ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2011) Volume 22, Issue 4
Cultivation of Intervertebral Disc Cells in Medium Fortified with Growth Factors Improved In Vitro Chondrogenesis
Manira M1, Shamsul BS1, Aminuddin BS1,2, and Ruszymah BHI1,3*
1Tissue Engineering Centre, Universiti Kebangsaan Malaysia Medical Centre Malaysia
2Ear Nose & Throat Consultant Clinic, Ampang Puteri Specialist Hospital, Selangor, Malaysia
3Department of Physiology, Faculty of Medicine, Universiti Kebangsaan Malaysia
- *Corresponding Author:
- Ruszymah Bt. Hj Idrus
Tissue Engineering Centre
Universiti Kebangsaan Malaysia Medical Centre
Jalan Yaacob Latiff, Bandar Tun Razak Cheras, 56000, Kuala Lumpur
Malaysia
Accepted date: May 07 2011
Various chondrogenic growth factors have been shown to promote proliferation, matrix production and early chondrogenesis of intervertebral disc (IVD). The objective of this study is to reconstruct IVD via tissue engineering technique and to evaluate in-vitro chon-drogenesis of chondrocytes cultured in basic growth medium with chondrogenic growth factors (FDGFs) and without chondrogenic growth factors (FD). The annulus fibrosus (AF) and nucleus pulposus (NP) were aseptically dissected from the lumbar discs of sheep and the tissues enzymatically digested and cultured in-vitro. Passage 1 cells were used to form in-vitro construct with autologous fibrin as the biomaterial. All constructs were stabi-lized for 14 days in their respective growth media. Histological and sulphated-glycosaminoglycan (sGAG) analysis were performed at the end of the experiment. The AF cells showed greater growth kinetic profile and higher viability in FDGFs while the NP cells showed no differences for both medium. The construct of AF and NP in FDGFs showed a bigger size construct compared to FD. The qualities of both constructs were simi-lar as evidence by Safranin O staining in both groups. In conclusion, FDGFs increase the viability and quantity of cells for AF and not in NP. However, FDGFs supplementation improved in-vitro constructs formation for both cells type.
Keywords
Intervertebral disk, annulus fibrosus, nucleus pulposus, sulphated-glycosaminoglycan (sGAG), chondro-genesis, tissue engineering
Introduction
The intervertebral discs (IVD) separate the vertebrae of the spine and account for approximately one-third of the height of the entire spinal column. The discs have unique structure that enables the bending and twisting motion of the spine [1]. The intervertebral disc (IVD) is the largest avascular tissue in the human body [2]. There are three distinct morphological regions of the IVD: the nucleus (a soft gel-like tissue), the inner annulus (both fibrous and cartilage like), and the outer annulus (ligmanetous) [3]. The AF consists of an extracellular matrix (ECM) composed both type I and type II collagen oriented in a lamellar structure. The outermost part of the AF is ligamentous, and is rich in type I collagen, whereas the inner part is fibrocartilagelike and is rich in type II collagen and proteoglycan [1]. The NP has an ECM composed primarily of type II collagen, large aggregating proteoglycan and a low concentration of chondrocytes [4]. The intervertebral disc (IVD) has a wide range of functions, including the provision of reversible resistance to compressive, rotational and tensile stresses applied to the vertebral column [5].Other than that, it also can act as a joint that allows movement between bones [6] such as within the cervical, thoracic and lumbar spine.
In human, the IVDs are separated from the vertebral bone by the cartilage endplates, which in the juvenile are responsible for longitudinal vertebral growth. In the adult, the IVDs are essentially avascular structures, which receive most of their nutrients from the vasculature of the vertebral bone by diffusion through the endplates. The size of the IVDs increases on progressing down the spine, with the largest lumbar discs being approximately 1 cm thick and 4 cm in diameter. The supply of nutrients to the cells at the center of these large discs is readily compromised, and is one factor leading to IVD degeneration [7]. The structure and function of the IVD may be altered by processes including normal physiological aging, mechanical factors, i.e., trauma and repetitive stress, segmental instability of the spine, inflammatory and biochemical factors [8]. In degeneration, there is disruption of the nucleus pulposus, including changes in the proportion and types of proteoglycan and collagens, a reduction in the number of chondrocytes, and the formation of permeative ‘slitlike’ spaces within the nucleus pulposus. Often, there is disruption of the collagen fiber arrays in the annulus fibrosus, traumatic damage to the end plate, and ingrowths of vessels and nerves into the nucleus pulposus [6]. The IVD requires proper nutrition, mainly by diffusion from the vertebral bodies and end plates, in order to maintain the steadystate metabolism of the cells [9].
