ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2022) Volume 33, Issue 3
Comparitive study of anti-ulcer activity of marketed preparations of Tinospora cardifolia (guduchi) and Glycerrhiza glabra (jyeshthmadh) with proton pump inhibitor (rabeprazole).
Ghanshyam M Chavan1*, Akash S Jain2, Hitendra S Chaudhari2, Divakar R. Patil2
1Department of Pharmacology, PSGVPM’s College of Pharmacy, Shahada, Maharashtra, India
2Department of Pharmaceutical Sciences, PSGVPM’s College of Pharmacy, Shahada, Maharashtra, India
- Corresponding Author:
- Ghanshyam M. Chavan
Department of Pharmacology
PSGVPM’s College of Pharmacy
Shahada
Maharashtra
India
Accepted date: March 14, 2022
Peptic ulcer, most common disorder of the GIT has multifactorial causes in its pathophysiology and cannot achieve a complete eradication with a single drug hence search of drugs from various systems of medicines like ayurveda, siddha, unani is a common practice.
Ulcers were induced in 48 hours fasted albino rats by pylorus legations. In each induction procedure there were four groups namely control, positive control and two tests and each group containing four animals. In all four separate groups, the groups received oral administration of guduchi (Tinosporacardifolia) (1000 mg/ kg) and jyeshthmadh (Glycerriza glabra) (1000 mg/kg) prior to ulcer induction showed significant reduction in the occurrence of gastric ulcers as compared to control received distilled water and positive control group received Rabeprozole (500 mg/kg).Both test drugs showed significant reduction in gastric juice volume and total acidity as well as significant increase in gastric pH. When anti-ulcer activity of Jyeshthmadh and Guduchi was compared, guduchi showed more potency than jyeshthmadh.
These results emphasize on the need to diversify alternative therapeutic approaches pertaining to herbal medicine. Wherein a single easily available plant may provide answer to several therapeutic challenges as observed in antiulcer activity shown by guduchi and jyeshthmadh.
Keywords
Anti-ulcer activity, Jyeshthmadh, Guduchi, Rabeprazole.
Introduction
Peptic ulcer is one of the major ailments affecting about 60% of human adults and nearly 80% of child population in tropical countries. Peptic ulcers one believed to develop because of an imbalance between aggressive factors like acid, pepsin, bile salts and defensive factors such as mucus, bicarbonate, blood flow, epithelial cell restoration and prostaglandin [1]. Most of the studies suggest that either the defect in antral-pylorus-duodenal motility or a general abnormal motility patterns permits duodenal contents to reflex into the stomach leading to damage to gastric mucosa [2].
Stress ulcers have been defined as acute ulcerations that is usually multiple and commonly located in gastric body and fundus which occur in majority of severely ill or injured patients. Patients at risk of developing stress ulcers are those who are severely ill with septicemia, shock, multiple trauma, burns, major surgery or multiple organ failure [3].
Several plants are known to possess antiulcer activity. The prominent among these herbs include guduchi (Tinospora cardifolia) and jyeshthmadh (Glycerrhiza glabra) [4]. The present work aims at comparing the antiulcer activity of guduchi (Tinospora cardifolia) and jyeshthmadh (Glycerrhiza glabra) using proton pump inhibitor (rabeprazole) as standard. The anti-ulcer activity was compared in pylorus ligated model in albino rats.
Materials and Methods
Plant material
The marketed preparations of guduchi (Tinospora cardifolia) and jyeshthmadh (Glycerrhiza glabra) were procured from “Phadke distributors, Sangli” The preparations were in powder form and both the drugs are known to have antiulcer activity. Rabeprazole used in the experiment was obtained from F.D.C. Pharmaceuticals.
Animals
Adult albino rats (Wister albino strain) of either sex weighing 150-200 gm were used for the study. They were housed in polypropylene cages and maintained under standard conditions (12 hours light/12 hours dark cycle, 25ºC ± 3ºC, 35%-60% humidity) Animals were fed with chow diet and water ad libitum. All experiment were carried out in the central animal house, PSGVPM’s College of Pharmacy, Shahada, Dist. Nandurbar, 425409, Maharashtra (India) [5-7].
