ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 16
Correlation between IL-33 and sST2 levels in sera of patients with acute myocardial infarction
Sahar Karimzadeh1, Somaye Zamani1, Fatemeh Amirzade-Fard1, Alireza Abdi Ardekani2 and Mehrnoosh Doroudchi1*
1Department of Immunology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
2Department of Cardiology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
- *Corresponding Author:
- Mehrnoosh Doroudchi
Department of Immunology
School of Medicine
Shiraz University of Medical Sciences, Shiraz, Iran
Accepted on July 11, 2017
Objective: We investigated the correlation between levels of IL-33 multifunctional inflammatory cytokine and its soluble receptor (sST2) in the sera of patients with myocardial infarction.
Methods: Blood samples were collected from 39 patients with ST Elevated Myocardial Infarction (STEMI) and 42 healthy blood donors. IL-33 and sST2 were measured in the sera by Enzyme Linked Immunosorbent Assay (ELISA) and their concentrations were compared between groups and according to clinical criteria.
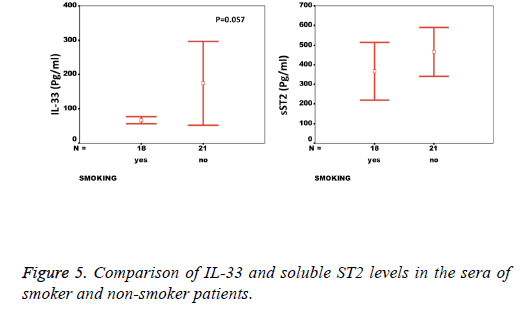
Results: While there was a significant difference between healthy individuals and patients based on sST2 (p=0.002, the level of IL-33 was not significantly different between groups (p=0.17). A significant correlation between the serum levels of sST2 and IL-33 in patient group was observed (p=0.022). There were significant negative correlations between serum levels of sST2 (p=0.028) and IL-33 (p=0.091) with Ejection Fraction. While there was no difference between diabetic and non-diabetic patients regarding sST2 levels (p=0.695), a trend of lower IL-33 level was observed in diabetic patients in comparison to non-diabetes ones (p=0.057). Similarly, IL-33 was lower in smokers compared to non-smokers, although the difference did not reach the significant level (p=0.057).
Conclusion: sST2 but not IL-33 levels increase in sera of patients with STEMI. However, the correlation between the two factors in patients group implies a complex interaction between IL-33 and its receptor (s) in STEMI. The higher levels of IL-33 in non-diabetic and non-smokers highlight a difference in the Th2 responses in patients and demands further investigation.
Keywords
Ejection fraction, Enzyme linked immunosorbent assay (ELISA), IL-33, sST2, ST elevated myocardial infarction.
Introduction
Interleukin-33 (IL-33) is a member of IL-1 family of cytokines, which is broadly expressed in different tissues [1]. Despite being in the same family with IL-1α, IL-1β, and IL-18, it demonstrates a different cellular pattern of expression. Accordingly, hematopoietic cells show only limited IL-33 mRNA expression [2]. Endothelial cells are the main producers of IL-33 in different tissue and organs including lung, colon, kidney, liver, skin, brain, and adipose tissues [3,4]. In addition to endothelial cells, epithelial cells, keratinocytes, Smooth Muscle Cells (SMC), and fibroblasts may express IL-33 [1].
IL-33 is a multi-functional immunodulatory cytokine that can act both as a pro or anti-inflammatory factor in different conditions depending on disease state or experimental conditions [5]. IL-33 binds to a receptor complex composed of toll like/IL-1 receptor family member (ST2) and IL-R Accessory Protein (IL-IRACP), which is expressed on T helper cell type 2 (Th2) lymphocytes [1,6]. ST2 exists is both soluble and membranous forms which are produced by alternative splicing and differential usage of proximal and distal promoters rather than protease-dependent cleavage [7,8]. The soluble form of the molecule can play the role of a decoy receptor by binding free IL-33 and preventing its signaling [9]. Soluble ST2 (sST2) is shown to decrease TNF-α, IL-33, and Toll like receptor-4 thereby modulating inflammation in acute lung injury. IL-33 as the natural ST2 ligand plays a role in the Th2 dependent immunopathology associated with IL-4, and IL-5 cytokines such as asthma, rheumatoid arthritis, collagen vascular disease, and lung cancer [10]. Chronic IL-33 exposure can cause epithelium hypertrophy and accumulation of mucus in bronchi as seen in Chronic Pulmonary Obstructive Disease (COPD) [11]. On the other hand, soluble ST2 is shown to increase in the sera of patients with systemic lupus erythematosus, advanced systemic sclerosis, Wegener granulomatosis and behcet’s disease [12]. In this regard,administration of Fc-sST2 is shown to reduce the symptoms in rheumatoid arthritis as well as reducing IFN-γ and TNF-α levels [13]. Thus much interest exists in sST2 as a new therapeutic molecule in the treatment of asthma and rheumatoid arthritis [14]. Increased sST2 levels in the sera of patients diagnosed with sepsis as well as in patients who were hospitalized in Emergency ward due to non-cardiac dyspnea are reported [15]. Similarly, administration of exogenous sST2 can reduce serum levels of IL-6, IL-12, and TNF-α associated with increased survival in mice with sepsis [15].