Growth factors are polypeptides that bind to cell membranes by means of specific receptors. They regulate matrix production and repair of various types of cells. Growth factors were originally discovered in body fluids, including blood and synovial fluid, and tissue extracts that had biological effects, such as stimulation of cell proliferation, differentiation and migration [10]. In this study, basic culture medium were supplemented with growth factors such as transforming growth factor (TGF-β2), basic fibroblast growth factor (bFGF) and insulin –like growth factor (IGF). TGF-β induced a significant enhancement of cell proliferation [10] and maintained the phenotype of the cells. Basic fibroblast growth factor (bFGF) has been recognised as an important factor in modulating chondrocyte mitogenesis and synthesis of cartilage matrix [11]. A culture medium (FD), which consists of essential growth factors such as ITS, IGF-1 together with bFGF and TGF- ß2 can promote proliferation and provide sufficient number of chondrocytes for tissue engineered cartilage formation [12].
In this study, fibrin was used as a scaffold for the construct formation. Although fibrin is unstable and quickly disintegrates, its use as a natural polymer scaffold for tissue engineering is increasing [13]. Fibrin is easily polymerized and moulded from its basic constituents. Moreover, fibrin is noncytotoxic, biocompatible and biodegradable [14]. Based on the study of Munirah et al. (2008), fibrin have proven to be an ideal cell carrier to enhance in vitro tissue formation by rabbit AF and NP cells [15].
The current treatment options for degenerative disk disease range from NSAIDS to invasive procedures including discectomy and spinal fusion. Unfortunately these treatments are not only remaining the origin of the problem which is the degeneration of IVD itself but also increasing the load of intact discs and joints located adjacently to the degenerated disc [8]. Mizuno et al. (2004) also reported that current surgical treatments which focus on fusion of the involved IVDs levels can eliminates pain but does not attempt to restore disc function [4]. Therefore, the field of tissue engineering may represent a new strategy for the treatment of intervertebral disc disease in that it involves generation of living tissues to restore organ function. Our approach in this study was to develop methods for the construction of AF and NP cells in two different medium and to evaluate the morphology, cellular proliferation, matrix synthesis and cartilaginous tissue formation of AF and NP cells cultured in the fibrin scaffold to determine the potential of IVD reconstruction.
Materials and Methods
This study has been approved by the Universiti Kebangsaan Malaysia Research and Ethical Committee with approval number of 02-01-02-SF0444. The study duration is from 9 June 2008 to 30 June 2009, approximately 12 months.
Cell preparation
Six Sheep intervertebral discs were used in this study. The muscles and tendons were removed and the column was sectioned transversally in the middle of each disc. The annulus fibrosus (AF) and nucleus pulposus (NP) tissue was aseptically dissected from the lumbar discs. The AF was separated from the NP with a scalpel and both were washed twice in phosphate-buffered saline (PBS) solution (Gibco,Grand Island, NY) containing 100 μg/ml penicillin and 100 μg/ml streptomycin. Each tissue was cut into small pieces and digested in 15 ml 0.6% w/v Collagenase type II (Gibco/Invitrogen) at 37°C in incubator shaker for 6 hours. The resulting cell suspension was centrifuged at 6000 rpm at 37°C for 5 min (Jouan centrifuge). Supernatant was decanted and the cells pellet was washed with PBS to remove the remaining enzyme. After final centrifugation, AF and NP cells were harvested and seeded in 6-well plates (Falcon, Franklin Lakes, NJ) with two different medium, F12:DMEM (1:1) + 10% FBS medium (FD) and F12:DMEM (1:1) + 10% FBS with the addition of growth factors (IGF-1, TGF-B2, BFGF) (FDGF). All cultures were maintained in 5% CO2 incubator (Galaxy R, CO2 incubator, RS Biotech) at 37°C with the medium changed alternate day. Once confluence, the primary cultured cells; P0, was trypsinized using 0.05% trypsin-EDTA (Gibco) and counted for total cell and viability.
