ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2018) Volume 29, Issue 17
Confirming oral bioavailability of novel oestradiol analogues by liquid chromatography-tandem mass spectrometry in a murine model
1Department of Physiology, Faculty of Health Sciences, School of Medicine, University of Pretoria, Pretoria, South Africa
2Department of Pharmacology, Faculty of Health Sciences, School of Medicine, University of Pretoria, Pretoria, South Africa
3Faculty of Veterinary Sciences, Biomedical Research Centre, Laboratory Animal Technology, University of Pretoria, Pretoria, South Africa
4Department of Pharmacology, Faculty of Veterinary Sciences, Biomedical Research Centre, University of Pretoria, Pretoria, South Africa
5Biostatistics Unit, Medical Research Council, Pretoria, South Africa
- *Corresponding Author:
- Joubert AM
Department of Physiology
Faculty of Health Sciences
School of Medicine, University of Pretoria
Pretoria, South Africa
Accepted date: February 12, 2018
DOI: 10.4066/biomedicalresearch.29-17-2838
Visit for more related articles at Biomedical Research2-methoxyestradiol (2ME) is an endogenous 17β-oestradiol metabolite that exerts antiproliferative, antiangiogenic and antitumour activity on cancer cells both in vitro and in vivo. However, the use of 2ME as a potential anticancer agent is limited due to its poor oral bioavailability coupled to a short elimination half-life. In an attempt to improve the oral bioavailability and expand the drug targets, three sulphamoylated 2ME analogues were designed using in silico modelling and subsequently synthesized. A screening limit of 5 μg/ml for each analogue using a liquid chromatography tandem mass spectrometer (LC-MS/MS) method for 2ME analogues in serum and solvent was established. Therapeutically relevant oral bioavailability was assessed for these 2ME analogues using a murine oral absorption model (CD-1 mice) where the presence of these synthetic analogues in serum samples was assessed at two hours post dosing at 150 mg/kg of individual compounds. Blood was collected and analysed for the presence of the dosed compound and potential metabolites via LC-MS/MS. Results indicated that these analogues were present in serum above the screening limit at two hours post dosing and that there is merit to further investigation into the mode and mechanism(s) of action of ESE-15-one and ESE-15-ol and ESE-16 in vivo.
Keywords
Oral bioavailability, LC-MS/MS, 2-methoxyestradiol analogues, Murine.
Abbreviations
APC: Anaphase-Promoting Complex; CAII: Carbonic Anhydrase II; CAD: Collision Activation Dissociation Gas Flow; CXP: Collision Cell Exit Potential; CUR: Curtain Gas; Da: Dalton; DP: De-Clustering Potential; EP: Entrance Potential; ESE-15-One: 2-Ethyl-3-O-Sulphamoyl-Oestra-1, 3, 5 (10), 15-Tetraene-3-Ol-17-One; ESE-15-ol: 2-Ethyl-3-OSulphamoyl- Oestra-1, 3, 5 (10), 15-Tetraene-3, 17-Diol; ESE-16: 2-Ethyl-3-O-Sulphamoyl-Oestra-1, 3, 5 (10), 16- Tetraene; IC50: Half Maximal Inhibitory Concentration; HPLC: High-Performance Liquid Chromatography; TEM: Interference Temperature; kV: Kilovolts; LC-MS/MS: Liquid Chromatography-Tandem Mass Spectrometer; L/h: Liters per Hour; m/z: Mass-to-Charge Ratio; M: Method; 2ME: 2- Methoxyestradiol; 2-MeOEbisMATE: 2-Methoxyoestradiol- Bis-Sulphamate; 2ME-BM: 2-Methoxyestradiol-Bis- Sulphamate; μg/ml: Microgram per Milliliter; μl: Microlitre; μm: Micrometre; mg/kg: Milligram per Kilogram; mg/ml: Milligram per Milliliter; ml: Millilitre; mm: Millimetre; min: Minutes; MRM: Multiple Reaction Monitoring; ESI-: Negative Ion Mode; SNO: Oesophageal Carcinoma Cells; PLGA: Poly- Lactic-Co-Glycolic Acid; ESI+: Positive Mode; PG: Propylene Glycol; QTOF: Quadrupole Time-of-Flight; RT: Retention Time; SRM: Selected Reaction Monitoring; THF: Tetrahydrofuran; x g: Times Gravity; TIC: Total Ion Chromatogram; GS2: TurboIonSpray Drying Gas Nitrogen; GS1: TurboIonSpray Nebulizer Gas Nitrogen; UP: University of Pretoria; UPBRC: University of Pretoria Biomedical Research Centre; V/eV: Volts per electron-Volts; v/v: Volume per Volume; W: Watt.
Introduction
The endogenous 17β-oestradiol metabolite, 2-methoxyestradiol (2ME) that is produced by sequential hepatic hydroxylation of β-oestradiol and 2-O-methylation catalysed by cytochrome P450 enzymes and catechol-O-methyltransferase respectively, has been identified as a potential anticancer agent [1,2]. 2ME binds to the colchicine-binding site of microtubules exerting antiproliferative-, antiangiogenic- and antimitotic effects both in vitro and in vivo [3-7]. Colchicine-binding site occupation by 2ME induces microtubule depolymerisation by binding to tubulin and inhibits microtubule assembly [8,9].
However, the major challenge of developing 2ME as a useful therapeutic anticancer drug is to overcome its poor oral bioavailability and undesirable pharmacokinetic properties encompassing a short elimination half-life [10]. In a phase I clinical trial using a capsule formulation of 2ME, the efficacy of the compound was reduced due to the 17-hydroxy group being targeted in 17-β-hydroxysteroid dehydrogenase-mediated metabolism resulting in its rapid metabolic breakdown [11-13]. Consequently, strategies such as developing 2ME analogues, designing NanoCrystal® Dispersion techniques (such as Panzem NCDTM), employing 2ME delivery systems such as hydrogel and poly-lactic-co-glycolic acid (PLGA) microspheres and producing injectable formulations were investigated in order to overcome 2ME pharmacokinetic limitations [7,8,13-17].