Of note, sST2 is significantly associated with stroke/transient ischemic attack even after adjustment for cardiovascular risk factors and increases the rate of re-hospitalization and mortality in patients with non-cardiac dyspnea [16,17]. With regard to stable coronary artery disease, elevated sST2 plasma concentrations at baseline correlate with poor survival of the patients [18]. Moreover, sST2 levels one day after MI, correlate with unfavourable outcome [19]. Pathological examinations of myocardial tissues after MI are indicator of advanced inflammatory reaction where fibrosis and scar formation mediated by cellular infiltrates are unfavourable responses to injury [20]. In animal models, manipulation of this cellular inflammatory process causes decrement in the intensity of MI [21]. Interestingly, IL-33/ST2 system can modulate the fibrosis associated with arrhythmia as well [14].
Although the prognostic values of circulating IL-33 in cardiovascular disease is not yet clear, increase in the levels of its soluble receptor is recognized as a marker of poor prognosis in patients with myocardial infarction and heart failure [19,22]. IL-33 and sST2 are shown to be induced upon biomechanical strain in cardiac fibroblasts where IL-33 exerts antihypertrophic effects on cardiomyocytes in a dose-dependent manner in rats [14].
It is therefore not surprising that new therapeutic approaches based on the IL-33/ST2 pathway are emerging [22-24]. IL-33 administration has been shown to decrease the size of atheromas in ApoE-/-mice, which were fed a high-fat diet and had high serum cholesterol [22]. The idea of using IL-33/ST2 pathway in therapeutic regimens is in its infancy but ST2 has already been recommended in the treatment of arthritis [13]. Administration of beta-blockers is recently shown to enhance IL-33/ST2 signaling by increasing IL-33 and decreasing sST2 [25].
Since sST2 can play as a decoy receptor for IL-33, we asked if the levels of IL-33 and its soluble receptor correlate with each other and the clinicopathological parameters after myocardial infarction.
Materials and Methods
The study population included 39 patients and 42 healthy individuals. The patients entered to this study by the convenient method of sampling from hospitals affiliated to the Shiraz University of Medical Science and controls selected from volunteers of Blood Transfusion Center of Shiraz. Serum samples from patients gathered after consideration of inclusion and exclusion criteria. Inclusion criteria of the patients were: Increase in TnT levels and ST elevation in anterolateral and anteroseptal leads. Diagnosis of myocardial infarction was made by the treating physician based on the presenting Electrocardiogram (ECG) in combination with serial TnT measurement. The diagnosis was later confirmed with selective coronary angiography in the hospital course. Patients with Chronic Renal Failure (CRF), autoimmune diseases, and cardiogenic shock were excluded from the study. Clinical data for each patient extracted from patient’s files and at the time of serum sampling using a questionnaire. The demographic and clinical information gathered included: Name, age, gender, history of ischemic diseases, family history of myocardial disease, smoking, medications, and history of coronary angiography, diabetes history, hypertension, valvular heart disease, fat blood, ECG findings, and echocardiography. The inclusion of control individuals was based on the lack of history of cancer and autoimmune diseases as well as their age and gender. The demographical and clinical characteristics of patients are shown in Table 1.