Measurement of cell proliferation activity
Cell numbers and viability were determined using a haemacytometer (Weber, UK) and trypan blue vital dye. Growth rate (cell /day/cm2) and population doubling time was measured for primary passage (P0) to passage 3 (P3) for both AF and NP cells in their respective growth media.
Preparation of sheep fibrin as biomaterial
Whole blood was withdrawn from sheep into 9 ml plasma collection tubes containing 3.2% Sodium Citrate (Bio-One,Greiner, USA). The whole blood was centrifuged at 6000 rpm for 5 min to collect the plasma. The plasma was sterile-filtered using 0.2 μm syringe filter (Sartorious, USA) to remove the cell debris that may cause spontaneous clotting of the plasma. Plasma was kept at -20°C until used.
Formation of in vitro constructs
AF and NP cells from the primary culture (P0) were harvested and subcultured, passage 1 (P1) in 75 cm2 culture flasks (Falcon) at a density of 5000 cells/cm2 before they were harvested and seeded into 3D fibrin scaffold. After the cells reach confluence, they were harvested by trypsinization, counted for total cell and viability. Four million cells were resuspended with fibrin and were polymerized within 10 minutes by the addition of calcium chloride (CaCl2) solution. Both AF and NP constructs were cultured for 14 days in vitro in their respective growth media. The resulting in vitro constructs were then harvested and evaluated.
Macroscopic observation and histological analysis
Approximately 4 x 106 cells per scaffold were cultured in the fibrin. Seeded cells adhered onto the scaffold, proliferated and produced matrices filling in the void spaces of the scaffolds. Each construct was observed grossly at room temperature without any fixation and the diameter was measured. After fixation with 10% phosphate buffered formalin for 24 hours, specimens were processed and stained with Haematoxylin and Eosin (H&E). Safranin O staining used to identify the presence of the proteoglycanrich matrix for detection of cartilage.
Sulphated glycosaminoglycan (sGAG) production assay
All sample were digested with papain digestion solution [125 μg/ml papain (Sigma Aldrich), 5mM L-cystein (Sigma Aldrich), 100 mMNa2HPO4 (Merck GaA, Germany), 5mM EDTA, pH 7.5] at 60°C for 16-24 hours. The sGAG content was analyzed using the 1,9-dimethylmethylene blue (DMMB) assay. Each sample which contains 50 μl papain solutions was mixed with 200 μl DMMB solution and the absorbance was measured at 525 nm wavelengths. Total amount of chondroitin sulfate or sGAG content in tissueengineered AF and NP was measured by extrapolated using a standard plot of shark chondroitin sulfate (Sigma-Aldrich) in the range of 0–50 μg/ml. Total sGAG production was normalized by the dried weight of each sample and represented as relative sGAG content in μg/mL according to standard curve for DMMB analysis. Data was expressed as mean ± standard error of the mean (SEM).
Statistical analysis
Results were analyzed using student’s t-test and the differences are considered significance when P <0.05.
Results
Monolayer culture of AF and NP cells
The monolayer-cultured AF and NP cells in FDGF medium reached confluence faster than the cells in FD medium at the end of P1. The morphology for AF cells showed the presence of fibroblast and chondrocytes while NP cells showed rounded chondrocytes.
Measurement of cell proliferation activity
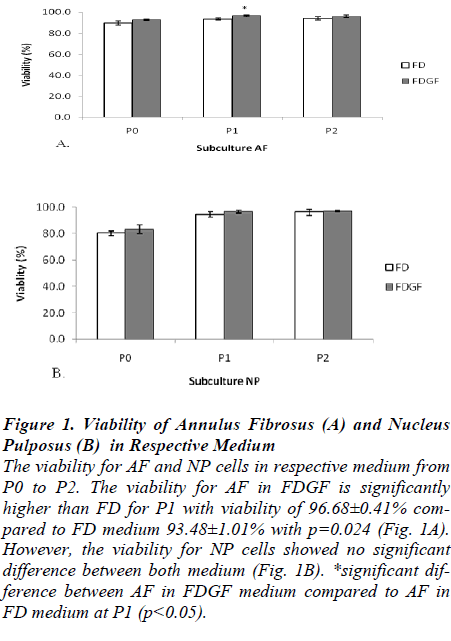
The viability of AF and NP cells was greater than 80% in every passage during the monolayer cultured. Figure 1 shows the viability for monolayer culture of AF and NP cells in FD and FDGF medium from P0 to P2. The viability for AF at P1 showed significantly higher in FDGF medium 96.68±0.41% compared to FD medium 93.48±1.01% with p=0.024 (Fig. 1A). However, the viability for NP cells showed no significant differences between both medium (Fig. 1B).