Evaluation of antiulcer properties
Healthy albino rats were taken and divided into groups. (Each group consists of 5 animals) for each pharmacological testing animal were divided into 4 groups.
Group I: Treated with control vehicle (0.2% agar 500 mg/kg).
Group II: Treated with Aq. suspension of guduchi (Tinospora cardifolia) (500 mg/kg in 0.2% agar).
Group III: Treated with Aq. Suspension of jyeshthmadh (Glycerrhiza glabra) (500 mg/kg in 0.2% agar).
Group IV: Treated with Rabeprazole (500 mg/kg in 0.2% agar).
Ulcer induction procedure
Pylorus ligation: All the animals were fasted for 48 hours prior to dosing. The control group animals (Group I) were administered with calculated dose of 0.2% agar (500 mg/kg) The positive control group animals (Group IV) were dosed with Rabeprazole (500 mg/kg) in 0.2% agar. Test Group II and Group III animals were dosed with aqueous suspension of guduchi (Tinospora cardifolia) and jyeshthmadh (Glycerrhiza glabra) (500 mg/kg) in 0.2% agar respectively. Dosing was carried out 30 minutes prior to the procedure to be carried out for ulcer induction. Throughout the experiment water was provided ad libitum and food was withdrawn from the animals [8,9] (Figure 1).
The rats were subjected to pylorus ligation under ether anesthesia 19 hours after pylorus ligation animals were sacrificed. Stomachs from the rats were removed carefully and cut along the greater curvature. The gastric content of each animal was emptied into graduated centrifuge tubes separately. The collected gastric content (gastric juice) was centrifuged at 1000 rpm for 30 minutes. The volume of gastric juice was measured and pH was determined. The total acidity was determined using supernatant liquid, with 0.01 N NaOH and expressed as m.Eq/l gastric juice [6,10].
Stomach of each animal was washed in distilled water.
The mucosal layer of the stomach was observed under magnifying lens and was checked for ulcers, Hemorrhagic areas or perforations [11].
The ulcer score was given as follows (Table 1).
| Score | Parameter |
|---|---|
| 0 | Normal coloured stomach |
| 0.5 | Red colouration |
| 1 | Spot ulcer |
| 1.5 | Haemorrhagic streaks |
| 2 | Ulcers ≥ 3 but ≤ 5 |
| 3 | Ulcers>5 |
Table 1. Ulcer score parameter.
Results and Discussion
As shown in Table 1, gastric content, total acidity, ulcer score and pH for guduchi (Tinospora cardifolia), jyeshthmadh (Glycerrhiza glabra) and rabeprazole were statistically significant (P<0.001) when compared with control (Table 2).
| Parameter | Group I (Treated with control vehicle) | Group II (Treated with guduchi) | Group III (Treated with jyeshthmadh) | Group IV (Treated with rabeprazole) |
|---|---|---|---|---|
| Volume of gastric content in ml | 7 ± 0.4243 | 4 ± 0.216 | 5.5 ± 0.2828 | 6.125 ± 0.2217 |
| Total acidity m.Eq./l | 72 ± 0.8165 | 30 ± 1.414 | 47 ± 2.16 | 51 ± 2.16 |
| Ulcer score | 2.625 ± 0.25 | 1.375 ± 0.4787 | 1.5 ± 0.4082 | 1.625 ± 0.25 |
| pH | 3.1 ± 0.216 | 4.8 ± 0.216 | 4.2 ± 0.09574 | 4.3 ± 0216 |
All values represent mean ± S.D.; n=5 in each group; ANOVA P<0.001 for all parameters with control and positive control.
Table 2. Effect of guduchi (Tinospora cardifolia), jyeshthmadh (Glycerrhiza glabra) and rabeprazole on volume of gastric content and pH, total acidity value and ulcer score in Pylorus ligation model.