One particular 2ME analogue, 2-methoxyoestradiol-bissulphamate (2-MeOEbisMATE), has proven to be more resistant to metabolism due to sulphamoylation on positions C3 and -17, and showed enhanced oral bioavailability [15]. The increased oral bioavailability was attributed to the reversible binding of the aryl sulphamoyl group to carbonic anhydrase II (CAII) present in erythrocytes, thereby reducing the rapid hepatic first pass metabolism of the steroid analogue. With its desirable profile as a potent anticancer, antiangiogenic drug that is orally available and resistant to metabolism it has been identified as a good candidate for development as a cancer therapeutic agent which still needs to be explored.
To address the challenge of 2ME’s poor bioavailability and short half-life, Stander et al. designed three sulphamoylated 2ME analogues in silico by modifying the carbon at the C2 and -17 positions with moieties known to improve the antimitotic activity and which would be expected to increase the compounds’ elimination half-life. These oestradiol analogues were specifically designed to both improve binding to tubulin, as well as to increase carbonic anhydrase binding affinity. The three in silico-designed sulphamoylated 2ME analogues namely 2-ethyl-3-O-sulphamoyl-oestra-1, 3, 5 (10), 15- tetraene-3-ol-17-one (ESE-15-one), 2-ethyl-3-O-sulphamoyloestra- 1, 3, 5 (10), 15-tetraene-3, 17-diol (ESE-15-ol) and 2- ethyl-3-O-sulphamoyl-oestra-1, 3, 5 (10), 16-tetraene (ESE-16) revealed enhanced antimitotic activity in vitro [18-22]. The oestradiol analogues caused abnormal spindle morphology, disrupted intracellular microtubule integrity leading to mitotic block and consequently inducted apoptosis. Generation of reactive oxygen species, an altered iron metabolism and inhibition of Bcl-2 phosphorylation were observed in vitro, which are all important for mitochondrial membrane depolarization [18-20,22]. These analogues also demonstrated binding affinity with CAII and CAIX, where ESE-15-ol and ESE-16 inhibited CAII at Nano molar concentrations, exhibiting a preferential selectivity for a mimic of CAIX [18,21,22].
This study investigated the oral bioavailability of the novel analogues and assessed whether therapeutically sufficient plasma concentrations of the compounds could be achieved using a murine oral absorption model with a new, sensitive LCMS/ MS screening method. A screening concentration limit of 5 μg/ml was determined for each of the 2ME analogues. Blood was collected from mice dosed with the individual 2ME analogues two hours (h) after oral gavage and analysed using the developed LC-MS/MS screening method for the presence of the compounds and potential related metabolites.
Materials and Methods
Logistics
Research was conducted at the Department of Physiology, School of Medicine, Faculty of Health Sciences at the University of Pretoria, Pretoria, South Africa. LC-MS/MS analysis for the investigation of screening limits was performed with an Agilent 1100 HPLC system coupled to a Sciex 4000QTrap tandem mass spectrometer (Concordia, Canada) at the Department of Pharmacology, University of Pretoria, South Africa.
Serum samples obtained from individual 2ME analogue dosed CD-1 mice, a commonly used murine model in cancer toxicology research, were analysed using a Waters® nanoACQUITY UPLC® SYNAPT® G2 high resolution mass spectrometry system and an ACQUITY UPLC BEH C18 100 × 2.1 mm, 1.7 μm column at the Department of Chemistry, University of Pretoria, South Africa. CD-1 mice were housed and maintained at the University of Pretoria Biomedical Research Centre (UPBRC), Conventional Rodent Unit, Faculty of Veterinary Sciences, University of Pretoria, and Pretoria, South Africa. CD-1 mice were sourced from Onderstepoort Biological Products SOC Ltd. (Onderstepoort, Pretoria, South Africa). Mice were group housed in Euro standard type II cages on wood shavings and received water ad libitum and irradiated animal food consisting of 22.7% protein, 5% fat, 4.5% fibre and 7.5% ash (Epol (Pty) Ltd., Pretoria, South Africa) [15,23,24]. Animals were maintained on a 12:12 h light-dark cycle at a room temperature of 22 ± 1°C to standardize behaviour and physiological effects of light intensity, wavelength and periodicity on the behaviour and physiology of the 8 mice. Mice were kept for a period of 7 d to allow for acclimatization prior to experimentation. Mice were monitored for relevant clinical signs of distress before, during and after treatment to determine their health, wellbeing and survival [25,26]. These signs specifically included rapid breathing, hunched posture, piloerection, hypoactivity, half- closed eyes, tremors, dyspnoea, agitation, fighting and feeding ability [27]. Additional environmental variables such as humidity, air quality, ventilation and noise were minimized and standardized [28].
Ethics statement
The study was approved by the Faculty of Health Sciences Research Ethics Committee, University of Pretoria, and Pretoria, South Africa (402/2016) and conducted under conditions that comply with institutional committee for research ethics and integrity requirements. For the mouse study, approval was also obtained from the Animal Ethics Committee (H006-12 and H004-14) and was in compliance with the South African National Standard for the use and care of animals in scientific experiments (SANS: 10386).
Materials
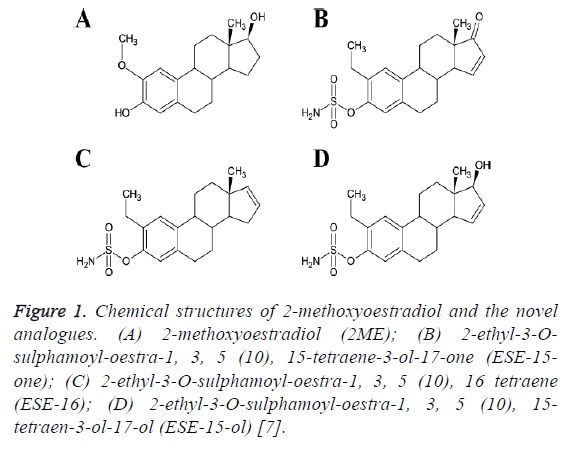
Drug synthesis: The three non-commercially available in silico-designed sulphamoylated 2ME analogues namely ESE-15-one, ESE-15-ol and ESE-16 were synthesized by Ithemba Pharmaceuticals (Pty) Ltd. (Modderfontein, Gauteng, South Africa). The structures of these compounds are shown in Figure 1.