Figure 1:. Viability of Annulus Fibrosus (A) and Nucleus Pulposus (B) in Respective Medium
The viability for AF and NP cells in respective medium from P0 to P2. The viability for AF in FDGF is significantly higher than FD for P1 with viability of 96.68±0.41% compared to FD medium 93.48±1.01% with p=0.024 (Fig. 1A). However, the viability for NP cells showed no significant difference between both medium (Fig. 1B). *significant difference between AF in FDGF medium compared to AF in FD medium at P1 (p<0.05).
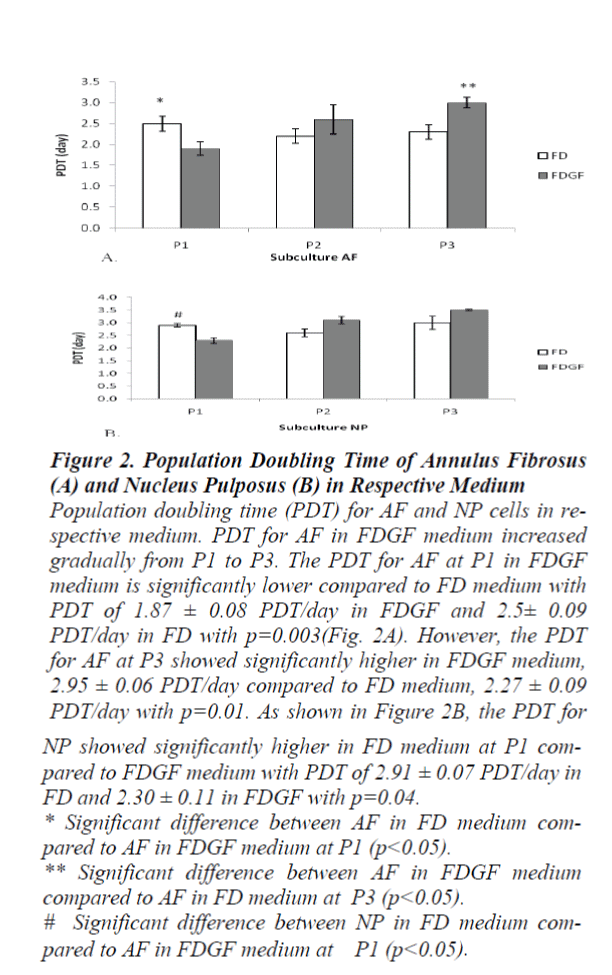
The population doubling time (PDT) for AF in the FDGF gradually increased from P1 to P3. The PDT for AF at P1 in FDGF medium showed significantly lower compared to FD medium with PDT of 1.87±0.08 PDT/day in FDGF and 2.5±0.09 PDT/day in FD with p=0.003 (Fig. 2A). However, the PDT for AF at P3 showed significantly higher in FDGF 2.95±0.06 PDT/day compared to FD 2.27±0.09 PDT/day with p=0.01. While for NP, the PDT in FDGF at P1 was significantly lower compared to FD with value 2.30±0.11 in FDGF and 2.91±0.07 in FD with p= 0.04 (Fig. 2B).