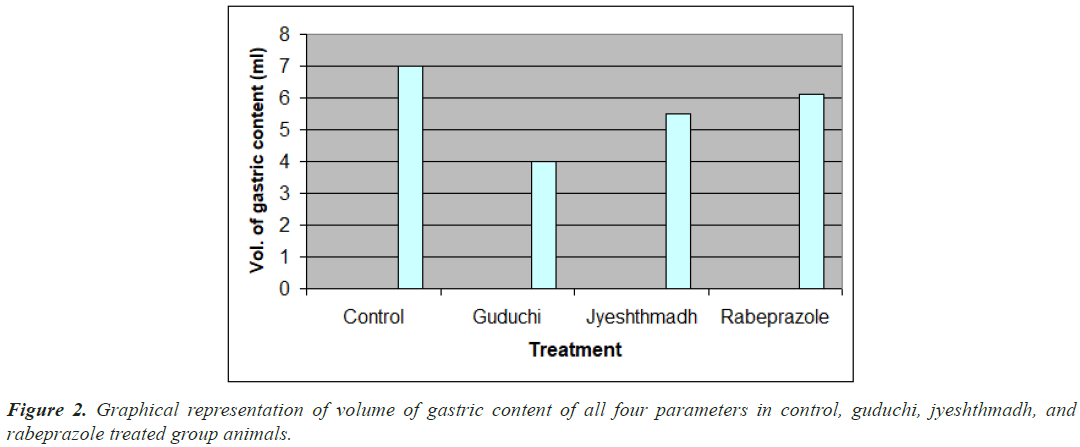
The rabeprazol treated group has shown the highest gastric content which is 6.1 ml as compare with jyeshthmadh 5.5 ml, guduchi 4 ml and control 7 ml as shown in Figure 2. Amount of gastric content secreted by stomach is directly proportional to hydrochloric acid released by parietal cells of stomach. And HCL secretion is responsible for ulceration produced on wall of mucosal layer.
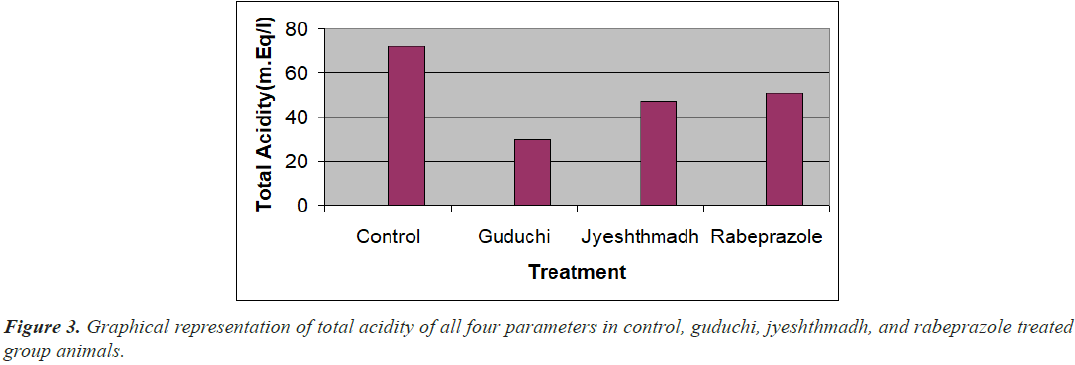
Similarly the volume of gastric content, and total acidity value of guduchi (Tinospora cardifolia), jyeshthmadh (Glycerrhiza glabra), and rabeprazole treated group animals have significantly reduced (P<0.001) while pH was increased (P<0.001) when compared with control as per Figure 3. If more gastric content secrete it is indication of more acid secretion in stomach, due to ligation acid content won’t go further, it stay remain in the stomach pouch.