Figure 1: Chemical structures of 2-methoxyoestradiol and the novel analogues. (A) 2-methoxyoestradiol (2ME); (B) 2-ethyl-3-Osulphamoyl- oestra-1, 3, 5 (10), 15-tetraene-3-ol-17-one (ESE-15- one); (C) 2-ethyl-3-O-sulphamoyl-oestra-1, 3, 5 (10), 16 tetraene (ESE-16); (D) 2-ethyl-3-O-sulphamoyl-oestra-1, 3, 5 (10), 15- tetraen-3-ol-17-ol (ESE-15-ol) [7].
Reagents
All required reagents were of analytical grade and were purchased from Sigma Aldrich (St. Louis, Missouri, USA) unless otherwise specified. Acetonitrile (ultragradient ROMILUltra Purity Solvent and acetonitrile 200 far UV ROMIL-Super Purity Solvent) was purchased from ROMIL (Waterbeach, Cambridge, United Kingdom). Isofluor was purchased from SAFELINE Pharmaceuticals (Pty) Ltd. (Sandton, Johannesburg, Gauteng, South Africa).
Methods
Screening limits using two different methods developed for both a triple quadrupole and a QTOF liquid chromatographytandem mass spectrometer (LC-MS/MS) system were assessed and confirmed. The methods could be used to achieve the required screening limits for the analytes in available sample volumes. This was a crucial step before performing animal studies to avoid the need for repeated animal studies [29]. As the quantitation limits of these two analytical methods for all the analytes in the serum samples was confirmed to be below 5 μg/ml in vivo, this meant oral bioavailability studies could be performed.
Determination of the optimal dose and bioavailability of oral administration of three novel in silico-designed compounds: The dose of 150 mg/kg body weight was selected due to previous literature that reported that doses of 2ME ranging from 50 to 150 mg/kg/d were effective against tumour growth, but were not toxic to mice [6,14,30,31].
In vivo bioavailability studies were conducted on CD-1 mice (n=12) treated with doses of 150 mg/kg body weight considering the body weights on Table 1 [12,32]. Nine mice were treated orally with 150 mg/kg compound in propylene glycol and tetrahydrofuran (PG: THF, 9:1 v/v), while three mice were administered with excipient only as controls (PG:THF, 9:1 v/v) [15,33]. Compound administration was done using the oral gavage technique to deposit the defined amount directly into the stomach [34].
| Experimental groups | ||||
|---|---|---|---|---|
| Body weight (kg) and dosing regimen (µl) | ESE-15-one | ESE-15-ol | ESE-16 | Control |
| 0.028 (93) | 0.025 (83) | 0.027 (90) | 0.029 (98) | |
| 0.025 (83) | 0.025 (83) | 0.025 (83) | 0.024 (80) | |
| 0.026 (87) | 0.025 (83) | 0.022 (75) | 0.024 (80) | |
Table 1: Actual body weights (kg) for CD-1 mice and the dosing volume (μl) to achieve 150 mg/kg body weight.
Oral treatment of animals with test compounds: Two h following oral administration of 150 mg/kg ESE-15-one, ESE-15-ol or ESE-16, mice were subjected to deep terminal anaesthesia using isoflurane and blood was collected by cardiac puncture using 26G (0.45 × 13 mm) gauge needle and 1 ml Luer syringe (Terumo Corporation, Shibuya, Tokyo, Japan) [15,33]. Blood samples from each e xperimental subject were transferred into anticoagulant free 5 ml tubes, allowed to clot and separated for 60 min at room temperature.
Serum was collected after centrifugation at 1800 × g at 25°C for 15 min and stored at -80°C until analysed. After a step wise extraction and protein precipitation the serum samples were analysed using a SYNAPT G2 HDMS liquid chromatographhybrid tandem (QTOF) Mass Spectrometer system using Masslynx 4.1 software (Waters, Milford Ma, USA). Samples were screened for the presence of compound and related metabolites using a threshold concentration of 5 μg/ml.
Serum collection for determination of screening limits of test compounds: Ten compound naive female CD-1 mice were subjected to deep terminal anaesthesia using isoflurane and blood collected by cardiac puncture using a 26G (0.45 × 13 mm) gauge needle and 1 ml-10 ml/Luer Solo syringe (Terumo Corporation, Shibuya, Tokyo, Japan) [33]. Blood samples from each experimental subject were placed in anticoagulant free 5 ml tubes. Approximately 0.8 ml of blood was collected from each animal. After blood was allowed to clot and separate for 60 min at room temperature, serum was separated by centrifugation at 1800 × g at 25°C for 15 min, harvested and stored in 2 ml centrifuge tubes at -80°C for screening studies.
Sample preparation for determining screening limits of test compounds: A stock solution concentration of 1.0 mg/ml for each compound was prepared. A Sartorius M2P microbalance (0.001 mg) was used to measure 0.148 mg of ESE-15-one, 0.130 mg of ESE15-ol and 0.140 mg of ESE-16. The excipient was prepared in a 10 ml measuring cylinder containing (PG:THF, 9:1 v/v). Each compound was dissolved in the excipient to a final concentration of 1.0 mg/ml. From the above concentrations, 1:10, 1:100 and 1:200 dilutions were prepared using the excipient or serum. Serum based samples were divided into two equal aliquots, the ‘same day’ serum sample (serum samples spiked with a series of dilutions of drug, prepared and analysed within 2 h of spiking) and the ‘overnight’ serum samples (serum samples spiked with drug stock solution in a dilution series and stored at -80°C for 24 h) and subsequently analysed. Excipient samples were exposed to the same two experimental storage conditions.