Figure 2:. Population Doubling Time of Annulus Fibrosus (A) and Nucleus Pulposus (B) in Respective Medium Population doubling time (PDT) for AF and NP cells in respective medium. PDT for AF in FDGF medium increased gradually from P1 to P3. The PDT for AF at P1 in FDGF medium is significantly lower compared to FD medium with PDT of 1.87 ± 0.08 PDT/day in FDGF and 2.5± 0.09 PDT/day in FD with p=0.003(Fig. 2A). However, the PDT for AF at P3 showed significantly higher in FDGF medium, 2.95 ± 0.06 PDT/day compared to FD medium, 2.27 ± 0.09 PDT/day with p=0.01. As shown in Figure 2B, the PDT for NP showed significantly higher in FD medium at P1 compared to FDGF medium with PDT of 2.91 ± 0.07 PDT/day in FD and 2.30 ± 0.11 in FDGF with p=0.04. * Significant difference between AF in FD medium compared to AF in FDGF medium at P1 (p<0.05). ** Significant difference between AF in FDGF medium compared to AF in FD medium at P3 (p<0.05). # Significant difference between NP in FD medium compared to AF in FDGF medium at P1 (p<0.05).
Macroscopic observation
As shown in Fig. 3, morphological appearances of AF and NP seeded with fibrin were comparable after 2 weeks in in-vitro culture. The construct for AF and NP showed smooth and glistening morphology. Fig. 4 shows the diameter for the AF and NP construct in both medium. The diameter of the AF and NP constructs in FDGF medium showed a bigger diameter of average 1 to 2 mm more than 3D construct for cells with FD media. The diameter for AF construct in medium FDGF was significantly bigger with diameter of 6.35±0.09 mm, compared to AF cultured in FD medium with diameter of 4.18±0.06 mm (p=0.001). For NP, the diameter of the construct also showed bigger in FDGF (6.15±0.09 mm) compared to FD medium (4.62±0.14 mm) with p=0.001.
Figure 4: Diameter of Annulus Fibrosus and Nucleus Pulposus Construct The diameter for AF and NP constructs after 2 weeks in vitro cultured. The diameter for AF construct in medium FDGF showed significantly bigger with diameter of 6.35±0.09 mm, compared to AF cultured in FD medium with diameter of 4.18±0.06 mm with p=0.001. As for NP, the diameter of the construct also showed bigger in FDGF (6.15±0.09 mm) compared to FD medium (4.62±0.14 mm) where p=0.001. * Diameter of AF in FDGF medium compared to FD medium (p<0.05) ** Diameter of NP in FDGF medium compared to FD medium (p<0.05)
Histological observation
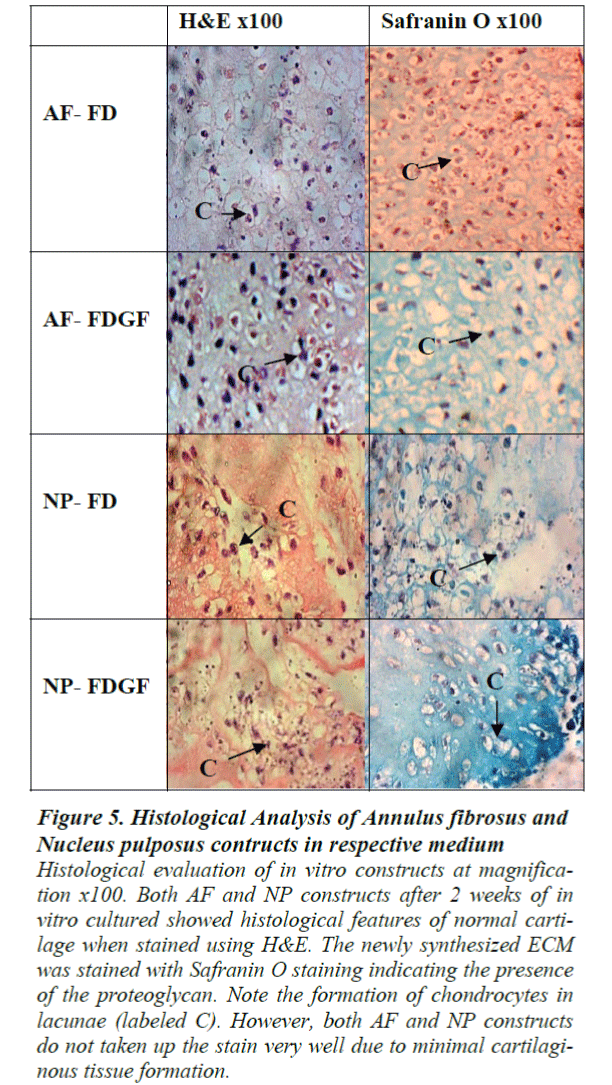
At 2 weeks of in vitro cultured, minimal cartilaginous tissue formation was observed when both AF and NP construct were stained using H&E staining. The newly synthesized ECM was slightly stained with Safranin Ostaining indicating the formation of cartilage with proteoglycanrich matrix. The formation of cartilaginous tissue in both AF and NP was evident by presence of rounded cell (chondrocytes) in lacunae. However, both AF and NP constructs showed lower intensity towards the H&E and Safranin O staining due to minimal cartilaginous tissue formation (Fig. 5).