| Characteristics | No. (%) | sST2 (pg/ml) | IL-33 (pg/ml) | |
|---|---|---|---|---|
| Gender | ||||
| Male | 16 (41.1) | 493.91 ± 277.11 | 74.11 ± 25.62 | |
| Female | 23 (58.9) | 386.30 ± 285.28 | 197.77 ± 307.24 | |
| Smoking status | ||||
| Smoker | 18 (46.1) | 456.43 ± 275.40 | 66.86 ± 19.37 | |
| Non-smoker | 21 (53.9) | 366.63 ± 295.03 | 174.55 ± 269.81 | |
| Diabetes | ||||
| Positive | 8 (20.5) | 401.63 ± 165.94 | 69.41 ± 28.75 | |
| Negative | 31 (79.5) | 424.53 ± 310.60 | 139.15 ± 226.42 | |
| Hypertension | ||||
| Positive | 18 (46.1) | 446.71 ± 269.27 | 92.72 ± 106.55 | |
| Negative | 21 (53.9) | 396.80 ± 302.86 | 152.38 ± 259.54 | |
| Hyperlipidemia | ||||
| Positive | 12 (30.7) | 475.93 ± 296.33 | 107.83 ± 130.81 | |
| Negative | 27 (69.3) | 394.90 ± 282.21 | 132.41 ± 230.5 | |
| Family history | ||||
| Positive | 4 (10.2) | 386.77 ± 153.22 | 77.13 ± 21.08 | |
| Negative | 35 (89.8) | 423.61 ± 297.83 | 130.3 ± 214.42 | |
Table 1. IL-33 and sST2 levels in patients based on their demographical criteria and risk factors.
After informed consent, 5 ml blood was collected by venipuncture method. After 30 min coagulation, the serum was separated, aliquoted in 50 μl volumes and kept in the -20°C until used. The IL-33 (BioLegend, San Diego, USA) and sST2 (Abcam, UK) were measured by Enzyme Linked Immunosorbent Assay (ELISA) according to the manufacturer’s instructions. The Optical Density (OD) values transformed to concentration levels by the standard curve. The results were then analyzed using the SPSS 11.5 software and discussed accordingly. For this purpose, student t-test was used to compare the serum levels of IL-33 and sST2 between patients and control group. Due to the lack of normality of data (Kolmogrov-Smirnov test) the results were confirmed by Mann-Whitney test. Correlation between IL-33 and sST2 levels in the sera of patients as well as each factor with Ejection Fraction was evaluated by Spearman rank test. Comparison between serum levels of IL-33 and sST2 in patients group based on clinical or demographical criteria such as Diabetes, smoking, etc. was performed by Mann-Whitney test. Chi-square and Kruskal-Wallis tests were used to evaluate the correlation between IL-33 and sST2 levels and clinical presentations of MI where applicable. P values of ≤ 0.05 were considered statistically significant.
Results
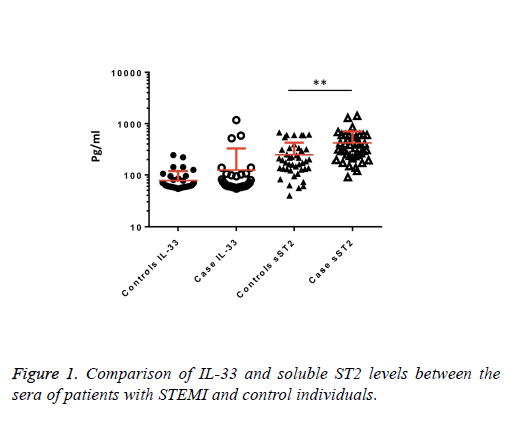
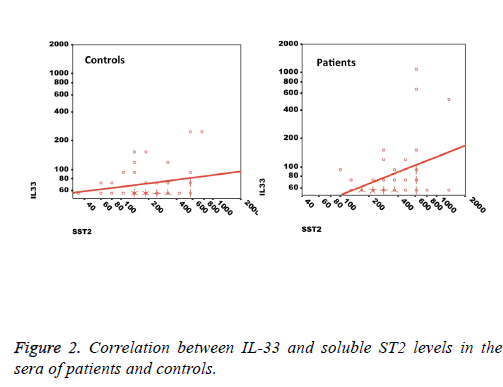
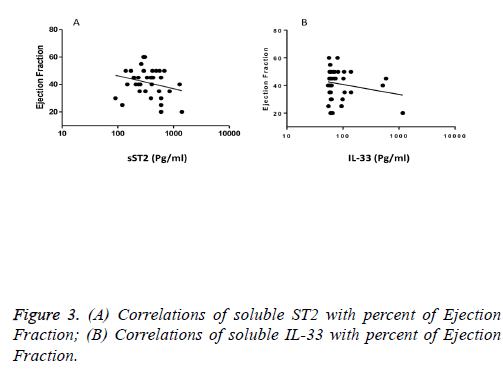
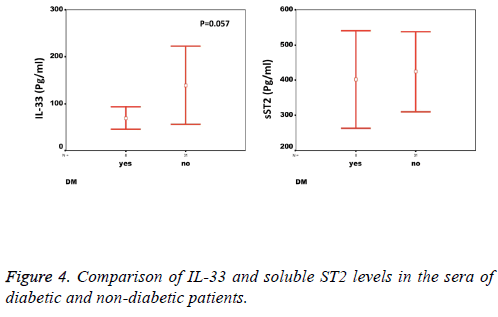
