ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 9
CTHRC1 promotes the proliferation and invasion of prostate cancer cells by regulating the activity of Wnt/PCP signalling pathway
This study aims to observe whether Collagen Triple Helix Repeat Containing-1 (CTHRC1) can promote the proliferation, migration and invasion of prostate cancer cells by regulating the activity of the Wnt/PCP signalling pathway. CTHRC1 expression in prostate cancer tissues and cells was detected by RT-PCR. PcDNA3.1-CTHRC1 and CTHRC1 siRNA was transfected into prostate cancer PC3 cells using electroporation method. The effect of CTHRC1 on the proliferation of prostate cancer PC3 cells was detected by clone formation assay; the effect of CTHRC1 on the migration of prostate cancer PC3 cells were detected by wound healing assay and Transwell test; and the effect of CTHRC1 on the activity of signalling pathway was detected by dual luciferase reporter assay. RT-PCR results revealed that CTHRC1 expression was significantly higher in prostate cancer tissue compared with normal tissues. Western blot revealed that transfection with CTHRC1 could significantly promote CTHRC1 expression in prostate cancer cells. Clone formation assay revealed that transfection with CTHRC1 could significantly promote the proliferation of prostate cancer cells. Wound healing assay and Transwell test revealed that transfection with CTHRC1 could significantly promote the migration of prostate cancer cells. Dual luciferase reporter assay revealed that CTHRC1 protein could significantly activate Wnt/PCP signalling pathway in prostate cancer PC3 cells. CTHRC1 could promote the proliferation, migration and invasion of prostate cancer cells by regulating the activity of the Wnt/PCP signalling pathway. This may be the mechanism by which CTHRC1 is involved in the occurrence and development of prostate cancer.
Keywords
Collagen triple helix repeat containing-1, Prostate cancer, Proliferation, Migration, Invasion.
Introduction
Prostate cancer is one of the most common malignant tumors in men; millions of people die of this disease every year [1]. Methods of treatment for prostate cancer include radical prostatectomy, radiation therapy, or Androgen Deprivation Therapy (ADT) combined with radiation therapy [2-4]. The growth and proliferation of prostate cancer are mainly dependent on the level of androgen. ADT treatment can reduce androgen levels in patients with prostate cancer; therefore it has become an effective method for the treatment of prostate cancer. However, almost all prostate cancer patients will eventually generate resistance to androgen deprivation, and the disease will progress into Castration-Resistant Prostate Cancer (CRPC) [5]. Most of the patients will encounter a recurrence of prostate cancer, and the disease will develop into CRPC, which eventually causes the patients’ death from cancer metastasis [6]. Based on the complex pathogenesis of prostate cancer, it is essential to explore and discover new molecules that regulate the development of prostate cancer, which may provide novel targeted molecular agents for the treatment of prostate cancer.
Collagen Triple Helix Repeat Containing-1 (CTHRC1) is a secreted glycoprotein of 25 kDa, which was first found in the search for artery balloon injury-related genes in rats [7]. CTHRC1 can promote tissue repair and cell migration by limiting deposition of collagen matrix [7]. Studies revealed that CTHRC1 is abnormally expressed in many tumors, including melanoma, gastrointestinal cancer, breast cancer, thyroid cancer, liver cancer and pancreatic cancer [8-13]. In addition, high expression of CTHRC1 has diagnostic value for many kinds of tumors, including liver cancer, gastric cancer, nonsmall- cell lung cancer and gastrointestinal stromal tumor [14]. Over-expression of CTHRC1 can increase the invasion ability of colorectal cancer cells [15].
At present, there are few literatures report the effects of CTHRC1 on the proliferation, migration and invasion of prostate cancer cells and the related mechanisms. In this study, we first detected the expression of CTHRC1 in prostate cancer tissues and cells. PcDNA3.1-CTHRC1 and CTHRC1 siRNA were transfected into prostate cancer PC3 cells by electroporation. The effects of CTHRC1 on the proliferation and invasion of PC3 cells were observed; and the effect of CTHRC1 on the downstream signalling pathway was detected by dual luciferase reporter assay. Thereby the role of CTHRC1 in the pathogenesis of prostate cancer was explored. The details are as follows.