Serum de-proteinisation for LC-MS/MS: ‘Overnight’ samples were allowed to thaw at room temperature for 5 min. Both ‘overnight’ (24 h) samples and ‘same day’ samples were centrifuged at 16000 × g for 5 min using a Microfuge 16 centrifuge (Beckman Coulter, California, United States). Spiked serum (50 μl) and excipient samples (50 μl) were partitioned by a series of additions (25 μl, 25 μl and 50 μl) of acetonitrile in a 2 ml micro-centrifuge tube giving a final volume of 150 μl. Samples were vortex-mixed for 5 min between additions using a Vortex Genie 2 mixer (Model G560) and then processed in a Branson sonication bath (120 W) (Danbury, CT. USA) for 10 min. This process was repeated for every addition of acetonitrile and samples were finally centrifuged for 5 min at 16000 × g. The supernatant was transferred into a vial and dried at 40°C overnight using a CentriVap concentrator (Labconco Corporation, Kansas City, Missouri, USA).
The sample residues were reconstituted in 50 μl of 25% acetonitrile in distilled water. Prepared serum and excipient samples were analysed using an Agilent 1100 HPLC system coupled to a Sciex 4000 QTrap tandem mass spectrometer (Sciex, Concordia, Canada).
LC-MS/MS method
In this method, the test compounds were extracted from serum using the extraction method as described above. An injection volume of 10 μl of the reconstituted extract was analysed using the Sciex 4000QTrap LC-MS/MS system. Separation was performed using a Gemini C18 100 × 2.1 mm 3 μm column (Phenomenex). The screening method was established in both positive- (ESI+) and negative ion mode (ESI-) combined with multiple reaction monitoring (MRM) or selected reaction monitoring (SRM) where multiple or one selected ion fragment is monitored in addition to the precursor ion respectively to ensure selectivity and sensitivity of the analytical method.
Serum and excipient samples were assayed at the Department of Pharmacology, University of Pretoria, Pretoria, South Africa using a developed and validated LC-MS/MS method for the screening of ESE-15-one, ESE15-ol or ESE-16 concentrations (M1 shows more details of the method).
Results
Observations
Mice were observed for clinical signs of toxicity in Euro standard type II cages before, during and after treatment to determine their health, well-being and any adverse effects [25,26]. Mice showed no signs of toxicity over the 2 h after dosing with the highest oral dose of 150 mg/kg.
Screening threshold determination of animal study
Three different blank serum samples and excipient samples (with no compound) were analysed using the developed LCMS/ MS screening method which proved to be selective for each analyte to evaluate chromatographic interference. No interferences with analyte peaks were detected when using the MRM technique.
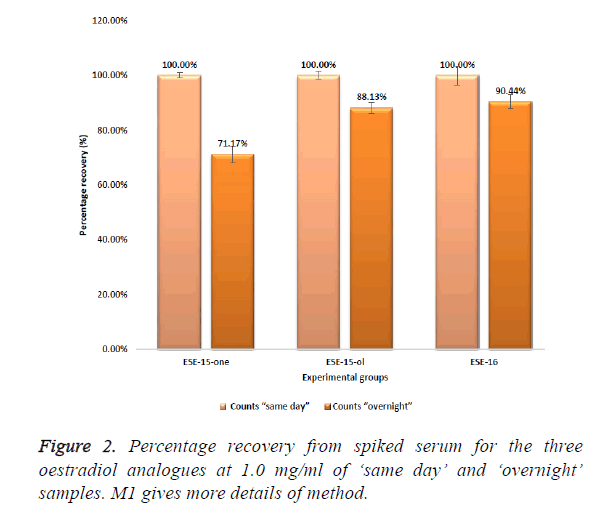
Initial screening studies to determine threshold limits of the compounds when spiked into serum were conducted and showed that ESE-15-one, ESE-15-ol and ESE-16 could all be positively identified in serum using the MRM based LCMS/ MS screening methods at concentrations of 5 μg/ml with a signal to noise ratio of greater than 30. Figure 2 represents the peak area counts of serum extracts of these oestradiol analogues spiked at 1.0 mg/ml each.
Test compound stability testing
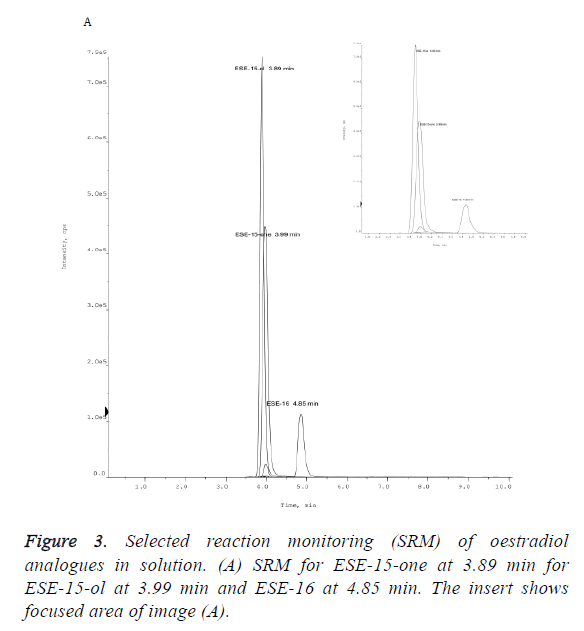
The peak area of a compound is a measure of the concentration. The measured mean peak area of ESE-15-one ‘overnight’ samples (1.58E+03) was 28.82% lower that the area of the equivalent ESE-15-one ‘same day’ samples (2.22E +03) (Figure 2). ESE-15-one eluted from the Gemini C18 100 × 2.00 mm, 3 μm column at a retention time (RT) of 3.99 min (Figure 3). ESE-15-ol eluted at a retention time 3.89 min, with a peak area of (1.60E+04) with ‘same day’ samples, 11.87% higher than the equivalent ESE-15-ol (1.41E+04) ‘overnight’ samples. Higher recovery was seen with ESE-16 samples when compared to other analogues. ESE-16 (2.72E+03) ‘same day’ samples were 9.55% higher than ESE-16 (2.46E+03) ‘overnight’, and eluted from the same column at a retention time of 4.85 min.
All three test compounds could be clearly identified without interference in spiked serum. A greater peak area was seen with ‘same day’ samples compared to equivalent overnight stored samples, indicating possible storage instability of the compound.