We observed a significant increase (p=0.002) in the serum levels of sST2 in patients with acute MI 419.83 ± 285.22 pg/ml compared to control individuals 248.01 ± 181.27 pg/ml (Figure 1). A trend of higher concentration of IL-33 in the patients sera 124.85 ± 203.5 pg/ml compared to controls 78.00 ± 41.39 pg/ml was also observed (p=0.17) (Figure 1). The patients were divided to two age groups under and over age 50 y and no significant differences in IL-33 (85.63 ± 33.73 vs. 134.97 ± 227.9 pg/ml, p=0.816) and sST2 (409.47 ± 149.23 vs. 422.51 ± 312.74 pg/ml, p=0.81) between groups were observed. Using Spearman rank test, we also observed a significant correlation between the serum levels of sST2 and IL-33 in patient group (p=0.022, Figure 2). Interestingly, there were negative correlations between serum levels of sST2 (p=0.028, Figure 3A) and IL-33 (p=0.091, Figure 3B) with Ejection Fraction. While there was no difference between diabetic and nondiabetic patients regarding sST2 levels (p=0.695), a trend of lower IL-33 level was observed in diabetic patients in comparison to non-diabetes ones (p=0.057, Figure 4). Similarly, IL-33 was lower in smokers 66.86 ± 19.37 pg/ml compared to non-smokers 174.55 ± 269.81 pg/ml, although the difference was only marginally significant (p=0.057, Figure 5).
There was no significant difference in the IL-33 or sST2 between patients regarding diastolic function (p>0.5 for both). No significant correlation between the type (location) of myocardial lesion and sST2 was observed. The level of IL-33 was higher in extensive anterior MI than other groups, however, the difference did not reach the significant level (p=0.28). No significant correlation between Hypertension, Hyperlipidaemia, family history of MI, family history of angiography, mechanical complications, rate, axis, MR, TR, and other clinical parameters (Table 2) with IL-33 or sST2 levels was found. Moreover, due to the small group sizes, no statistical analysis was performed on the IL-33 or sST2 levels between patients based on consumption of ACE Inhibitors, beta blockers, Statins, Streptokinase and LCX. However, the average levels in the groups are shown in Table 3. There was no significant difference in the level of IL-33 or sST2 based on ASA prescription.
| Characteristics | No. (%) | sST2 (pg/ml) | IL-33 (pg/ml) | |
|---|---|---|---|---|
| MI type | ||||
| Anterior | 4 (10.3) | 491.12 ± 182.16 | 64.78 ± 6.58 | |
| Anterolateral | 2 (5.1) | 575.20 ± 35.07 | 104.94 ± 48.94 | |
| Anteroseptal | 6 (15.4) | 281.43 ± 181.88 | 67.31 ± 11.83 | |
| Antreoseptal/ Anterolateral |
27 (69.2) | 428.52 ± 318.62 | 148.01 ± 241.94 | |
| History of angiography | ||||
| Positive | 5 (12.8) | 407.36 ± 244.48 | 133.4 ± 216.99 | |
| Negative | 34 (87.2) | 504.65 ± 519.01 | 66.71 ± 19.85 | |
| ECG rate | ||||
| <60 | 2 (5.1) | 197.40 ± 69.69 | 58.59 ± 1.45 | |
| 60-90 | 11 (28.2) | 457.70 ± 310.63 | 117.12 ± 134.77 | |
| >90 | 26 (66.6) | 420.92 ± 283.32 | 133.21 ± 235.10 | |
| ECG axis | ||||
| Natural | 25 (64.1) | 429.08 ± 299.93 | 133.02 ± 240.19 | |
