ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 15
Correlation between the expression of BRCA-1 and BAG-1 proteins in triple negative breast cancer and its sensitivity to platinum-based chemotherapy
Wei Wang1, Qu Chen1, Ruo-Bing Wang2, Zheng-Yan Wang1, Li-Xin Jiao1, Xiang-Dong Li1, Wei Li1 and Li Zhang3*
1Department of Oncology, Jinzhou Central Hospital, Jinzhou, PR China
2Department of Oncology, the 307th Hospital of Chinese People’s Liberation Army, Beijing, PR China
3Experiment Center, Jinzhou Central Hospital, Jinzhou, PR China
Accepted on June 16, 2017
The aim of this study was to detect the expression of BRCA-1 and BAG-1 in Triple Negative Breast Cancer (TNBC), and analyse the correlation between protein expression and sensitivity to Platinumbased Chemotherapy (PbC). Malignant tissues from 72 TNBC patients who were treated according to the NP and GP programs; then, the relationships between BRCA-1 and BAG-1 protein expression and the survival period (median Overall Survival (OS) and Progression-Free Survival (PFS)) were examined. In TNBC, BRCA-1 expression was related to lymph node metastasis and clinical stage (P<0.01); whereas BAG-1 expression was only related to lymph node metastasis. In TNBC tissues, the expression of BRCA-1 and BAG-1 proteins was significantly positively correlated (r=0.845, P=0.001), and the median OS and PFS of the BRCA-1 and BAG-1-negative group were significantly longer than those of the BRCA-1 and BAG-1-positive group after PbC (P<0.05). In TNBC, the expression of BRCA-1 and BAG-1 was significantly positively correlated, suggesting that these two have synergistic roles in the development of TNBC. The survival rate of TNBC patients with non-BRCA-1 and BAG-1-expressing tumours was higher than that of patients with BRCA-1 and BAG-1-expressing tumours; thus, negativity for BRCA-1 and BAG-1 expression could be used to predict chemo sensitivity of TNBC to PbC.
Keywords
BRCA-1, BAG-1, Triple-negative breast cancer, Platinum-based program, Chemo sensitivity.
Introduction
Breast cancer is a highly heterogeneous disease, and its clinical course varies tremendously [1]. While some of this variability can be explained by traditional clinicopathological factors, molecular profiling studies have defined breast cancer subtypes with distinct clinical outcomes, including Luminal A type, Luminal B type (including HER-2-negative and HER-2 positive), HER-2-overexpressing type, and triple-negative type, according to immunohistochemistry [2,3].
The prognosis of Triple-Negative Breast Cancer (TNBC) has been shown to be related to different genes; in addition, survival after metastatic relapse with TNBC is shorter than with other breast cancer subtypes, the treatment options are few, and the response rates are poor and lack durability [4,5]. Currently, Platinum-based Chemotherapy (PbC) is the most popular treatment for TNBC, and the pathological complete remission rate (pCR) of carboplatin-containing chemotherapy for TNBC is 15% [6,7].
BRCA-1 is a tumour suppressor gene, and methylation of BRCA-1 initiation factor can reduce its expression. BRCA-1 expression is associated with high histological grades and a triple-negative phenotype [8-10]. Many studies have shown that PbC exhibits significant activities in TNBC patients with BRCA-1 gene defects and BRCA-1 mutation carriers benefit more from neoadjuvant PbC than non-carriers [11-13]. Misregulation and reduced expression of BRCA-1 also contribute to sporadic forms of breast cancer. The primary tumour-suppressing role of BRCA-1 is related to the maintenance of genomic integrity through regulation of DNA replication, repair, and transcription as well as the maintenance of various cell cycle checkpoints that ensure the survival of healthy cells. The important role of BRCA-1 in TNBC has also been demonstrated.
BAG-1 is an anti-apoptotic gene, and some researchers have evaluated BAG-1 expression in BC by immunohistochemistry (IHC), and showed that about two-thirds or more patients showed high BAG-1 expression, which could inhibit the apoptosis of BC cells, enhance the resistance of tumour cells to radiation, hypoxia, and chemotherapeutic drugs, stop external stimuli-induced cell growth inhibition, and prolong the life of cells. Thus, BAG-1 expression might be associated with resistance to PbC. In addition, BAG-1 has been used as a biomarker for predicting long-term survival in early-stage BC, as increased levels of BAG-1 are associated with better outcomes. Furthermore, the expression of BRCA-1 and BAG-1 in TNBC patients may be a more reliable biomarker than either one alone.