Figure 5: Histological Analysis of Annulus fibrosus and Nucleus pulposus contructs in respective medium
Histological evaluation of in vitro constructs at magnification x100. Both AF and NP constructs after 2 weeks of in vitro cultured showed histological features of normal cartilage when stained using H&E. The newly synthesized ECM was stained with Safranin O staining indicating the presence of the proteoglycan. Note the formation of chondrocytes in lacunae (labeled C). However, both AF and NP constructs do not taken up the stain very well due to minimal cartilaginous tissue formation.
Sulphated glycosaminoglycan (sGAG) production assay
After 2 weeks in vitro cultured, the sGAG content in both AF and NP showed no differences for both medium (Fig. 6)
Discussion
Our aimed was to reconstruct the IVD via tissue engineering technique and to evaluate in-vitro chondrogenesis of chondrocytes cultured in basic growth medium with chondrogenic growth factors (FDGFs) and without chondrogenic factors (FD). The AF cells have higher viability in FDGF medium due to the nature of the cells. In AF, the cells consist of a mixture of fibro-cartilage which response well with the growth factor as compare to NP which consists of mainly cartilage. The growth factor that influences this would be fibroblast growth factor (FGF), since the FGF is a potent inducer of the fibroblast proliferation [16]. A culture medium (FD), which consists of essential growth factors such as ITS, IGF-1 together with bFGF and TGF- ß2 can promote proliferation and provide sufficient number of chondrocytes for tissue engineered cartilage formation [12]. These growth factors prove to maintain the cells phenotype during cultivation.
Ideally, good quality cells play a major role in successful tissue reconstruction. Previous studies using monolayer cultured chondrocytes harvested from various sources, such as human articular cartilage [17], nasal septum and auricular cartilage [13] demonstrated that the most proliferative state of cells was at passage 1 than any other passages. Fröhlich et al. (2007) believed that harvesting cells in later passages is not recommended due to cell senescence plus the accompanying dedifferentiation [18]. Hence, we harvested P1 cells for in vitro 3D culture to ensure its full potential.
After 2 weeks of in vitro culture, the cells were reconstructed into 3 dimension (3D) construct using sheep’s own fibrin. Fibrin was applied as a carrier for cells, growth factors and pharmacologic substances especially in tissue engineering techniques. Therefore the construct that have been formed was fully autologous which can reduce the risk of immune rejection and this method can be used for future clinical application in human by using patient’s own fibrin. The diameter of the AF and NP constructs supplemented with growth factors showed a bigger diameter compared to FD medium. This showed that growth factors not only have a quantity effect on the 2D cell cultures but also have a significant role in forming a bigger 3D construct. Interestingly, the NP showed a bigger construct compare to AF although in 2D culture, the proliferation rate of the cells is higher in AF. This showed that the growth factor have an impact on the in vitro 3D reconstruction especially in the matrix production as shown in our GAG study.
After 2 weeks in vitro culture, the GAG contents showed no differences between both medium. Both AF and NP showed presence of lacunae in both medium which showed that there is formation of cartilage tissue. In Safranin O staining showed both cells were lightly stained due to the possibility of immature cartilage formation.
In conclusion, growth factors supplement is necessary in cells culture of AF and NP to produce better chondrogenesis of in vitro construct which is important in future clinical application. Our research generated important preliminary results in regenerating the IVD. However, this study only focuses on in-vitro evaluation. Further studies using this tissue engineered IVD on in vivo model has to be carried out.