| Left axis | 14 (35.8) | 414.66 ± 282.84 | 110.24 ± 119.45 | |
| Left ventricular hypertrophy | ||||
| Positive | 9 (23) | 489.26 ± 333.06 | 123.9 ± 148.46 | |
| Negative | 30 (77) | 399.00 ± 272.12 | 125.13 ± 219.59 | |
| Bundle branch block | ||||
| Negative | 31 (79.4) | 398.49 ± 260.36 | 124.47 ± 215.95 | |
| Right BBB | 2 (5.1) | 345.29 ± 360.21 | 80.02 ± 26.51 | |
| Left BBB | 6 (15.3) | 554.99 ± 398.29 | 141.75 ± 183.94 | |
| Ejection fraction | ||||
| <35% | 12 (30.7) | 531.41 ± 358.10 | 169.32 ± 316.21 | |
| >35% | 27 (69.3) | 370.25 ± 237.37 | 105.08 ± 130.17 | |
| Diastolic dysfunction | ||||
| positive | 12 (30.7) | 404.97 ± 336.97 | 136.90 ± 229.62 | |
| negative | 27 (69.3) | 379.25 ± 212.37 | 97.73 ± 11.79 | |
| Mitral regurgitation | ||||
| Negative | 24 (61.5) | 444.44 ± 281.61 | 93.01 ± 106.86 | |
| Mild MR | 15 (38.4) | 380.47 ± 296 | 175.78 ± 298.67 | |
| Tricuspid regurgitation | ||||
| Negative | 29 (74.3) | 456.30 ± 304.74 | 128.04 ± 217.69 | |
| Mild TR | 10 (25.6) | 314.08 ± 194.51 | 115.57 ± 165.55 | |
| Left anterior descending artery | ||||
| Normal | 2 (5.1) | 278.28 ± 20.67 | 60.086 ± 2.33 | |
| Significant lesion | 37 (94.8) | 428.82 ± 231.71 | 128.30 ± 208.57 | |
| Posterior descending artery | ||||
| Normal | 34 (87.2) | 368.31 ± 297.40 | 132.22 ± 217.33 | |
| Significant lesion | 5 (12.8) | 450.32 ± 266.9 | 74.70 ± 20.32 | |
| Left circumflex artery | ||||
| Normal | 22 (56.4) | 407.74 ± 273.66 | 93.84 ± 111.09 | |
| Significant lesion | 17 (43.5) | 412.5 ± 274.89 | 164.97 ± 281.40 | |
| Right coronary artery | ||||
| Normal | 29 (74.3) | 156.37 ± 51.68 | 105.70 ± 125.72 | |
| Significant lesion | 10 (25.6) | 439.98 ± 273.43 | 180.38 ± 348.11 | |
| LAD thrombosis | ||||
| Positive | 6 (15.3) | 493.88 ± 480.99 | 146.90 ± 214.80 | |
| Negative | 33 (84.6) | 406.37 ± 243.39 | 120.84 ± 204.67 | |
Table 2. IL-33 and sST2 levels in patients based on the clinical presentations of MI.
| Medication | No. (%) | sST2 (pg/ml) | IL-33 (pg/ml) | |
|---|---|---|---|---|
| ACE inhibitors | ||||
| Positive | 7 (17.9) | 374.64 ± 186.13 | 65.51 ± 17.99 | |
| Negative | 32 (82.1) | 429.72 ± 304.06 | 173.83 ± 223.08 | |
| STATIN | ||||
| Positive | 3 (7.6) | 311.76 ± 226.90 | 64.84 ± 14.79 | |
| Negative | 36 (92.4) | 428.84 ± 290.34 | 129.85 ± 21.29 | |
| ASA | ||||
| Positive | 12 (30.7) | 386.40 ± 322.24 | 99.31 ± 131.45 | |
| Negative | 27 (69.3) | 434.69 ± 272.43 | 136.19 ± 229.83 | |
| B-blocker | ||||
| Positive | 6 (15.3) | 485.31 ± 421.55 | 99.31 ± 131.45 | |
| Negative | 33 (84.6) | 407.93 ± 260.55 | 136.19 ± 229.83 | |
| Streptokinase | ||||
| Positive | 36 (92.3) | 394.15 ± 254.08 | 117.47 ± 200.83 | |
| Negative | 3 (7.6) | 728.06 ± 511.36 | 213.30 ± 262.25 | |
Table 3. IL-33 and sST2 levels in patients based on their medication.
