- Xie T, Vigil J, MacCracken E, Gasparaitis A, Young J, Kang W, Bernard J, Warnke P, Kang UJ. Low-frequency stimulation of STN-DBS reduces aspiration and freezing of gait in patients with PD. Neurology 2015; 84: 415-420.
- Vercruysse S, Vandenberghe W, Munks L, Nuttin B, Devos H, Nieuwboer A. Effects of deep brain stimulation of the subthalamic nucleus on freezing of gait in Parkinsons disease: a prospective controlled study. J Neurol Neurosurg Psychiatry 2014; 85: 871-877.
- Fling BW, Cohen RG, Mancini M, Carpenter SD, Fair DA, Nutt JG, Horak FB. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS One 2014; 9: 100291.
- Johnsen EL, Sunde N, Mogensen PH, Ostergaard K. MRI verified STN stimulation site-gait improvement and clinical outcome. Eur J Neurol 2010; 17: 746-753.
- Potter-Nerger M, Volkmann J. Deep brain stimulation for gait and postural symptoms in Parkinsons disease. Mov Disord 2013; 28: 1609-1615.
- Hamani C, Richter E, Schwalb J M, Lozano AM. Bilateral subthalamic nucleus stimulation for Parkinsons disease: a systematic review of the clinical literature. Neurosurgery 2005; 56: 1313-1324.
- Bejjani BP, Gervais D, Arnulf I, Papadopoulos S, Demeret S, Bonnet AM, Cornu P, Damier P, Agid Y. Axial Parkinsonian symptoms can be improved: the role of levodopa and bilateral subthalamic stimulation. J Neurol Neurosurg Psychiatry 2000; 68: 595-600.
- Guehl D, Dehail P, de Sèze MP, Cuny E, Faux P, Tison F, Barat M, Bioulac B, Burbaud P. Evolution of postural stability after subthalamic nucleus stimulation in Parkinsons disease: a combined clinical and posturometric study. Exp Brain Res 2006; 170: 206-215.
- Khan S, Gill SS, Mooney L, White P, Whone A, Brooks DJ, Pavese N. Combined pedunculopontine-subthalamic stimulation in Parkinson disease. Neurology 2012; 78: 1090-1095.
- Moreau C, Defebvre L, Destee A, Bleuse S, Clement F, Blatt JL, Krystkowiak P, Devos D. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology 2008; 71, 80-84.
- Peppe A, Pierantozzi M, Chiavalon C, Marchetti F, Caltagirone C, Musicco M, Stanzione P, Stefani A. Deep brain stimulation of the pedunculopontine tegmentum and subthalamic nucleus: effects on gait in Parkinsons disease. Gait Posture 2010; 32: 512-518.
- Zanini S, Moschella V, Stefani A, Peppe A, Pierantozzi M, Galati S, Costa A, Mazzone P, Stanzione P. Grammar improvement following deep brain stimulation of the subthalamic and the pedunculopontine nuclei in advanced Parkinsons disease: A pilot study. Parkinsonism Relat Disord 2009; 15: 606-609.
- Peppe A, Pierantozzi M, Baiamonte V, Moschella V, Caltagirone C, Stanzione P, Stefani A. Deep brain stimulation of pedunculopontine tegmental nucleus: role in sleep modulation in advanced Parkinson disease patients-one-year follow-up. Sleep 2012; 35: 1637-1642.
- Khan S, Javed S, Mooney L, White P, Plaha P, Whone A, Gill SS. Clinical outcomes from bilateral versus unilateral stimulation of the pedunculopontine nucleus with and without concomitant caudal zona incerta region stimulation in Parkinsons disease. Br J Neurosurg 2012; 26: 722-725.
- Moreau C, Defebvre L, Devos D, Marchetti F, Destee A, Stefani A, Peppe A. STN versus PPN-DBS for alleviating freezing of gait: Toward a frequency modulation approach? Mov Disord 2009; 24: 2164-2166.
- Weiss PH, Herzog J, Potter-Nerger M, Falk D, Herzog H, Deuschl G, Volkmann J, Fink GR. Subthalamic nucleus stimulation improves parkinsonian gait via brainstem locomotor centers. Mov Disord 2015; 30:1121-1125.
- Jafari N, Pahwa R, Nazzaro JM, Arnold PM, Lyons KE. MDS-UPDRS to assess non-motor symptoms after STN DBS for Parkinsons disease. Int J Neurosci 2016; 126: 25-29.
- Goetz CG, Tilley BC, Shaftman SR. Movement disorder society-sponsored revision of the unified parkinsons disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008; 23: 2129-2170.
- Goetz CG, Poewe W, Rascol O. Movement disorder society task force on rating scales for Parkinsons disease, the Unified Parkinsons Disease Rating Scale (UPDRS): Status and recommendations. Mov Disord 2003; 18: 738-750.
- Lau B, Welter ML, Belaid H, Fernandez Vidal S, Bardinet E. The integrative role of the pedunculopontine nucleus in human gait. Brain 2015; 138: 1284-1296.
- Liu Y, Li W, Tan C, Liu X, Wang X, Gui Y, Qin L, Deng F, Hu C, Chen L. Meta-analysis comparing deep brain stimulation of the globus pallidus and subthalamic nucleus to treat advanced Parkinson disease: A review. J Neurosurg 2014; 121: 709-718.
- Fasano A, Daniele A, Albanese A. Treatment of motor and non-motor features of Parkinsons disease with deep brain stimulation. Lancet Neurol 2012; 11: 429-442.
- Ferraye MU, Debû B, Fraix V, Krack P, Charbardès S, Seigneuret E, Benabid AL, Pollak P. Subthalamic nucleus versus pedunculopontine nucleus stimulation in Parkinson disease: synergy or antagonism? J Neural Transm (Vienna) 2011; 118: 1469-1475.
- Johnson L, Rodrigues J, Teo WP, Walters S, Stell R, Thickbroom G, Mastaglia F. Interactive effects of GPI stimulation and levodopa on postural control in Parkinsons disease. Gait Posture 2015; 41: 929-934.
- Guzzi G, Della Torre A, Chirchiglia D, Volpentesta G, Lavano A. Critical reappraisal of DBS targeting for movement disorders. J Neurosurg Sci 2016; 60: 181-188.
- Liu HG, Zhang K, Yang AC, Zhang JG. Deep brain stimulation of the subthalamic and pedunculopontine nucleus in a patient with Parkinsons disease. J Korean Neurosurg Soc. 2015; 57: 303-306.
- Benabid AL, Torres N. New targets for DBS. Parkinsonism Relat Disord 2012; 18: 21-23.
- Moreau C, Delval A, Defebvre L. Methylphenidate for gait hypokinesia and freezing in patients with Parkinsons disease undergoing subthalamic stimulation: a multicentre, parallel, randomised, placebo-controlled trial. Lancet Neurol 2012; 11: 589-596.