Acknowledgement
This study was made possible by research grant from the Ministry of Science, Technology and Innovation of Malaysia (06-02-02-003 BTK/ER/022). The authors wish to thank Dr. Angela Ng Min Hwei and Dr. Chua Kien Hui for expert technical support.
References
- Wan Yuqing, Feng G, Shen FH, et al. Biphasic scaffold for annulus fibrosus tissue regeneration. Biomaterials 2008; 29: 643-652.
- Helen W, Gough JE. In vitro studies of annulus fibrosus disc cell attachment, differentiation and matrix production on PDLLA/45S5 Bioglasss composite films. Biomaterials 2006; 27: 5220-5229.
- 3 Urban JPG, Roberts S. Structure and function of the extracellular matrix. Comper W. The intervertebral disc. United Kingdom: Gordon and Breach Publishers 1996.
- Mizuno H, Amit KR, Charles A, et al. Tissueengineered composites of annulus fibrosus and nucleus pulposus for intervertebral disc replacement. Spine 2004; 29 (12): 1290-1298.
- Helen W, Merry CLR, Blaker JJ, et al. Threedimensional culture of annulus fibrosus cells within PDLLA/ Bioglasss composite foam scaffolds: Assessment of cell attachment, proliferation and extracellular matrix production. Biomaterials 2007; 28: 2010-2020.
- Freemont TJ, Le Maitre C, Watkins A, et al. Degeneration of intervertebral discs: current understanding of cellular and molecular events, and implications for novel therapies. Cambridge University 2001.
- Roberts S, & Urban JPG. Degeneration of interverte-bral disc. Arthritis Research and Therapy 2003; 5: 120-130.
- Munirah S, Sun JY, Youn KK, et al. Fibrin promotes proliferation and matrix production of intervertebral disc cells cultured in three-dimensional poly (lactic-co-glycolic acid) scaffold. Journal Biomaterial Science Polymer 2008; 19 (9): 1219-1237.
- Urban JP, Holm S, Maroudas A, et al. Nutrition of the intervertebral disk. An in vivo study of solute transport. Clin Orthop 1977; 129: 101-114.
- Masuda K & An HS. Growth factors and the intervertebral disc. The Spine Journal 2004; 4: 330S-340S.
- Chua KH, Aminuddin BS, Fuzina NH, et al. Basic fibroblast growth factor with human serum supplementation: enhancement of human chondrocyte proliferation and promotion of cartilage regeneration. Singapore Med J 2007; 48 (4): 324.
- Chua KH, Aminuddin BS, Fuzina NH, et al. Insulintransferrin-selenium prevent human chondrocyte dedifferentiation and promote the formation of high quality tissue engineered human hyaline cartilage. European Cells and Materials 2005; 9: 58-67.
- Yang Wang, Yu-Zhu Bian, Qiong Wu, et al. Evaluation of three-dimensional scaffolds prepared from poly (3-hydroxybutyrateco-3-hydroxyhexanoate) for growth of allogeneic chondrocytes for cartilage repair in rabbits. Biomaterials 2008; 29: 2858-2868.
- Ruszymah BHI, Lokman BS, AsmaA, et al. Pediatric auricular chondrocytes gene expression analysis in monolayer culture and engineered elastic cartilage. International Journal of Pediatric Otorhinolaryngology 2007; 71: 1225-1234.
- Munirah S, Kim SH, Ruszymah BHI, et al. The use of fibrin and poly (lactic-co-glycolic acid) hybrid scaffold for articular cartilage tissue engineering: an in vivo analysis. European Cells and Materials 2008; 15: 41- 52.
- Strutz F, Zeisberg M, Renziehausen A, et al. TGF-b1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2). Kidney International 2001; 59: 579-592.
- Munirah S, Aminuddin BS, Samsudin OC, et al. The re-expression of collagen type II, aggrecan and SOX 9 in tissue-engineered human articular cartilage. Tissue Engineering and Regenerative Medicine 2005; 2 (4): 347-355.
- Fro¨hlich M, Mali_cev E, Gorensˇek M, et al. Evaluation of rabbit auricular chondrocyte isolation and growth parameters in cell culture. Cell Biology International 2007; 31: 620- 625.