Discussion
Our first finding in this study is that sST2 levels increase in sera of patients with ST Segment Elevated MI (STEMI) and negatively correlate with ejection fraction on admission. Previous studies have shown that sST2 is a valuable marker in prognosis of heart failure and Acute Coronary Syndrome (ACS) in patients [26-29].
A previous study has beautifully shown that elevated levels of either of IL-33 or sST2 in STEMI patients between 3-5 d is associated with increased risk of mortality in short and longterm follow up [30]. sST2 is an independent factor which participates in the regulation of extra cellular matrix remodelling and inflammation and it can lead to arrhythmia and other coronary cardiac diseases [7,9,14,28-32]. Moreover, sST2 elevation and its prognostic value in patients with ST segment elevated AMI is shown in another study [19]. However, its value to predict fatal and non-fatal heart failure, coronary heart disease and stroke in a large population of healthy adults in Finland was not significant [33].
A recent study has shown a higher but not significant increase in the baseline sST2 of patients admitted with primary Percutaneous Coronary Intervention (PCI) for acute MI [30]. In our hands too, there was no significant difference between the levels of sST2 in patients who were referred for PCI and those who were not.
Interestingly, IL-33 showed only a marginal increase in the patient’s sera which was negatively but non-significantly correlated with ejection fraction. Due to the harmful effects of low ejection fraction, our finding is in accordance with previous studies that showed elevated IL-33 was correlated with increased mortality in patients with STEMI, but not NSTEMI cardiac condition.
The finding that the IL-33 and sST2 levels are in direct correlation implies that the crosstalk between IL-33 and its receptor both on the cell membrane and as a soluble factor is a complex interaction. On one hand we expect that IL-33 binding to surface ST2 results in down-regulation of the receptor and therefore, one may expect that increased IL-33 would decrease the surface and soluble ST2 molecules [31]. On the other hand, it is suggested that IL-33 can induce the synthesis and release of sST2 in allergic inflammation and sST2 plays a role as a decoy receptor for IL-33 and can modulate the functions of this cytokine while in other conditions, nuclear IL-33 but not free IL-33 regulates the expression of sST2 [32,34]. In fact it is possible that the differential usage of promoters for expression of sST2 in different cells create diverse cellular sources of sST2, which do not correspond to membranous ST2 expression levels [8].
In general, IL-33 can induce Th2 cell to produce cytokines and inhibits inflammation and myocardial hypertrophy. Moreover, the possible variation in the receptor signaling and function based on genetic differences in related genes such as Glycogen Synthase Kinase-3 (GSK-3) may contribute to differences in the correlation coefficient we observed between patients and controls [35]. This point would make sense in the context of the role Akt/GSK-3β pathway plays in cardiomyopathies [36,37]. However, there is no direct evidence on the significance of functional differences of the molecules downstream of IL-33/ST2 signaling pathway in myocardial infarction.
Another interesting finding that calls for further investigation is the lower levels of IL-33 but not sST2 in diabetic patients with MI as compared with non-diabetic patients. While both diabetic and non-diabetic patients had 2-folds higher sST2 in their sera compared to healthy individuals, the IL-33 levels were only increased in non-diabetic patients (Table 1). Our data are in line with the study by Rui et al. who showed that the levels of IL-33 decreased in myocardium of mice with diabetes mellitus and this decrease exacerbated the injury and increased infarction size [38]. Since elevated levels of IL-33 in type I diabetes patients is also reported, investigating IL-33 levels in CAD patients with and without type I diabetes would be of great value [23].
The decreased levels of IL-33 in the sera of smoker patients is contrary to our anticipation, as the handful of studies on the relation of smoking and IL-33 levels have shown that cigarette smoke induces IL-33 expression. Having said so, we should consider that the majority of studies have investigated COPD in mice models, which may not be completely relevant in human MI [39,40].
Conclusion
Increased levels of sST2 in sera of patients with STEMI and its correlation with the IL-33 levels in patients group not only underscores the significance of this receptor in the immunopathology of Myocardial Infarction but also implies a complex interaction between IL-33 and its receptor(s) in STEMI. The higher levels of IL-33 in non-diabetic and nonsmokers highlight a difference in the Th2 responses in patients and demands further investigation.
Acknowledgement
This work was financially supported by grants (2966 and 2967) from Shiraz University of Medical Sciences, Shiraz, Iran.
References
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005; 23: 479-490.