In vivo studies
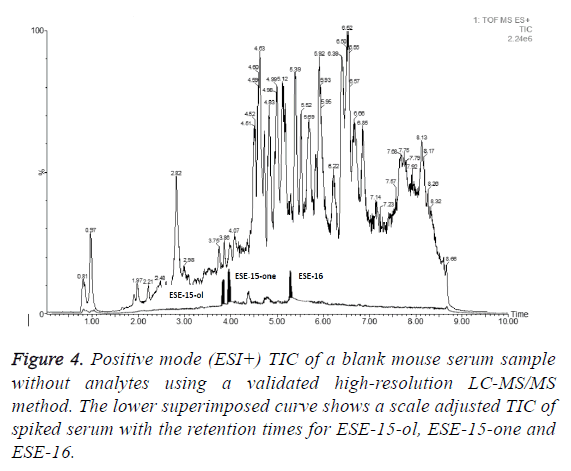
Mouse serum samples (n=3) spiked with PG/THF (9:1 v/v) alone were used as control samples to assess the presence of any interferences eluting at the same retention times as the test compounds during chromatography of the serum extracts. No interferences were observed using a multiple reaction monitoring (MRM) or selected reaction monitoring (SRM) based analysis. Figure 4 shows a total ion chromatogram (TIC) for a representative blank serum sample (serum sample from test compound naïve mouse) with a scale adjusted and superimposed TIC of serum sample spiked with 10 μg/ml of ESE-15-one, ESE-15-ol and ESE-16 and where the test compound peaks have been highlighted in black.
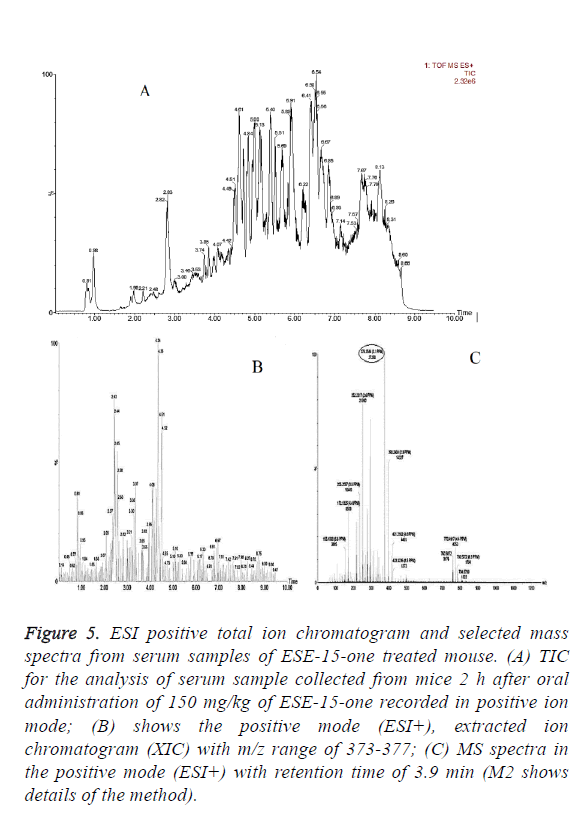
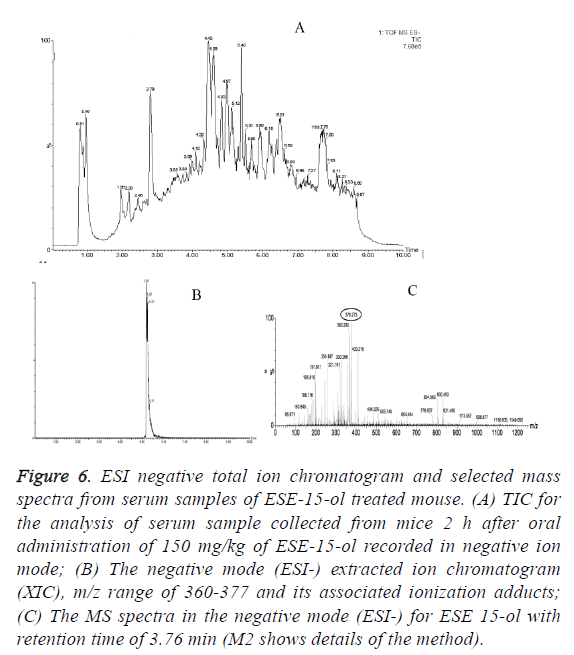
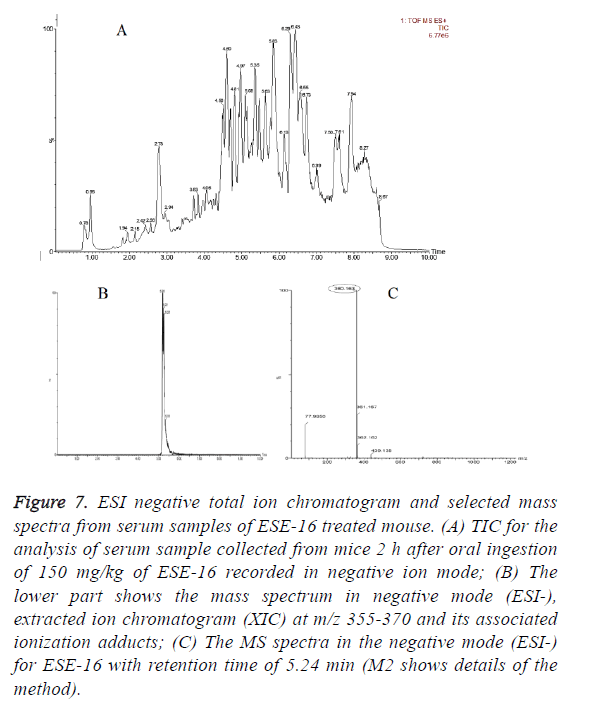
In vivo oral bioavailability studies revealed above threshold level concentrations of the test compounds ESE-15-one, ESE-15-ol and ESE-16 in serum samples collected 2 h after oral drug administration (Figures 5-7). Data show the TIC and selected mass spectra of serum samples from mice dosed at 150 mg/kg of ESE-15-one, ESE-15-ol or ESE-16 respectively. The mass-to-charge ratio (m/z) 355-377 and associated ionization adducts were collected for each compound. Analyses of serum samples using LC-MS/MS showed presence of the test compounds above the threshold level of 5 μg/ml for all three test compounds following oral gavage.