Materials and Methods
Clinical data
A total of 20 prostate cancer patients who hospitalization and received surgery in our hospital from November 1, 2014 to December 30, 2015 were included into this study. Patients had not been treated with chemotherapy, radiation therapy or immunotherapy before surgery. Cancer tissue specimens were collected from these patients, and 20 paracancerous tissue specimens were collected as controls. These specimens were stored in liquid nitrogen within 10 minutes after being obtained. Each specimen was confirmed as prostate cancer by histopathological diagnosis. The average age of these 20 patients was 61.13 ± 18.91 years old. The study was approved by the Medical Ethics Committee of the hospital, and all participants signed informed consent.
Cells and reagents
Human prostate cancer cell line LNCap, PC3 and DU145 were purchased from the Institute of Preclinical Medicine in Chinese Academy of Medical Sciences; all these cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum. Electroporation transfection reagent was purchased from Lonza; anti-CTHRC1 was purchased from Cell signalling technology; actin antibody was purchased from Sigma Company; si-CTHRC1 and CTHRC1 overexpression plasmids were synthesized by Invitrogen Company; Transwell used for migration and Boyden chambers used in invasion assay were purchased from Millipore. Lipofectamin 2000 was purchased from Invitrogen; protease inhibitor was purchased from Cell Signaling Technology (CST); and protein extraction reagent CytoBuster was purchased from Merck.
RT-PCR
Total cell RNA was extracted with Trizol, and reversely transcribed into cDNA. The reaction condition of PCR: 95°C for five minutes, 95°C for 10 sec, 60°C for 20 seconds, a total of 40 cycles were performed. CTHRC1 upstream primer: 5'- GCATGCTGTCAGCGTTGGTA-3'; downstream primer: 5'- TCAATGGGAAGAGGTCCTGAA-3'. GAPDH upstream primer: 5'-GTCGGAGGCAACATCACC-3'; downstream primer: 5'-GTCCAAATGCGGGAACAG-3'. The relative expression level of CTHRC1 was calculated using the 2-Ct method, and all the data is the absolute value of the removed GAPDH.
Electroporation transfection
All operations were conducted strictly according to the kit instructions.
Extraction of cell total protein
Prostate cancer PC3 cells were washed for two times with iced PBS, centrifuged at 400 g for five minutes. The supernatant was discarded, protease inhibitor and 100 μl of CytoBuster were added to the precipitate, and pipetting was repeated for uniform mixing. Then the extraction was placed at room temperature for 15 minutes, centrifuged at 12,000 g, 4°C, for 15 minutes. The obtained supernatant was the total cell protein. Part of the total protein was used for concentration detection. The remaining was packaged, 15 μl for each tube, and stored at-80°C.
Determination of protein concentration by BCA method
The dilution of standard Bovine Serum Albumin (BSA) was carried out strictly according to Bicinchoninic Acid (BCA) kit instructions and relevant steps in strict accordance with the kit instructions. After completion of the assay, the standard curve was drawn according to the OD value and the concentration gradient of the standards. The concentration of sample was calculated according to the standard curve and the OD value of the sample.
Western-blot
The equivalent amounts of the extracted proteins were separated by 8-10% separating gel for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and 5% concentrated gel, transferred to nitrocellulose film in a half-dry state, and blocked at room temperature for two hours with Tris- Buffered Saline and Tween 20 (TBST) containing 5% BSA. Mouse anti-human CTHRC1 antibody was added, and was incubated at 4°C overnight. On the next day, the film was washed with 0.1% TBST for three times, with five minutes each time. Corresponding Horseradish Peroxidase (HRP)- labelled secondary antibody was added, and was incubated at room temperature for one hour. After the film was washed with 0.1% TBST, the bands on the film were colored with Supersignal West Femto HRP-sensitive chemiluminescence substrate. Actin was used as an internal control. All the experiments were repeated for at least three times.
Clone formation test
These PC3 cells transfected with pcDNA3.1-CTHRC1 and CTHRC1 siRNA, 5 × 103 cells for each type, were inoculated in six-well plates. After 72 h of culture, the formed clones were fixed with methanol, and stained with 20% methanol containing 0.1% crystal violet for 30 minutes. The clones were counted.
Migration and invasion assay
Cell migration ability was detected by wound healing assay, and the specific operations were based on a related literature [10]. Invasion ability of cells was detected using Boyden chambers. Specific steps: The cells used in this experiment were stained with trypan blue, counted, and then prepared into suspension. A total of 1 × 105 cells were placed in the upper chamber after gel preparation, and culture medium was added to 300 μl. Then, 500 μl of culture medium containing 10% fetal bovine serum was added to the lower chamber. After 12 h of incubation, the uninvaded cells were removed from the upper chamber with a cotton swab. The cells in the lower chamber were fixed and stained with 0.1% crystal violet, and the number of invaded cells was counted.