- Küchler AM, Pollheimer J, Balogh J, Sponheim J, Manley L, Sorensen DR, De Angelis PM, Scott H, Haraldsen G. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am J Pathol 2008; 173: 1229-1242.
- Yasuoka S, Kawanokuchi J, Parajuli B, Jin S, Doi Y, Noda M, Sonobe Y, Takeuchi H, Mizuno T, Suzumura A. Production and functions of IL-33 in the central nervous system. Brain Res 2011; 1385: 8-17.
- Zeyda M, Wernly B, Demyanets S, Kaun C, Hämmerle M, Hantusch B, Schranz M, Neuhofer A, Itariu BK, Keck M, Prager G, Wojta J, Stulnig TM. Severe obesity increases adipose tissue expression of interleukin-33 and its receptor ST2, both predominantly detectable in endothelial cells of human adipose tissue. Int J Obes 2013; 37: 658-665.
- Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PLoS One 2008; 3: e3331.
- Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol 2007; 179: 2551-2555.
- Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation 2002; 106: 2961-2966.
- Iwahana H, Yanagisawa K, Ito-Kosaka A, Kuroiwa K, Tago K, Komatsu N, Katashima R, Itakura M, Tominaga S. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur J Biochem 1999; 264: 397-406.
- Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest 2007; 117: 1538-1549.
- Miller AM. Role of IL-33 in inflammation and disease. J Inflamm 2011; 8: 22.
- Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, Wu K, You Y, Alevy Y, Girard JP, Stappenbeck TS, Patterson GA, Pierce RA, Brody SL, Holtzman MJ. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest 2013; 123: 3967-3982.
- Kuroiwa K, Arai T, Okazaki H, Minota S, Tominaga S. Identification of human ST2 protein in the sera of patients with autoimmune diseases. Biochem Biophys Res Commun 2001; 284: 1104-1108.
- Leung BP, Xu D, Culshaw S, McInnes IB, Liew FY. A novel therapy of murine collagen-induced arthritis with soluble T1/ST2. J Immunol 2004; 173: 145-150.
- Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov 2008; 7: 827-840.
- Hur M, Kim H, Kim HJ, Yang HS, Magrini L, Marino R, Cardelli P, Di Somma S. Soluble ST2 has a prognostic role in patients with suspected sepsis. Ann Lab Med 2015; 35: 570-577.
- Benoit JL, Hicks CW, Engineer RS, Hart KW, Lindsell CJ, Peacock WF. ST2 in emergency department patients with non-cardiac dyspnea. Acad Emerg Med 2013; 20: 1207-1210.
- Andersson C, Preis SR, Beiser A, DeCarli C, Wollert KC, Wang TJ, Januzzi JL Jr, Vasan RS, Seshadri S. Associations of circulating growth differentiation factor-15 and ST2 concentrations with subclinical vascular brain injury and incident stroke. Stroke 2015; 46: 2568-2575.
- Dieplinger B, Egger M, Haltmayer M, Kleber ME, Scharnagl H, Silbernagel G, de Boer RA, Maerz W, Mueller T. Increased soluble ST2 predicts long-term mortality in patients with stable coronary artery disease: results from the Ludwigshafen risk and cardiovascular health study. Clin Chem 2014; 60: 530-540.
- Barbarash O, Gruzdeva O, Uchasova E, Dyleva Y, Belik E, Akbasheva O, Karetnikova V, Shilov A. Prognostic value of soluble ST2 during hospitalization for ST-segment elevation myocardial infarction. Ann Lab Med 2016; 36: 313-319.
- Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 2002; 53: 31-47.
- Díez J, González A, López B, Querejeta R. Mechanisms of disease: pathologic structural remodelling is more than adaptive hypertrophy in hypertensive heart disease. Nat Clin Pract Cardiovasc Med 2005; 2: 209-216.
- McLaren JE, Michael DR, Salter RC, Ashlin TG, Calder CJ, Miller AM, Liew FY, Ramji DP. IL-33 reduces macrophage foam cell formation. J Exp Med 2008; 205: 339-346.
- Rui T, Zhang J, Xu X, Yao Y, Kao R, Martin CM. Reduction in IL-33 expression exaggerates ischaemia/reperfusion-induced myocardial injury in mice with diabetes mellitus. Cardiovasc Res 2012; 94: 370-378.