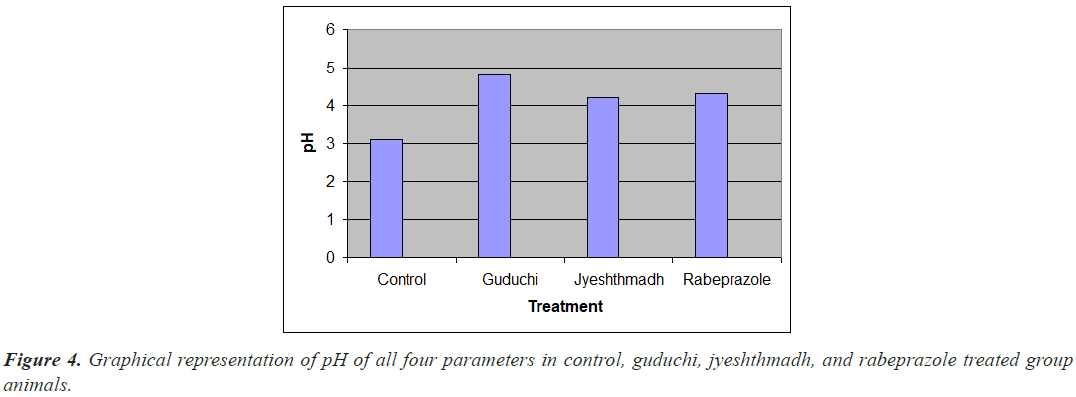
Guduchi has shown the highest increase in pH that is 4.8 where as jyesthmadh and rebeprazol has shown pH 4.2 and 4.3 respectively as compare to the control 3.1. (Figure 4).
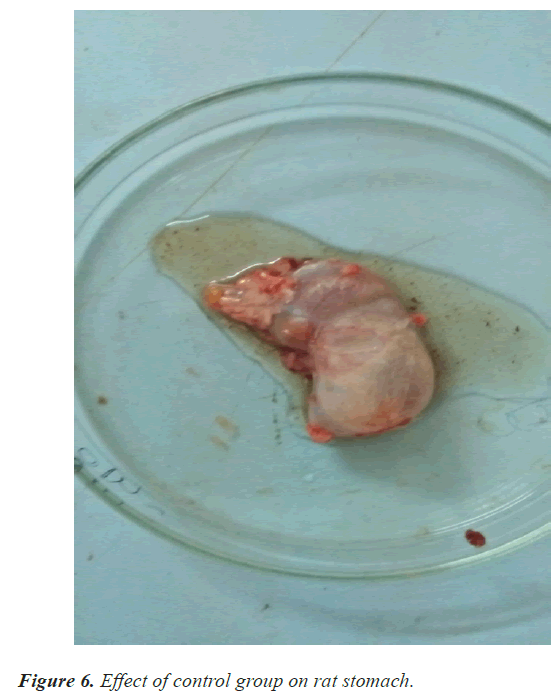
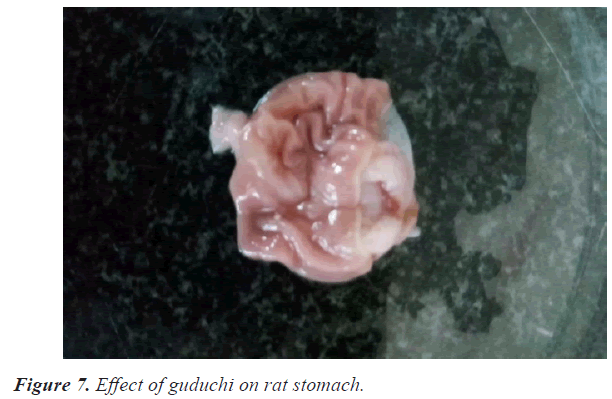
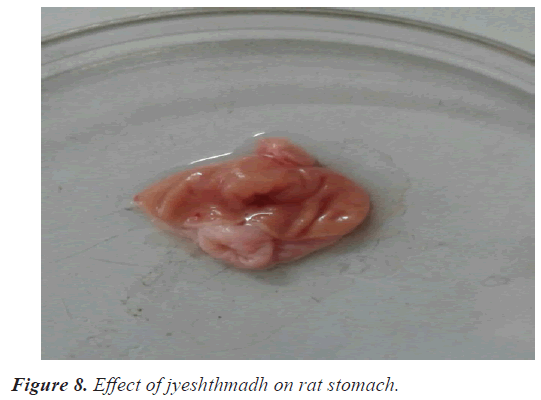
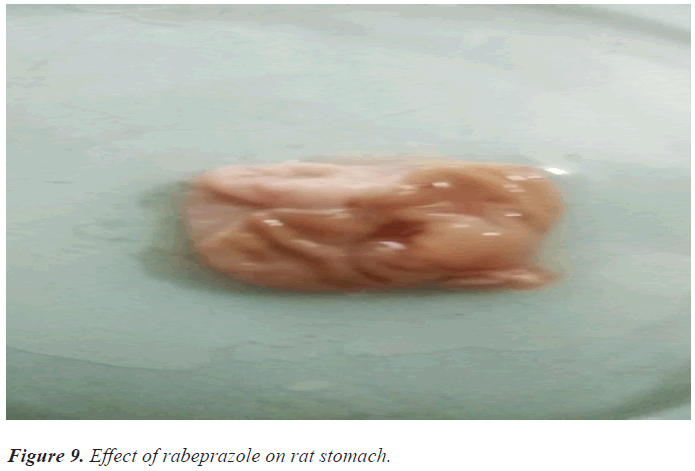
In the ulcer score rabeprazole has shown slightly high ulcer score 1.6 as compare with guduchi 1.3 and jyeshthmadh 1.5 and with control 2.6. The guduchi (Tinospora cardifolia) and jyeshthmadh (Glycerrhiza glabra) treated group animals have shown significant reduction in all 3 parameters (volume of gastric content, total acidity in % m.Eq/l, ulcer score). Higher ulcer score means more ulceration has been produced to the epithelial layer of mucosa of stomach due to high secretion of HCL (Figures 5-9).