In this study, the expression of BRCA-1 and BAG-1 in TNBC was evaluated and combined with a retrospective analysis of clinical follow-up to examine the relationship of BRCA-1 and BAG-1 expression with chemosensitivity to PbC. The correlations among these variables were studied with an aim to provide experimental and clinical evidence for individualized TNBC treatment.
Materials and Methods
General clinical information
For this study, patients with BC, diagnosed in the department of general surgery of the First Affiliated Hospital of Liaoning Medical College from Jan 2004 to Dec 2010, were selected, who had immunohistochemically confirmed ER (-), PR (-) and HER-2 (-) BC by two senior pathologists. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Jinzhou City Central Hospital. Written informed consent was obtained from all participants. All patients were female and aged 32-73 y (median, 47 y). Among the 72 patients there 56 cases of invasive ductal carcinoma, 11 cases of invasive lobular carcinoma and 5 cases of other. There were 43 patients with lymph node metastasis, and 29 patients without lymph node metastasis. The patients were staged according to the AJCC staging system (2003), and there were 10 cases of stage I, 36 cases of stage II and 26 cases of stage III/IV. The patients did not receive preoperative chemotherapy, biological therapy, or immunotherapy, and postoperative PbC was performed in the department of oncology before enrolment. The patients were also evaluated by colour ultrasound of the breast and axillary lymph nodes, chest and abdominal CT, head CT or MRI, and other tests, and their clinical data were complete. The PbC regimen was carried out for at least two cycles, and only when the patient provided written informed consent. In addition, 30 normal breast tissues were randomly sampled (≥ 5 cm away from the edge of the cancerous tissue) from the above-mentioned 72 TNBC patients, another 25 patients with benign breast diseases were included as controls.
BRCA-1 and BAG-1 detection
Immunohistochemistry was used to evaluate BRCA-1 and BAG-1 expression, and the immunoreactive scores were calculated according to the degree of staining and the percentage of stained cells. A rabbit anti-human BRCA-1 polyclonal antibody (Proteintech Group, USA) and a rabbit anti-human BAG-1 polyclonal antibody (Beijing Biosynthesis Biotechnology Co. Ltd., China) were used in these experiments.
Clinical treatment
All patients were administered at least 2 cycles of PbC. The two regimens used were the NP program: vinorelbine, 40 mg/m2 on days 1 and 8 and cisplatin, 25 mg/m2 on days 1-3 and the GP program: gemcitabine, 1000 mg/m2 on days 1 and 8 and cisplatin, 25 mg/m2 on days 1-3. Efficacy was evaluated as described in similar studies conducted abroad, by determining the Overall Survival (OS), Median OS, Progression-Free Survival (PFS) and median PFS.
Follow-up protocol
All patients were followed up in the clinic or by telephone, and the follow-up start time was the end of PbC, and the end of follow-up was Dec 2010 or the date of recurrence, metastasis, or death by any cause. Follow-up analyses included color ultrasound of breast and lymph nodes, chest and abdominal CT, head CT, MRI, etc., and all included patients had complete clinical and follow-up data. Patients unwilling to continue and those who were lost were recorded as lost.
Statistical analysis
All data and statistical analyses were performed using SPSS17.0 statistical analysis software. Expression among the experimental groups was evaluated by the chi-square test and Fisher’s exact test. The correlation of BRCA-1 with BAG-1 was analysed with Spearman’s correlation analysis, and intergroup differences were compared with the log-rank test. Survival data were analysed with the Kaplan-Meier method and the statistical significant of differences was evaluated with a two-sided test. P values less than 0.05 were considered statistically significant.