Dual luciferase reporter assay
Suitable amount of prostate cancer PC3 cells were inoculated in 96-well plates. Lipofectamin 2000 transfection system was used to transfect the prostate cancer PC3 cells with 100 ng of the mixture of TOPFLASH and FOPFLASH (the materials for determination of intracellular β-catenin-mediated transcriptional activity), or transfect with 100 ng of pFR and 10 ng of ATF2 (Wnt/PCP reporter plasmid), as well as 10 ng of fluorescein. Parts of these cells were activated by CTHRC1 (20 nm). After 72 h, the luciferase activity in cell lysis solution was detected by dual luciferase reporter system (Promega, Madison, WI).
Statistical methods
Statistical analysis was conducted using statistical software SPSS16.0. Data were compared between two groups using t-test. P<0.05 was considered statistically significant.
Results
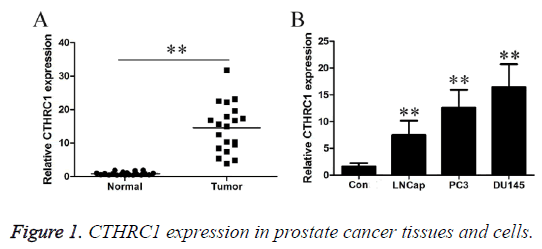
CTHRC1 expression in prostate cancer tissues and cells
As shown in Figure 1A, RT-PCR results revealed that CTHRC1 expression level was 0.87 ± 0.49 in the control group, and 14.58 ± 7.24 in the prostate cancer tissues. CTHRC1 expression level was significantly higher in prostate cancer tissue than in normal tissues (P<0.01). As shown in Figure 1B, RT-PCR results revealed that CTHRC1 expression level was 1.64 ± 0.63 in normal human prostate cells, and were 7.46 ± 2.71, 12.64 ± 3.3 and 16.46 ± 4.26 in three types of different metastatic potential prostate cancer cells, LNCap, PC3 and DU145, respectively. CTHRC1 expression level was significantly higher in prostate cancer cells than in control cells (P<0.01). Additionally, with the gradual increase in metastatic potential of prostate cancer cells, CTHRC1 expression level gradually increased (P<0.01).
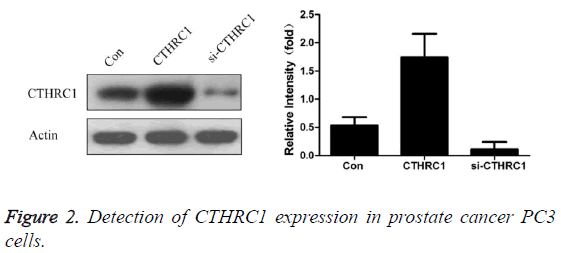
Effect of transfection with CTHRC1 and CTHRC1 siRNA on the expression of CTHRC1 in prostate cancer PC3 cells
As shown in Figure 2, the results revealed that CTHRC1 expression level was 0.53 ± 0.15 in prostate cancer PC3 cells in control group, 1.74 ± 0.42 in prostate cancer PC3 cells transfected with CTHRC1, and 0.11 ± 0.13 in prostate cancer PC3 cells transfected with CTHRC1siRNA. Transfection with CTHRC1 can significantly promote CTHRC1 expression in prostate cancer cells (P<0.01); while transfection with si- CTHRC1 can significantly inhibit it (P<0.01).
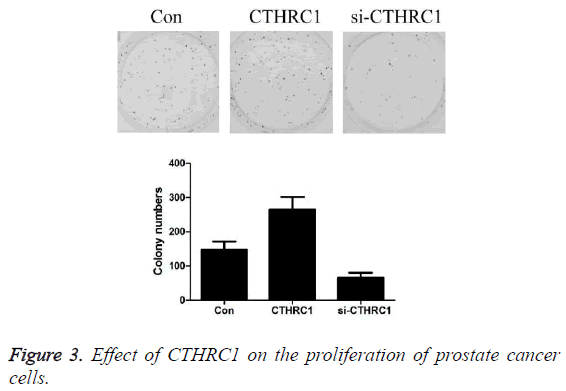
Effect of CTHRC1 on the proliferation of prostate cancer cells detected by clone formation assay
As shown in Figure 3, the results revealed that the number of clones of prostate cancer PC3 cells was 146.85 ± 24.53 in the control group, 264.84 ± 37.25 in CTHRC1 group, and 65.42 ± 15.03 in CTHRC1siRNA group. Transfection with CTHRC1 can significantly promote the proliferation of prostate cancer cells (P<0.01); while transfection with si-CTHRC1 can significantly inhibit it (P<0.01).