While guduchi (Tinospora cardifolia) treated group (II) animals have shown more significant results as compared to jyeshthmadh (Glycerrhiza glabra).
This indicates that guduchi (Tinospora cardifolia) may have more anti-ulcer activity than jyeshthmadh (Glycerrhiza glabra); so the study provokes the investigation to be carried out on the phytoconstituents of guduchi for isolation of a potent anti-ulcer molecule.
These results emphasize on the need to diversify alternative therapeutic approaches pertaining to herbal medicine. Wherein a single easily available plant may provide answer to several therapeutic challenges as observed in antiulcer activity shown by guduchi and jyeshthmadh. Hence the study supports the strong medicinal potential of herbal drugs.
Conclusion
The herbal formulation containing guduchi and jyeshthmadh has shown significant antiulcer activity when we consider all four aspects to evaluate activity. More or less in all four parameter gastric content, total acidity, ulcer score and pH guduchi and jyeshthmadh has shown promising results. There is need to isolate key drugs from guduchi and jyeshthmadh which are responsible for antiulcer activity. As both guduchi (Tinospora cardifolia) and jyeshthmadh (Glycerrhiza glabra) are herbal drugs, hence they possesses less or no side effects as compare with the allopathic drugs like, rabeprazol; these herbal drugs proved to be a good alternative for conventional allopathic drugs.
References
- Jana U. Anti-ulcer activity of digitrall: A polyherbal drug in rats. Ind J Pharmacol 2005; 37: 406-407.
- Gowrishankar NL. A preliminary study on gastric antiulcer activity of Commiphora berryi (Arn) Engl in rats. Indian Drugs 2004; 41: 97-100.
- Toshi Fukai. Anti-Helicobacter pylori flavonoids from liquorice extract. Life sci 2002; 71: 1449-1463.
[Crossref] [Google Scholar] [PubMed]
- Nadkarni KM. Indian materia medica. Popular Prakashan 1999; 279-281.
- Ghanshyam M Chavan, Mamata D. Ranalkar, Swapnil K. Bagul. Evaluation of Anti-ulcer activity of leaves of alstonia scholaris (L) R Br by using pylorus ligation method. J Seybold Rep 2020; 15: 6768-6782.
- Vogel. Drug Discovery and Biological Evaluation. 2nd Ed 2019; 867-868.
- Kulkarni SK. handbook of experimental pharmacology. Vallabh Prakashan 2005; 148-150.
- Ghosh MN. Fundamental of experimental pharmacology. Scientific Book Agency 2nd Ed 1984; 177-182.
- Nivsarkar M, Kothari A, Padh H. Antiulcer activity of methanol extract of eclipta alba. Indian J Pharm Sci 2005; 67: 165-168.
- Bafna PA. Anti-ulcer and antioxidant activity of pepticare, a herbal mineral formulation. Phytomedicine 2005; 12: 264-270.
[Crossref] [Google Scholar] [PubMed]
- Sisodia SS. Gastric anti-ulcer activity of rutin and quercetin. The Indian Pharm 2005: 89-92.