Results
Pathomorphology
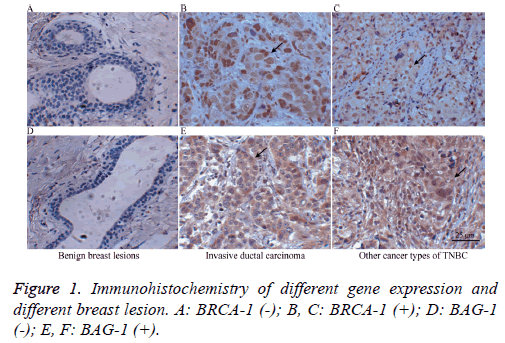
Among the 72 TNBC patients, there were 56 case of invasive ductal carcinoma, 11 cases of invasive lobular carcinoma, and 5 case of other; 43 patients had lymph node metastasis, while 29 patients did not have lymph node metastasis. The cases were staged according to the AJCC staging system (2003) and there were 10 cases of stage I, 36 cases of stage II, and 26 cases of stage III/IV. As controls, 30 tumour-adjacent normal breast tissues were randomly selected from the BC patients as well as another 25 benign breast lesions. The panels in Figure 1 show BRCA-1 (-) and BAG-1 (-) benign breast lesion (A and D), BRCA-1 (+) and BAG-1 (+) invasive ductal carcinoma (B and E) and other types of BRCA-1 (+) and BAG-1 (+) TNBC (C and F).
Expression of BRCA-1 and BAG-1 in different breast tissues
As shown in Table 1, the expression levels of BRCA-1 and BAG-1 in TNBC were 76.4% and 69.4% higher, respectively, than those in benign breast lesions and adjacent normal breast tissues. The intergroup comparison showed that these differences were statistically significant (P<0.01).
| Group | Cases | BRCA-1 | BAG-1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| + | % | χ2 | P | + | % | χ2 | P | |||
| Benign breast lesions | 25 | 1 | 4 | 2 | 8 | |||||
| Adjacent normal breast tissues | 30 | 7 | 23.3 | 49.744 | 0 | 6 | 20 | 38.666 | 0 | |
| TNBC | 72 | 55 | 76.4 | 50 | 69.4 | |||||
Table 1. Expressions of BRCA-1 and BAG-1 in different breast tissues.
Relationships between clinicopathological features and the protein expression of BRCA-1 and BAG-1
As shown in Table 2, BRCA-1 expression in TNBC was related to lymph node metastasis and clinical stage, as the rate of BRCA-1 expression in the lymph node metastasis group (93.0%) was significantly higher than that the non-lymph node metastasis group (51.7%). In addition, more advanced clinical stages were significantly associated with higher expression (P<0.01). However, BRCA-1 expression was also significant correlated with other clinicopathological features (P<0.05).
| Group | Cases | BRCA-1 | BAG-1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| + | % | χ2 | P | + | % | χ2 | P | |||
| Age (y) | 0.002 | 0.963 | 2.7 | 0.1 | ||||||
| <50 | 42 | 32 | 76.2 | 26 | 61.9 | |||||
| ≥ 50 | 30 | 23 | 76.7 | 24 | 80 | |||||
| Menstruation | 1.849 | 0.174 | 1.799 | 0.18 | ||||||
| Before menostasis | 44 | 36 | 81.8 | 28 | 63.6 | |||||
| After menostasis | 28 | 19 | 67.9 | 22 | 78.6 | |||||
| Lymph node metastasis | 16.378 | 0.001 | 4.661 | 0.031 | ||||||
| Negative | 29 | 15 | 51.7 | 16 | 55.2 | |||||
| Positive | 43 | 40 | 93 | 34 | 79.1 | |||||
| Pathological type | 0.974 | 0.614 | 0.413 | 0.813 | ||||||
| Invasive ductal carcinoma | 56 | 44 | 78.6 | 38 | 67.9 | |||||
| Invasive lobular carcinoma | 11 | 8 | 72.2 | 9 | 81.8 | |||||
| Others | 5 | 3 | 60 | 3 | 60 | |||||
| Clinical stage | 9.481 | 0.009 | 5.077 | 0.079 | ||||||
| Stage I | 10 | 4 | 40 | 7 | 70 | |||||
| Stage II | 36 | 28 | 77.8 | 29 | 80.6 | |||||
| Stage III/IV | 26 | 23 | 88.5 | 14 | 53.8 | |||||
Table 2. Relationship between the expressions of BRCA-1 and BAG-1 with the clinicopathological features.
BAG-1 expression in TNBC was related to lymph node metastasis, as the expression rate in the lymph node metastasis group (79.1%) was significantly higher than that in the group without lymph node metastasis (55.2%), and the difference was statistically significant (P<0.05). However, BAG-1 expression was also significant correlated with other clinicopathological features (P<0.05).