Figure 5: ESI positive total ion chromatogram and selected mass spectra from serum samples of ESE-15-one treated mouse. (A) TIC for the analysis of serum sample collected from mice 2 h after oral administration of 150 mg/kg of ESE-15-one recorded in positive ion mode; (B) shows the positive mode (ESI+), extracted ion chromatogram (XIC) with m/z range of 373-377; (C) MS spectra in the positive mode (ESI+) with retention time of 3.9 min (M2 shows details of the method).
Figure 6: ESI negative total ion chromatogram and selected mass spectra from serum samples of ESE-15-ol treated mouse. (A) TIC for the analysis of serum sample collected from mice 2 h after oral administration of 150 mg/kg of ESE-15-ol recorded in negative ion mode; (B) The negative mode (ESI-) extracted ion chromatogram (XIC), m/z range of 360-377 and its associated ionization adducts; (C) The MS spectra in the negative mode (ESI-) for ESE 15-ol with retention time of 3.76 min (M2 shows details of the method).
Figure 7: ESI negative total ion chromatogram and selected mass spectra from serum samples of ESE-16 treated mouse. (A) TIC for the analysis of serum sample collected from mice 2 h after oral ingestion of 150 mg/kg of ESE-16 recorded in negative ion mode; (B) The lower part shows the mass spectrum in negative mode (ESI-), extracted ion chromatogram (XIC) at m/z 355-370 and its associated ionization adducts; (C) The MS spectra in the negative mode (ESI-) for ESE-16 with retention time of 5.24 min (M2 shows details of the method).
Discussion
Literature has shown that 2ME (2 μM) inhibited the anaphasepromoting complex (APC) in MDA-MB-435 breast cells [14]. In previous studies, the 2ME analogue, 2-MeOEbisMATE was shown to inhibit growth of human breast adenocarcinoma cell line (MDA-MB-231) with a half maximal inhibitory concentration (IC50) of 0.33 μM [35]. Additionally 2- MeOE2bisMATE, 2-methoxyestradiol-bis-sulphamate (2MEBM) and ESE-15-one, analogues of 2ME had inhibitory effects on cell growth in the range of 0.2 μM-1 μM in several cell lines including the MCF-7 cell line and oesophageal carcinoma SNO cells, showing that the sulphamoylated derivatives of 2ME are more potent inhibitors of the cancer cell than the parent compound 2ME [19,36,37].
Since steroids have a hydrophobic scaffold, solubility is generally a concern in excipients. Theron et al. reported sulphomylation of oestradiol analogues should therefore increase their solubility in aqueous media. To determine whether the three sulphomoylated in silico-designed 2ME analogues have potential for further clinical anticancer investigation, their stability in excipient and bioavailability needed to be determined.
A mass spectrometry method to screen and identify these sulphamoylated 2ME analogues in serum and excipient was established using serial dilutions created from the 1 mg/ml stock solutions.
This in vivo study aimed to determine whether the three novels in silico-designed drugs namely ESE-15-one, ESE-15-ol and ESE-16 could be recovered from and detected in serum following oral administration. The screening assay of the three 2ME analogues was determined at 5 μg/ml from both excipient and serum.
Research has shown that doses of 2ME ranging from 50 to 150 mg/kg/d were effective against various types of cancer in mice, without reporting serious systemic toxicity [4,14,31,38-40]. 2- MeOEbisMATE administered orally had a maximum tolerated dose of 150 mg/kg body weight [15]. In this study, 150 mg/kg body weight as a dose was therefore selected based on these previous studies where 2ME and its analogues were assessed [5,30,31].
In vivo studies in mice were conducted to assess relevant clinical signs of distress before, during and after oral administration of oestradiol analogues. Health, wellbeing and survival of the mice were therefore monitored [25,26]. No adverse effects of rapid breathing, hunched posture, piloerection, hypoactivity, half-closed eyes, tremors, dyspnoea, agitation, fighting and feeding ability were observed amongst the treated mice. Thus, in vivo studies gave no clinical indication of systemic drug toxicity [27].
Our research focuses on improving treatment systems via enhanced oral bioavailability of the in silico-designed potential anticancer drug, to enable the drug to only affect the cancer cells and at low dosages with less frequent treatment intervals. Studies are currently being conducted using the Rapid Equilibrium Dialysis system to evaluate the binding efficacy of these sulphamoylated analogues to CAII within erythrocytes which would facilitate bypassing the first pass hepatic degradation of the steroid compounds.
Conclusion
To determine whether the three in silico-designed compounds will have potential in vivo anticancer therapeutic applications it was important to analyse their stability in blood and to determine the oral bioavailability in vivo. In order to measure the oral bioavailability, a rapid screening mass spectrometric method to identify sulphamoylated 2ME analogues in serum and solvent excipient was used. Compound stability and the ability to identify the analytes after dilution in serum was demonstrated with recovery of these compounds from ‘overnight’ stored samples that included a freeze/thaw cycle. In vivo studies showed therapeutically relevant concentrations of the 2ME analogues in serum samples 2 h after oral administration using a LC-MS/MS validated method. This is the first study to determine whether ESE-15-one, ESE-15-ol and ESE-16 could be detected in circulation in vivo after oral administration of these three different 2ME derivatives. Results from this study merit further investigation of these potential anticancer compounds in vivo.
Supporting Information
Method 1 (M1) (Table 2)
Analytical column: Gemini C18 100 × 2.1 mm, 3 μm column (Phenomenex).
Tandem mass spectrometry conditions for assay: Ionisation voltage: 5500; Curtain gas (CUR): 23.00; Interference temperature (TEM): 450.0; De-clustering potential (DP): 50.00; Entrance potential (EP): 10.0; TurboIonSpray nebulizer gas nitrogen (GS1): 36.00; TurboIonSpray drying gas nitrogen (GS2): 35.00; Collision activation dissociation gas flow CAD: High; Collision cell exit potential (CXP): 10.0; Collision energy: 34.
| Total time (min) | Flow rate (µl/min) | A (%) | B (%) |
|---|---|---|---|
| 0 | 250 | 65 | 35 |
| 0.5 | 250 | 65 | 35 |
| 1 | 250 | 5 | 95 |
| 6 | 250 | 5 | 95 |
| 6.5 | 250 | 65 | 35 |
| 10 | 250 | 65 | 35 |
Table 2: Gradient method of method 1.