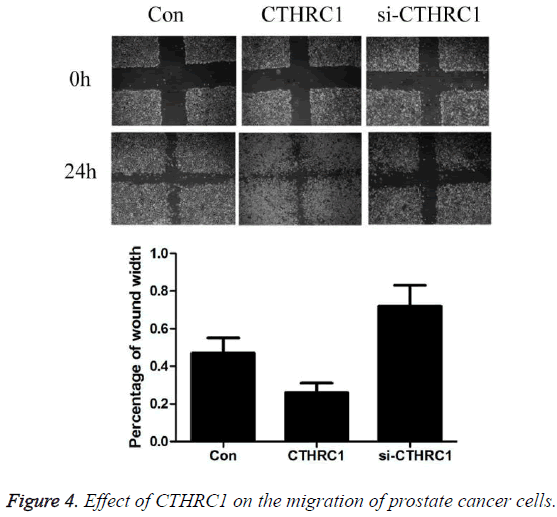
Effect of CTHRC1 on the migration of prostate cancer cells detected by wound healing assay
As shown in Figure 4, the results revealed that after 24 hours of culture, the percentage of wound width was 0.47 ± 0.08 in prostate cancer PC3 cells in control group, 0.26 ± 0.05 in CTHRC1 group, and 0.72 ± 0.11 in CTHRC1siRNA group. Transfection with CTHRC1 can significantly promote the migration of prostate cancer cells (P<0.01); while transfection with si-CTHRC1 can significantly inhibit it (P<0.01).
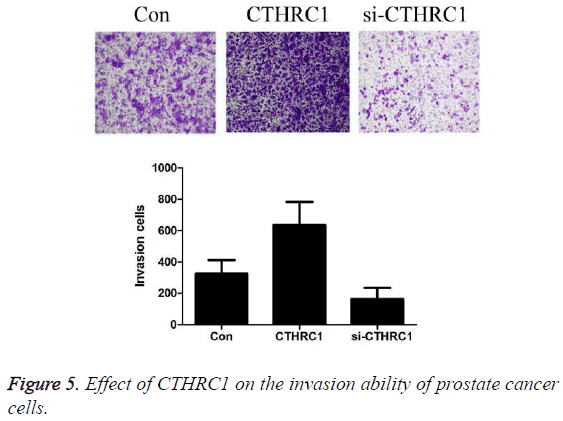
Effect of CTHRC1 on the invasion ability of prostate cancer cells detected by Transwell
As shown in Figure 5, the results revealed that the number of invaded cells by prostate cancer PC3 cells was 326.42 ± 85.35 in the control group, 636.24 ± 147.32 in CTHRC1 group, and 164.94 ± 68.46 in CTHRC1siRNA group. Transfection with CTHRC1 can significantly promote the invasion of prostate cancer cells (P<0.01); while transfection with si-CTHRC1 can significantly inhibit it (P<0.01).
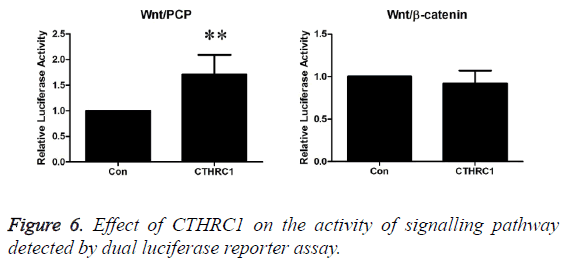
Effect of CTHRC1 on the activity of signalling pathway detected by dual luciferase reporter assay
As shown in Figure 6, the experimental results revealed that CTHRC1 could significantly activate Wnt/PCP signalling pathway in prostate cancer PC3 cells (P<0.01), but it has no significant effect on Wnt/β-catenin signalling pathway (P>0.05).