Correlation of BRCA-1 with BAG-1
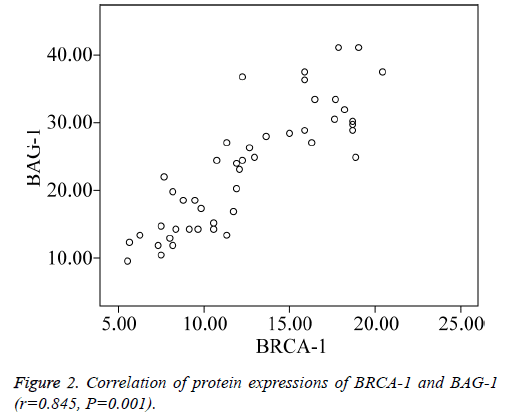
Spearman’s rank correlation test showed that BRCA-1 and BAG-1 were significantly positively correlated in TNBC (Figure 2) (r=0.845, P=0.001).
Expression of BRCA-1 and BAG-1 and the efficacies of treatment in TNBC
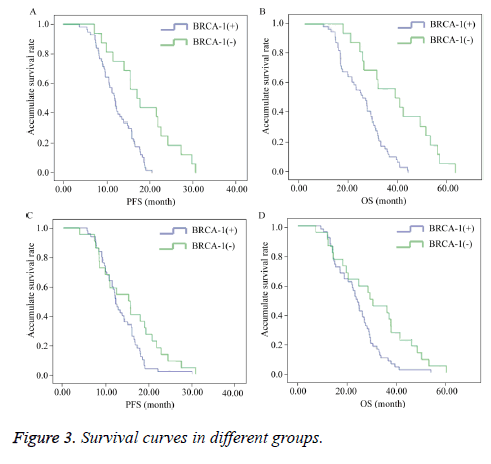
Of the 72 TMBC patients, 5 were lost to follow-up; therefore, 67 patients completed follow-up. The survival analysis of these 67 patient revealed that the median PFS and median OS of the BRCA-1-negative group (median PFS=17.08 months; 95% CI=12.592-21.568 and median OS=36.10 months; 95% CI=19.303-52.897) were longer than those of the BRCA-1- positive group (median PFS=11.90 months; 95% CI=10.538-13.262 and median OS=23.10 months; 95% CI=17.844-28.356) and the intergroup comparison of median PFS showed a statistically significant difference (P=0.000) (Figures 3A and 3B).
The median PFS and median OS of the BAG-1-negative group (median PFS=12.10 months; 95% CI=12.592-21.568 and median OS=29.23 months; 95% CI=16.245-42.215) were longer than those of the BAG-1-positive group (median PFS=12.00 months; 95% CI=10.637-13.363 and median OS=23.56 months; 95% CI=20.788-26.332) and the intergroup comparison of median PFS and median OS showed a statistically significant difference (P=0.046) (Figures 3C and 3D).
Discussion
BC’s are a heterogeneous group of tumours with large individual differences. Over the past decade, remarkable progresses in BC treatment have been made with endocrine therapy targeting hormone receptor-positive tumours and HER-2-positive targeted therapy. In contrast, because of a lack of viable molecular targets, progress in the treatment of TNBC has been comparatively slow. This subtype of BC is aggressive, and the prognosis is poor; although it is sensitive to chemotherapeutic drugs, recurrence is early and rapid, and the survival period is short. “Triple negative” is highly regarded as a risk factor when selecting treatment. Recently, in-depth studies of the pathophysiology and molecular characteristics of TNBC have provided the basis for new treatments. Current and future clinical trials are expected to optimize and improve the treatment options for TNBC.
Currently, combined chemotherapy with PbC is used to rescue patients with tumours that are resistant to anthracycline and (or) taxane chemotherapy; However, the effects are not consistent. PbC has recently received much attention for the treatment of TNBC because it can act directly on DNA. PbC can cross-link double-stranded DNA (dsDNA), resulting in dsDNA breaks, which impede the replication and transcription of DNA, and ultimately lead to tumour cell death [14,15]. A series of studies had demonstrated that pCR of TNBC patients after treatment with PbC was about 23-90%, which was higher than that obtained after anthracycline-taxane sequential chemotherapy (19-34%) [16]. As for the susceptibility to PbC, studies continued to find meaningful predictors in different tumours. BRCA-1 and BAG-1 expression was associated with TNBC, and related to the mechanism of PbC. We aimed to investigate the sensitivities of tumours to PbC according to BRCA-1 and BAG-1 expression, which might provide the foundation for future prospective studies.