Method 2 (M2) (Table 3)
Analytical column: ACQUITY UPLC BEH C18 100 × 2.1 mm, 1.7 μm column.
Tandem mass spectrometry conditions for assay: Mass range: Mass 50 Da to 1200 Da.
Lock spray configuration: Reference scan frequency (s): 10.000; Reference cone voltage (V): 40.000; Reference capillary voltage (kV): 3.100; Reference trap collision energy 4.000.
Experimental instrument parameters: Polarity ES+.
Analyser resolution mode: Capillary (kV): 2.5000; Source temperature (°C): 120; Sampling cone: (V/eV) 30.0000; Extraction cone (V/eV): 4.0000; De-solvation temperature (°C): 450; Cone gas flow (l/h): 10.0.
| Total time (min) | Flow rate (µl/min) | A (%) | B (%) |
|---|---|---|---|
| 0 | 300 | 90 | 10 |
| 1 | 300 | 90 | 10 |
| 6 | 300 | 10 | 90 |
| 7.5 | 300 | 10 | 90 |
| 7.75 | 300 | 90 | 10 |
| 10 | 300 | 90 | 10 |
Table 3: Gradient method of method 2.
Conflict of Interest
All authors declare that there are no ‘actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the submitted work that could inappropriately influence, or be perceived to influence, their work’.
Acknowledgement
This study was supported by grants from the Cancer Association of South Africa (A0V741, A0W228, A0B741), the Struwig Germeshuysen Trust (A0N074), Research Committee, School of Medicine (University of Pretoria) (A0H561), National Research Foundation (N00375, N00591) and the Medical Research Council (A0W110) awarded to Prof. AM Joubert. The mass spectrometers used in this study were both purchased with financial support from the National Research Foundation.
References
- Lakhani NJ, Sarkar MA, Venitz J, Figg WD. 2-Methoxyestradiol, a promising anticancer agent. Pharmacotherapy 2003; 23: 165-172.
- Lee JS, Kim YK, Yang H, Kang HY, Ahn C, Jeung EB. Two faces of the estrogen metabolite 2-methoxyestradiol in vitro and in vivo. Mol Med Rep 2015; 12: 5375-5382.
- D'Amato RJ, Lin CM, Flynn E, Folkman J, Hamel E. 2-Methoxyestradiol, an endogenous mammalian metabolite, inhibits tubulin polymerization by interacting at the colchicine site. Proc Natl Acad Sci U S A 1994; 91: 3964-3968.
- Fotsis T, Zhang Y, Pepper MS, Adlercreutz H, Montesano R, Nawroth PP, Schweigerer L. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature 1994; 368: 237-239.
- Klauber N, Parangi S, Flynn E, Hamel E, D'Amato RJ. Inhibition of angiogenesis and breast cancer in mice by the microtubule inhibitors 2-methoxyestradiol and taxol. Cancer Res 1997; 57: 81-86.
- Pribluda VS, Gubish ER, Jr., Lavallee TM, Treston A, Swartz GM, Green SJ. 2-Methoxyestradiol: an endogenous antiangiogenic and antiproliferative drug candidate. Cancer Metastasis Rev 2000; 19: 173-179.
- Stander BA, Marais S, Vorster CJ, Joubert AM. In vitro effects of 2-methoxyestradiol on morphology, cell cycle progression, cell death and gene expression changes in the tumorigenic MCF-7 breast epithelial cell line. J Steroid Biochem Mol Biol 2010; 119: 149-160.
- Lu Y, Chen J, Xiao M, Li W, Miller DD. An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm Res 2012; 29: 2943-2971.
- Ravelli RBG, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004; 428: 198-202.
- Lakhani NJ, Sparreboom A, Xu X, Veenstra TD, Venitz J, Dahut WL, Figg WD. Characterization of in vitro and in vivo metabolic pathways of the investigational anticancer agent, 2-methoxyestradiol. J Pharm Sci 2007; 96: 1821-1831.
- Dahut WL, Lakhani NJ, Gulley JL, Arlen PM, Kohn EC, Kotz H, McNally D, Parr A, Nguyen D, Yang SX, Steinberg SM, Venitz J, Sparreboom A, Figg WD. Phase I clinical trial of oral 2-methoxyestradiol, an antiangiogenic and apoptotic agent, in patients with solid tumors. Cancer Biol Ther 2006; 5: 22-27.
- Newman SP, Ireson CR, Tutill HJ, Day JM, Parsons MF, Leese MP, Potter BV, Reed MJ, Purohit A. The role of 17beta-hydroxysteroid dehydrogenases in modulating the activity of 2-methoxyestradiol in breast cancer cells. Cancer Res 2006; 66: 324-330.
- Tevaarwerk AJ, Holen KD, Alberti DB, Sidor C, Arnott J, Quon C, Wilding G, Liu G. Phase I trial of 2-methoxyestradiol NanoCrystal dispersion in advanced solid malignancies. Clin Cancer Res 2009; 15: 1460-1465.
- Bhati R, Gokmen-Polar Y, Sledge GW, Jr., Fan C, Nakshatri H, Ketelsen D, Borchers CH, Dial MJ, Patterson C, Klauber-DeMore N. 2-Methoxyestradiol inhibits the anaphase-promoting complex and protein translation in human breast cancer cells. Cancer Res 2007; 67: 702-708.
- Ireson CR, Chander SK, Purohit A, Perera S, Newman SP, Parish D, Smith AC, Potter BV, Reed MJ. Pharmacokinetics and efficacy of 2-methoxyoestradiol and 2-methoxyoestradiol-bis-sulphamate in vivo in rodents. Br J Cancer 2004; 90: 932-937.
- Pasquier E, Sinnappan S, Munoz MA, Kavallaris M. ENMD-1198, a new analogue of 2-methoxyestradiol, displays both antiangiogenic and vascular-disrupting properties. Cancer Ther 2010; 9: 1408-1418.