- Pascual-Figal DA, Lax A, Perez-Martinez MT, del Carmen Asensio-Lopez M, Sanchez-Mas J. Clinical relevance of sST2 in cardiac diseases. Clin Chem Lab Med 2016; 54: 29-35.
- Xia J, Qu Y, Yin C, Xu D. Preliminary study of beta-blocker therapy on modulation of interleukin-33/ST2 signaling during ventricular remodelling after acute myocardial infarction. Cardiol J 2017; 24: 188-194.
- Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, Lee RT. Serum levels of the interleukin-1 receptor family member ST2 predicts mortality and clinical outcome in acute myocardial infarction. Circulation 2004; 109: 2186-2190.
- Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation 2003; 107: 721-726.
- Eggers KM, Armstrong PW, Califf RM, Simoons ML, Venge P, Wallentin L, James SK. ST2 and mortality in non-ST-segment elevation acute coronary syndrome. Am Heart J 2010; 159: 788-794.
- Sims JE. IL-1 and IL-18 receptors and their extended family. Curr Opin Immunol 2002; 14: 117-122.
- van der Velde AR, Lexis CP, Meijers WC, van der Horst IC, Lipsic E, Dokter MM, van Veldhuisen DJ, van der Harst P, de Boer RA. Galectin-3 and sST2 in prediction of left ventricular ejection fraction after myocardial infarction. Clinica Chimica Acta 2016; 452: 50-57.
- Zhao J, Wei J, Bowser RK, Traister RS, Fan MH, Zhao Y. Focal adhesion kinase-mediated activation of glycogen synthase kinase 3β regulates IL-33 receptor internalization and IL-33 signaling. J Immunol 2015; 194: 795-802.
- Bandara G, Beaven MA, Olivera A, Gilfillan AM, Metcalfe DD. Activated mast cells synthesize and release soluble ST2-a decoy receptor for IL-33. Eur J Immunol 2015; 45: 3034-3044.
- Hughes MF, Appelbaum S, Havulinna AS, Jagodzinski A, Zeller T, Kee F, Blankenberg S, Salomaa V. ST2 may not be a useful predictor for incident cardiovascular events, heart failure and mortality. Heart 2014; 100: 1715-1721.
- Shao D, Perros F, Caramori G, Meng C, Dormuller P, Chou PC, Church C, Papi A, Casolari P, Welsh D, Peacock A, Humbert M, Adcock IM, Wort SJ. Nuclear IL-33 regulates soluble ST2 receptor and IL-6 expression in primary human arterial endothelial cells and is decreased in idiopathic pulmonary arterial hypertension. Biochem Biophys Res Commun 2014; 451: 8-14.
- Schaffer BA, Bertram L, Miller BL, Mullin K, Weintraub S, Johnson N, Bigio EH, Mesulam M, Wiedau-Pazos M, Jackson GR, Cummings JL, Cantor RM, Levey AI, Tanzi RE, Geschwind DH. Association of GSK3B with Alzheimer disease and frontotemporal dementia. Arch Neurol 2008; 65: 1368-1374.
- Morisco C, Zebrowski D, Condorelli G, Tsichlis P, Vatner SF, Sadoshima J. The Akt-glycogen synthase kinase 3beta pathway regulates transcription of atrial natriuretic factor induced by beta-adrenergic receptor stimulation in cardiac myocytes. J Biol Chem 2000; 275: 14466-14475.
- Luckey SW, Walker LA, Smyth T, Mansoori J, Messmer-Kratzsch A, Rosenzweig A, Olson EN, Leinwand LA. The role of Akt/GSK-3beta signaling in familial hypertrophic cardiomyopathy. J Mol Cell Cardiol 2009; 46: 739-747.
- Shruthi S, Mohan V, Amutha A, Aravindhan V. Increased serum levels of novel T cell cytokines IL-33, IL-9 and IL-17 in subjects with type-1 diabetes. Cytokine 2016; 86: 6-9.
- Wu H, Yang S, Wu X, Zhao J, Zhao J, Ning Q, Xu Y, Xie J. Interleukin-33/ST2 signaling promotes production of interleukin-6 and interleukin-8 in systemic inflammation in cigarette smoke-induced chronic obstructive pulmonary disease mice. Biochem Biophys Res Commun 2014; 450: 110-116.
- Qiu C, Li Y, Li M, Li M, Liu X, McSharry C, Xu D. Anti-interleukin-33 inhibits cigarette smoke-induced lung inflammation in mice. Immunol 2013; 138: 76-82.