Discussion
Tumor cells can alter the surrounding microenvironment by regulating the expression of some proteins, thus to establish an environment which is propitious to tumor cell survival, proliferation, invasion and metastasis. CTHRC1 is a secreted protein. It is known that CTHRC1 is closely related to the invasion and metastasis of tumors. CTHRC1 expression was up-regulated in a variety of cancers, including breast cancer, stomach cancer and liver cancer. Tang et al. [11] revealed that CTHRC1 was silenced or lowly expressed in benign moles and non-invasive tissues, and CTHRC1 expression was significantly increased in high invasive melanoma tissues. Overexpression of CTHRC1 in melanoma cells may promote migration and adhesion of melanoma cells and may inhibit melanoma cell apoptosis [16]. Ke et al. [17] revealed that overexpression of CTHRC1 was related to the invasion and poor prognosis of non-small-cell lung cancer. And Liu et al. [16] revealed that CTHRC1 alone could be used as biomarker for assessment of the prognosis of non-small-cell lung cancer. Therefore, CTHRC1 plays an important role in the occurrence and development of human cancers, and is a possible mechanism from which cancer cells obtain a drug resistant phenotype. At present, few literatures have reported the studies on CTHRC1 expression in prostate cancer tissues and its relationship with the occurrence and development of prostate cancer.
In this study, CTHRC1 expression in prostate cancer tissues and cells was observed at first. The results revealed that CTHRC1 expression was significantly higher in prostate cancer tissues and cells than in normal tissues (P<0.01). Additionally, with the gradual increase in metastatic potential of prostate cancer cells, CTHRC1 expression level gradually increased (P<0.01). This result roughly suggested that high expression of CTHRC1 in prostate cancer tissues may be closely related to the occurrence and development of prostate cancer. In order to further verify the role of CTHRC1 in the occurrence and development of prostate cancer, PcDNA3.1- CTHRC1 and CTHRC1 siRNA were transfected into prostate cancer PC3 cells by transfection method. Effects of CTHRC1 on the proliferation, migration and invasion of prostate cancer cells were also observed. The results revealed that CTHRC1 expression was significantly lower in prostate cancer PC3 cells transfected with CTHRC1 siRNA (P<0.01); and was significantly higher in prostate cancer cells transfected with pcDNA3.1-CTHRC1 (P<0.01). After CTHRC1 expression was knocked-down in prostate cancer PC3 cells, the proliferation, migration and invasion of prostate cancer PC3 cells were significantly decreased (P<0.01). Meanwhile, after the expression of CTHRC1 in prostate cancer PC3 cells was upregulated, the proliferation, migration and invasion of prostate cancer PC3 cells were significantly increased (P<0.01). This result further suggested that high expression of CTHRC1 in prostate cancer tissues may be closely related to the occurrence and development of prostate cancer.
At present, few studies have reported the molecular mechanism of CTHRC1. In arterial smooth muscle, CTHRC1 could inhibit transforming growth factor-β (TGFβ) signaling [18]. A recent study revealed that, CTHRC1 could selectively activate the Wnt signaling pathway by stabilizing the Wnt receptor complex [19]. In tumors, CTHRC1 could promote the invasion of gastrointestinal stromal tumor by activating the Wnt/PCPRho signalling pathway [20]. At present, no study has been reported about by which pathway CTHRC1 regulates the proliferation, migration and invasion of prostate cancer cells during the occurrence and development of the prostate cancer. In this study, the effect of CTHRC1 on the activity of signalling pathway was detected by dual luciferase reporter assay. The results revealed that CTHRC1 could significantly activate Wnt/PCP signalling pathway in prostate cancer PC3 cells (P<0.01), but will have no significant effect on Wnt/β- catenin signalling pathway (P>0.05).
Conclusion
In summary, the results of this study revealed that CTHRC1 expression was significantly increased in prostate cancer tissues and cell lines, and CTHRC1 could promote the proliferation, migration and invasion of prostate cancer cells. Increase in CTHRC1 expression in prostate cancer tissues may be related to the occurrence and development of prostate cancer. By regulating the activity of the Wnt/PCP signalling pathway, CTHRC1 will promote the proliferation, migration and invasion of prostate cancer cells. This study provides a certain scientific theoretical basis for the clinical search of new therapeutic targets of prostate cancer.
Acknowledgement
None
Declaration of Interest
The authors declare no conflict of interest.
References
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359-386.