BRCA-1 is a breast cancer susceptibility gene located on human chromosome 17q12-21. The BRCA-1 genome is approximately 100 kb is length, containing 24 exons and encoding a single protein of 1863 amino acid residues with a molecular weight of 220 kDa [17]. The gene product plays a variety of roles; it is involved in repairing dsDNA damage and maintaining genomic stability, including telomeric allelic imbalance, heterozygosity loss, and massive displacement etc., which are biomarkers of homologous recombination defects [18]. This also provides a theoretical basis for BRCA-1 as a predictor of PbC sensitivity in TNBC [19-21]. When the DNA in normal cells is damaged, it activates a DNA repair system, including BRCA-1, thus regulating the DNA repair process. When BRCA-1 is mutated, it cannot help repair dsDNA breaks, which is one of the pathogenic pathways underlying TNBC [22]. Lee et al. studied 1,167 BC patients (20-49 y old), and 48% of patients with a BRCA-1 gene mutation had TNBC, whereas only 12% of patients without a BRCA-1 mutation had TNBC [23]. This study showed that the BRCA-1 expression rate in TNBC tissues was 76.4%, which was significantly higher than the rates in normal breast tissues and benign breast tissues (P<0.01). Thus, we speculate that BRCA-1 is involved in the formation of TNBC. This study showed that in TNBC, BRCA-1 expression was associated with lymph node metastasis, as the expression rate in the lymph node metastasis group (93.0%) was higher than that in the non-lymph node metastasis group (51.7%), and this difference was statistically significant (P<0.01), which was not the same as the rate reported above, perhaps because our study included a relatively small number of cases and all the cases were TNBC.
The in vitro experiments by Husain et al. showed that up regulated expression of BRCA-1 could lead to cisplatin resistance, and in the cisplatin-resistant MCF7 BC cells, BRCA-1 was highly expressed [24]. Another study showed that low BRCA-1 levels could improve cisplatin sensitivity and paclitaxel resistance, whereas normal and high BRCA-1 level had the opposite effects. The results of the present study showed that the median PFS and median OS in the BRCA-1- negative group were longer than those of the BRCA-1-positive group after administration of PbC, and the difference was statistically significant (P<0.01), indicating that tumours with down regulated BRCA-1 expression would be much more sensitive to PbC, similar to the above findings. The difference in the results of the studies might be due to the fact that inactivation of BRCA-1 function is related to impairment of dsDNA breakage repair. Thus, such tumours are sensitive to DNA cross-linking agents and platinum-based drugs that can induce dsDNA breaks.
The BAG-1 gene is located on chromosome 9p12 and its full name is Bcl-2-associated anti-apoptotic gene. It was first reported in 1995 when Takayama screened Bcl-2 proteinassociated proteins from the Jurkat human lymphocyte cell line [25]. A number of studies have shown that BAG-1 was highly expressed in breast, lung, colorectal, prostate, and other malignant cancers, but was not expressed or expressed at a low level in normal tissues, and the BAG-1 gene might be a predictor of sensitivity to platinum drugs in advanced nonsmall- cell lung cancer [26]. Several studies have used immunohistochemical methods to detect the expression of BAG-1 protein, and the results showed that BAG-1 was largely expressed in BC. In this study, we showed that BAG-1 protein was expressed in 69.4% of TNBC tissues, and this rate was significantly higher than the rates in benign breast lesions and adjacent normal breast tissues (P<0.01), suggesting that BAG-1 plays a role in the occurrence and development of TNBC and could be used as early diagnostic indicator [27,28]. Different results have been reported on the correlations between the expression of BAG-1 with the malignancy of TNBC [25,29]. This study showed that BAG-1 expression was correlated with lymph node metastasis (P<0.05), as the expression rate in the lymph node metastasis group (79.1%) was significantly higher than the rate in the non-lymph node metastasis group (55.2%).