- Cho JK, Hong KY, Park JW, Yang HK, Song SC. Injectable delivery system of 2-methoxyestradiol for breast cancer therapy using biodegradable thermosensitive poly (organophosphazene) hydrogel. J Drug Target 2011; 19: 270-280.
- Theron AE, Nolte EM, Lafanechère L, Joubert AM. Molecular crosstalk between apoptosis and autophagy induced by a novel 2-methoxyestradiol analogue in cervical adenocarcinoma cells. Cancer Cell Int 2013; 13: 87.
- Visagie M, Theron A, Mqoco T, Vieira W, Prudent R, Martinez A, Lafanechère L, Joubert A. Sulphamoylated 2-methoxyestradiol analogues induce apoptosis in adenocarcinoma cell lines. PLoS One 2013; 8: e71935.
- Theron A, Prudent R, Nolte E, Bout I, Punchoo R, Marais S, Toit P, Hlophe Y, Papendorp D, Lafanechère L, Joubert A. Novel in silico‑designed estradiol analogues are cytotoxic to a multidrug‑resistant cell line at nanomolar concentrations. Cancer Chemother Pharmacol 2015; 75: 431-437.
- Stander BA, Joubert F, Tu C, Sippel KH, McKenna R, Joubert AM, Stanton R, Gernert K, Nettles J, Aneja R, Stander A, Joubert F, Joubert A. In vitro evaluation of ESE-15-ol, an estradiol analogue with nanomolar antimitotic and carbonic anhydrase inhibitory activity. PLoS One 2012; 7: e52205.
- Stander BA, Joubert F, Tu C, Sippel KH, McKenna R, Joubert AM. Signaling pathways of ESE-16, an antimitotic and anticarbonic anhydrase estradiol analogue, in breast cancer cells. PloS One 2013; 8: e53853.
- Meyer-Losic F, Newman SP, Day JM, Reed MJ, Kasprzyk PG, Purohit A, Foster PA. STX140, but not paclitaxel, inhibits mammary tumour initiation and progression in C3 (1)/SV40 T/t-antigen transgenic mice. PloS One 2013; 8: e80305.
- Horny C, Balasubashini MS, Komanduri K, Ganapathy M, Yeh IT, Ghosh R, Kumar AP. 2-Methoxyestradiol prevents LNCaP tumor development in nude mice: Potential role of G2/M regulatory proteins. J Cell Death 2009; 2: 1-8.
- Burkholder T, Foltz C, Karlsson E, Linton CG, Smith JM. Health evaluation of experimental laboratory mice. Curr Protoc Mouse Biol 2012; 145-165.
- Jiang Z, Liu Y, Wan C, Yang B, Guo L, Wang Y, Wang Y, Zhou W, Liu Y, Wang Z. Different light-dark cycles affect growth rate and food intake of mice. Biol Rhythm Res 2006; 37: 11-19.
- Prudent R, Vassal-Stermann E, Nguyen CH, Pillet C, Martinez A, Prunier C, Barette C, Soleilhac E, Filhol O, Beghin A. Pharmacological inhibition of LIM kinase stabilizes microtubules and inhibits neoplastic growth. Cancer Res 2012; 72: 4429-4439.
- Castelhano-Carlos M, Baumans V. The impact of light, noise, cage cleaning and in-house transport on welfare and stress of laboratory rats. Lab Anim 2009; 43: 311-327.
- Zhang D, Luo G, Ding X, Lu C. Preclinical experimental models of drug metabolism and disposition in drug discovery and development. Acta Pharm Sin B 2012; 2: 549-561.
- LaVallee TM, Burke PA, Swartz GM, Hamel E, Agoston GE, Shah J, Suwandi L, Hanson AD, Fogler WE, Sidor CF, Treston AM. Significant antitumor activity in vivo following treatment with the microtubule agent ENMD-1198. Mol Cancer Ther 2008; 7: 1472-1482.
- Huh J-I, Calvo A, Charles R, Green JE. Distinct tumor stage-specific inhibitory effects of 2-methoxyestradiol in a breast cancer mouse model associated with Id-1 expression. Cancer Res 2006; 66: 3495-3503.
- Tinley TL, Leal RM, Randall-Hlubek DA, Cessac JW, Wilkens LR, Rao PN, Mooberry SL. Novel 2-methoxyestradiol analogues with antitumor activity. Cancer Res 2003; 63: 1538-1549.
- Newman SP, Foster PA, Stengel C, Day JM, Ho YT, Judde J-G, Lassalle M, Prevost G, Leese MP, Potter BV. STX140 is efficacious in vitro and in vivo in taxane-resistant breast carcinoma cells. Clinical Cancer Res 2008; 14: 597-606.
- UBC Animal Care Guidelines. Oral dosing (gavage) in adult mice and rats SOP. Laboratory Animal Med 2015.
- Suzuki R, Newman S, Purohit A, Leese M, Potter B, Reed M. Growth inhibition of multi-drug-resistant breast cancer cells by 2-methoxyoestradiol-bis-sulphamate and 2-ethyloestradiol-bis-sulphamate. J Steroid Biochem 2003; 84: 269-278.
- Visagie M, Joubert A. The in vitro effects of 2-methoxyestradiol-bis-sulphamate on cell numbers, membrane integrity and cell morphology, and the possible induction of apoptosis and autophagy in a non-tumorigenic breast epithelial cell line. Cell Mol Biol Lett 2010; 15: 564-581.
- Vorster C, Joubert A. In vitro effects of 2-methoxyestradiol-bis-sulphamate on cell growth, morphology and cell cycle dynamics in the MCF-7 breast adenocarcinoma cell line. Biocell 2010; 34: 71-79.
- Chiche J, Ilc K, Laferrière J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouysségur J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res 2009; 69: 358-368.
- Wassberg E, Christofferson R. Angiostatic treatment of neuroblastoma. Eur J Cancer 1997; 33: 2020-2023.
- LaVallee TM, Zhan XH, Herbstritt CJ, Kough EC, Green SJ, Pribluda VS. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors alpha and beta. Cancer Res 2002; 62: 3691-3697.