- Van Poppel H, De Meerleer G, Joniau S. Oligometastatic prostate cancer: Metastases-directed therapy? Arab J Urol 2016; 14: 179-182.
- Van HM, Garmo H, Adolfsson J, Stattin P, Adi J, Klil D, Hui Y, Vicky T, Armen A, Laurent A. Androgen deprivation therapy for prostate cancer and risk of venous thromboembolism. Eur Urol 2016; 70: 56-61.
- Chen YW, Muralidhar V, Mahal BA, Nezolosky MD, Beard CJ, Choueiri TK, Hoffman KE, Martin NE, Orio PF, Sweeney CJ, Feng FY, Trinh QD, Nguyen PL. Factors associated with the omission of androgen deprivation therapy in radiation-managed high-risk prostate cancer. Brachytherapy 2016; 15: 695-700.
- Friedlander TW, Ryan CJ. Targeting the androgen receptor. Urol Clin North Am 2012; 39: 453-464.
- Ryan CJ, Smith MR, De Bono JS. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368: 138-148.
- Pyagay P, Heroult M, Wang Q, Lehnert W, Belden J, Liaw L, Friesel RE, Lindner V. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ Res 2005; 96: 261-268.
- Diatchenko L, Lau YF, Campbell AP, Chenchik A, Mogadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov ED, Siebert PD. Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci USA 1996; 93: 6025-6030.
- Durmus T, LeClair RJ, Park KS, Terzic A, Yoon JK. Expression analysis of the novel gene collagen triple helix repeat containing-1 (Cthrc1). Gene Expr Patterns 2006; 6: 935-940.
- LeClair R, Lindner V. The role of collagen triple helix repeat containing 1 in injured arteries, collagen expression, and transforming growth factor beta signaling. Trends Cardiovasc Med 2007; 17: 202-205.
- Tang L, Dai DL, Su M, Martinka M, Li G. Aberrant expression of collagen triple helix repeat containing 1 in human solid cancers. Clin Cancer Res 2006; 12: 3716-3722.
- Turashvili G, Bouchal J, Baumforth K. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer 2007, 7: 55-75.
- Ip W, Wellman-Labadie O, Tang L, Su M, Yu R, Dutz J, Wang Y, Huang S, Zhang X, Huang C, Zhou Y. Collagen triple helix repeat containing 1 promotes melanoma cell adhesion and survival. J Cutan Med Surg 2011; 15: 103-110.
- Yang XM, You HY, Li Q, Ma H, Wang YH. CTHRC1 promotes human colorectal cancer cell proliferation and invasiveness by activating Wnt/PCP signaling. Int J Clin Exp Pathol 2015; 8: 12793-12801.
- Kim HC, Kim YS, Oh HW, Kim K, Oh SS, Kim JT, Kim BY, Lee SJ, Choe YK, Kim SH, Chae SW, Kim KD, Lee HG. Collagen triple helix repeat containing 1 (CTHRC1) acts via ERK-dependent induction of MMP9 to promote invasion of colorectal cancer cells. Oncotarget 2014; 5: 519-529.
- Liu X, Liu B, Cui Y, Wang F, Sun H, Lv F. Collagen triple helix repeat containing 1 (Cthrc1) is an independently prognostic biomarker of non-small cell lung cancers with cigarette smoke. Tumour Biol 2014; 35: 11677-11683.
- Ke Z, He W, Lai Y, Guo XF, Chen SR, Li SH, Wang YF, Wang LT. Overexpression of collagen triple helix repeat containing 1 (CTHRC1) is associated with tumour aggressiveness and poor prognosis in human non-small cell lung cancer. Oncotarget 2014; 5: 9410-9424.
- LeClair RJ, Durmus T, Wang Q, Pyagay P, Terzic A. Cthrc1 is a novel inhibitor of transforming growth factor-beta signaling and neointimal lesion formation. Circ Res 2007; 100: 826-833.
- Yamamoto S, Nishimura O, Misaki K, Nishita M, Minami Y. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell 2008; 15: 23-36.
- Ma MZ, Zhuang C, Yang XM, Zhang ZZ, Ma H, Zhang WM, You H, Qin W, Gu J, Yang S, Cao H, Zhang ZG. CTHRC1 acts as a prognostic factor and promotes invasiveness of gastrointestinal stromal tumors by activating Wnt/PCP-Rho signaling. Neoplasia 2014; 16: 265-278.