The reason for this might be that BAG-1 is involved in the metastasis of TNBC, a phenomenon that requires further study. The main function of BAG-1 is anti-apoptosis, and its overexpression can inhibit the apoptosis of normal cells. Thus, in BAG-1 overexpressing tissues, the number of cells might abnormally increase and accumulate, increasing the rate of genetic mutations, and leading to the malignization of cells. Meanwhile, it might also increase the resistance of tumour cells to chemotherapeutic drugs, which could be a mechanism associated with resistance to platinum drugs. One study showed that synthesis of the BAG-1 protein was initiated by both cap-dependent and non-cap-dependent structures, and the synthesis of BAG-1 is dependent on IRES binding to the 5ʹ- untranslated region of BAG-1 mRNA [30]. When exposed to cisplatin, IRES could not be synthesized; therefore, the expression of BAG-1 was down regulated. There have been rare reports on the relationship between BAG-1 and platinum drug sensitivity in TNBC. The results of this study showed that in TNBC, the median PFS and median OS in the BAG-1- negative group were significantly longer than those in the BAG-1-positive group (P<0.05). Theoretically, as BAG-1 is an anti-apoptotic protein, if BAG-1 is highly expressed in cells, it would increase the probability of mutation, thus promoting the recurrence and metastasis of tumours, and affecting OS. The results of this study support this hypothesis.
In the present study, the correlation analysis of BRCA-1 and BAG-1 showed that their expression was positively correlated (r=0.845, P=0.001). These data seemed to contradict the idea that Bcl-2 itself is the bridge between the above two factors, as paradoxical relationships exist during the anti-apoptotic process. BRCA-1 is involved in transcriptional regulation, and can regulate cell cycle checkpoints and induce apoptosis; thus, its functions are closely related to those of other apoptosisregulating proteins, such as C-myc, p53, and Bcl-2. Laulier et al. reported that the occurrence of ionizing radiation-induced BRCA-1 mutations was inhibited when Bcl-2 was ectopically expressed [31].
Another study showed that in BRCA-1-mutated BC cells, the expression of the apoptosis inhibition gene Bcl-2 was decreased [32]. BAG-1 is an anti-apoptotic gene, and it encodes a multifunctional binding protein. Although it does not belong to the Bcl-2 family, BAG-1 can bind to Bcl-2 and form a complex; thus, promoting its anti-apoptotic abilities [33].
Further research is needed to further clarify the correlations between BRCA-1 and BAG-1, as well we the mechanisms underlying their synergic roles in the occurrence and development of TNBC, which may guide future treatments.
In this study, immunohistochemical methods were used to analyse the expression of BRCA-1 and BAG-1 in TNBC and the results showed that the expression of BRCA-1 and BAG-1 was related to the PbC sensitivity of TNBC. Down regulation or the absence of BRCA-1 and BAG-1 expression might indicate greater effects when these drugs are applied. With the continual emergence of new research findings, additional, more specific biological markers could be found to guide individual treatment. The application of new molecular techniques might provide invaluable assistance for identifying the factors that affect cell survival as well as possible targets for treatment, so that ultimately, the quality of life of patients with TNBC might be improved and their survival prolonged.
Conclusions
The study used immunohistochemical methods to detect the protein expression of BRCA-1 and BAG-1 and these dates were combined with clinical follow-up to comprehensively analyse the relationships of BRCA-1 and BAG-1 expression with survival after PbC. We arrived at the following conclusions:
(1) In TNBC, the rates of BRCA-1 and BAG-1 protein expression were higher than those in adjacent normal tissues and benign breast lesions, and were related to lymph node metastasis, suggesting that BRCA-1 and BAG-1 play roles in the development and early metastasis of TNBC. Thus, they could be used as potential prognostic factors. (2) In TNBC, the protein expression of BRCA-1 and BAG-1 was significantly positively correlated, suggesting that they had synergistic effects in the occurrence and development of TNBC. (3) The clinical follow-up revealed that among patients with TNBC, the survival period of patient with tumours that were negative for BRCA-1 and BAG-1 protein expression was longer than that of patients with tumours that were positive for BRCA-1 and BAG-1 protein expression, suggesting that BRCA-1 and BAG-1 could be potential predictors of PbC sensitivity, and could be used to guide the individualized treatment of TNBC patients.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Baird RD, Caldas C. Genetic heterogeneity in breast cancer: the road to personalized medicine? BMC Med 2013; 11: 151.
- Kulka J, Szekely B, Lukacs LV, Kiss O, Tokes AM, Vincze E, Turanyi E, Fillinger J, Hanzely Z, Arato G, Szendroi M, Gyorffy B, Szasz AM. Comparison of predictive immunohistochemical marker expression of primary breast cancer and paired distant metastasis using surgical material: a practice-based study. J Histochem Cytochem 2016; 64: 256-267.
- Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ. Personalizing the treatment of women with early breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol 2013; 24: 2206-2223.
- Sparano JA, Goldstein LJ, Childs BH, Shak S, Brassard D, Badve S, Baehner FL, Bugarini R, Rowley S, Perez EA, Shulman LN, Martino S, Davidson NE, Kenny PA, Sledge GW Jr, Gray R. Relationship between quantitative GRB7 RNA expression and recurrence after adjuvant anthracycline chemotherapy in triple-negative breast cancer. Clin Cancer Res 2011; 17: 7194-7203.
- Kumar P, Aggarwal R. An overview of triple-negative breast cancer. Arch Gynecol Obstet 2016; 293: 247-269.
- von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, Blohmer JU, Jackisch C, Paepke S, Gerber B, Zahm DM, Kummel S, Eidtmann H, Klare P, Huober J, Costa S, Tesch H, Hanusch C, Hilfrich J, Khandan F, Fasching PA, Sinn BV, Engels K, Mehta K, Nekljudova V, Untch M. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 2014; 15: 747-756.
- Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, Kuzma CS, Pluard TJ, Somlo G, Port ER, Golshan M, Bellon JR, Collyar D, Hahn OM, Carey LA, Hudis CA, Winer EP. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 2015; 33: 13-21.
- Esteller M, Silva JM, Dominguez G, Bonilla F, Matias-Guiu X, Lerma E, Bussaglia E, Prat J, Harkes IC, Repasky EA, Gabrielson E, Schutte M, Baylin SB, Herman JG. Promoter hyper methylation and BRCA-1 inactivation in sporadic breast and ovarian tumours. J Natl Cancer Inst 2000; 92: 564-569.
- Catteau A, Harris WH, Xu CF, Solomon E. Methylation of the BRCA-1 promoter region in sporadic breast and ovarian cancer: correlation with disease characteristics. Oncogene 1999; 18: 1957-1965.
- Rice JC, Ozcelik H, Maxeiner P, Andrulis I, Futscher BW. Methylation of the BRCA-1 promoter is associated with decreased BRCA-1 mRNA levels in clinical breast cancer specimens. Carcinogenesis 2000; 21: 1761-1765.
- Byrski T, Huzarski T, Dent R, Marczyk E, Jasiowka M, Gronwald J, Jakubowicz J, Cybulski C, Wisniowski R, Godlewski D, Lubinski J, Narod SA. Pathologic complete response to neoadjuvant cisplatin in BRCA-1 positive breast cancer patients. Breast Cancer Res Treat 2014; 147: 401-405.
- von Minckwitz G, Hahnen E, Fasching PA, Hauke J, Schneeweiss A, Salat C, Rezai M, Blohmer JU, Zahm DM, Jackisch C, Gerber B, Klare P, Kummel S, Eidtmann H, Paepke S, Nekljudova V, Loibl S, Untch M, Schmutzler RK. Pathological complete response (pCR) rates after carboplatin-containing neoadjuvant chemotherapy in patients with germline BRCA (gBRCA) mutation and triple-negative breast cancer (TNBC): Results from GeparSixto. J Clin Oncol 2014; 32: 5.
- Telli ML, Jensen KC, Kurian AW, Vinayak S, Lipson JA, Schackmann EA, Wapnir I, Carlson RW, Sparano JA, Head B, Goldstein LJ, Haley BB, Dakhil SR, Manola J, Ford J. Final efficacy results from a phase II study of gemcitabine (G) and carboplatin (C) plus iniparib (BSI-201) as neoadjuvant therapy for triple-negative (TN) and BRCA1/2 mutation-associated breast cancer. J Clin Oncol 2013; 31: 1003.
- Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repairs pathways. Clin Cancer Res 2008; 14: 1291-1295.
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 2007; 7: 573-584.
- Uhm JE, Park YH, Yi SY, Cho EY, Choi YL, Lee SJ, Park MJ, Lee SH, Jun HJ, Ahn JS, Kang WK, Park K, Im YH. Treatment outcomes and clinicopathologic characteristics of triple-negative breast cancer patients who received platinum-containing chemotherapy. Int J Cancer 2009; 124: 1457-1462.
- Wiwanitkit V. Interaction between BRCA-1 and human papilloma virus E7: an ontology study. Arch Gynecol Obstet 2006; 274: 146-149.
- Birkbak NJ, Wang ZC, Kim JY, Eklund AC, Li Q, Tian R, Bowman-Colin C, Li Y, Greene-Colozzi A, Iglehart JD, Tung N, Ryan PD, Garber JE, Silver DP, Szallasi Z, Richardson AL. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov 2012; 2: 366-375.
- Popova T, Manie E, Rieunier G, Caux-MV, Tirapo C, Dubois T, Delattre O, Sigal-ZB, Bollet M, Longy M, Houdayer C, Sastre-GX, Vincent-SA, Stoppa-LD, Stern MH. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res 2012; 72: 5454-5462.
- Vollebergh MA, Lips EH, Nederlof PM, Wessels LF, Schmidt MK, van Beers EH, Cornelissen S, Holtkamp M, Froklage FE, de Vries EG, Schrama JG, Wesseling J, van de Vijver MJ, van Tinteren H, de Bruin M, Hauptmann M, Rodenhuis S, Linn SC. An aCGH classifier derived from BRCA-1 mutated breast cancer and benefit of high-dose platinum-based chemotherapy in HER2-negative breast cancer patients. Ann Oncol 2011; 22: 1561-1570.
- Telli ML, Jensen KC, Abkevich V, Hartman AR, Vinayak S, Lanchbury J, Gutin A, Timms K, Ford JM. Homologous recombination deficiency (HRD) score predicts pathologic response following neoadjuvant platinum based therapy in triple-negative and BRCA1/2 mutation associated breast cancer. Cancer Res 2012; 72: 9-4.
- Narod SA. BRCA mutations in the management of breast cancer: the state of the art. Net Rev Clin Oncol 2010; 7: 702-707.
- Lee E, McKean-Cowdin R, Ma H, Spicer DV, Van Den Berg D, Bernstein L, Ursin G. Characteristics of triple-negative breast cancer in patients with a BRCA-1 mutation: results from a population-based study of young women. J Clin Oncol 2011; 29: 4373-4380.
- Husain A, He G, Venkatraman ES, Spriggs DR. BRCA1 up-regulation is associated with repair-mediated resistance to cis-diamminedichloroplatinum (II). Cancer Res 1998; 58: 1120-1123.
- Takayama S, Sato T, Krajewski S, Kochel K, Irie S, Millan JA, Reed JC. Cloning and functional analysis of BAG-1: anovel Bcl-2-binding protein with anti-cell death activity. Cell 1995; 80: 279-284.
- Wang YD, Ha MW, Cheng J, Zhang WL, Cong X, Tong CY, Sun J. The role of expression and polymorphism of the BAG-1 gene in response to platinum-based chemotherapeutics in NSCLC. Oncol Rep 2012; 27: 979-986.
- Townsend PA, Dublin E, Hart IR, Kao RH, Hanby AM, Cutress RI, Poulsom R, Ryder K, Barnes DM, Packham G. BAG-i expression in human breast cancer: interrelationship between BAG-1 RNA, protein, HSC70 expression and clinicopathological data. J Pathol 2002; 197: 51-59.
- Tang SC, Beck J, Murphy S, Chernenko G, Robb D, Watson P, Khalifa M. BAG-1 expression correlates with Bcl-2, p53, differentiation, estrogen and progester one receptors in invasive breast carcinoma. Breast Cancer Res Treat 2004; 84: 203-213.
- Tang SC, Shaheta N, Chemenko G, Khalifa M, Wang X. Expression of BAG-1 in invasive breast carcinomas. J Clin Oncol 1999; 17: 1710-1719.
- Dobbyn HC, Hill K, Hamilton TL, Spriggs KA, Pickering BM, Coldwell MJ, de Moor CH, Bushell M, Willis AE. Regulation of BAG-1 IRES-mediated translation following chemotoxic stress. Oncogene 2008; 27: 1167-1174.
- Laulier C, Barascu A, Guirouilh-Barbat J, Pennarun G, Le Chalony C, Chevalier F, Palierne G, Bertrand P, Verbavatz JM, Lopez BS. Bcl-2 inhibits nuclear homologous recombination by localizing BRCA-1 to the endomembrane. Cancer Res 2011; 71: 3590-3602.
- Freneaux P, Stoppa-Lyonnet D, Mouret E, Kambouchner M, Nicolas A, Zafrani B, Vincent-SA, Fourquet A, Magdelenat H, Sastre-GX. Low expression of Bcl-2 in BRCA-1 associated breast cancers. Br J Cancer 2000; 83: 1318-1322.
- Boise LH, Gonzalez-GM, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB. Bcl-x, a Bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993; 74: 597-608.