ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2005) Volume 16, Issue 3
Curative role of the aqueous extract of the herb, Phyllanthus niruri, against nimesulide induced oxidative stress in murine liver
Department of Chemistry, Bose Institute, 93/1, Acharya Prafulla Chandra Road, Kolkata, India
- *Corresponding Author:
- Dr. Parames C. Sil
Department of Chemistry Bose Institute 93/1
A.P.C. Road Calcutta-700009 West Bengal, India
Phone : 9133-23506619/2402/2403; # 412/ 413
Fax: +91-33-2350-6790
e-mail: parames ( at ) bosemain.boseinst.ac.in parames_95 ( at ) yahoo.co.in
Accepted date: October 14 2005
The present study was conducted to evaluate whether the aqueous extract of the herb, Phyllanthus niruri (PN) can effectively cure liver from nimesulide (NIM) induced oxidative stress in vivo. In our experiments, we have seen that administration of PN through intraperitoneal route is more effective in hepato-protection than oral administration. PN (100 mg/kg body weight) was, therefore, administered intraperitoneally for 5 days post to NIM application (10 mg/kg body weight/twice daily) for 7 days in mice. Levels of antioxidant enzymes like superoxide dismutase (SOD) and catalase (CAT), non-protein thiol reduced glutathione (GSH) and lipid peroxidation end-products were then determined. NIM administration caused significant depletion of the levels of SOD, CAT and GSH along with the increased levels of lipid peroxidation. Post-treatment with PN rapidly restored most of the NIM-induced oxidative changes compared to those obtained by the self recovery of liver. Histological studies supported these results. In addition, studies showed that PN could scavenge free radicals. Antioxidant property of PN has been compared with that of potent antioxidant, vitamin E. Besides, the effect of a non-relevant protein, BSA, has also been included in the study. Combining, data suggest that NIM induced oxidative stress in the liver and that could be cured by the beneficial effect of PN.
Keywords
Phyllanthus niruri, nimesulide, oxidative stress, antioxidants, curative role
Introduction
Herbal plants have been found beneficial to humans as they are the source of various medicinal compounds [1-3]. The Phyllanthus species have been shown to play benefi-cial roles against various pathological states [4-5]. Clinical studies with this herb in humans have been shown to have no side effects [6-7]. Although this herb has been reported to be effective against a number of drug and toxin-induced hepatic disorder [8-9], no report has yet been found describing the effect of this herb on NIM in-duced hepatic problems. The nonsteroidal anti-inflammatory drug, NIM, is a selective cyclooxygenase-2 (COX-2) inhibitor and is almost exclusively metabolized and cleared by the liver [10]. Recently, Sbeit et al. [11], and Schattner et al. [12], reported that NIM can cause several types of liver damage, ranging from mild abnor-mal function to severe organ injuries. Knowledge on the protective mechanisms against drug and toxin induced toxicity leads scientists to look for biologically active relevant compounds from herbal plants which can either increase the efficacy of the antioxidants or possess intrinsic antioxidant activity. In this particular study, curative role of PN was evaluated by determining the effect of the post treatment of PN on the levels of i) anti-oxidant enzymes, (namely, SOD and CAT); ii) ROS scavenger, GSH and iii) extent of lipid peroxidation (expressed as malondialdehyde equivalents), induced by NIM. Effect of a non-relevant protein, BSA, and a known antioxidant, vitamin E, were also included in the study. In addition, histological studies were carried out on the liver sections of normal mice, mice treated with NIM and mice treated with PN post to NIM administration for the determination of the ultrastructural changes of the liver due to post-treatment of PN on NIM induced oxidative stress.
Materials and Methods
Preparation of PN
P. niruri, a traditional herbaceous plant of Euphorbiaceae family, was collected from Bose Institute Experimental Farm and local markets. The fresh leaves and stems of the young plants were homogenized in 50 mM sodium phos-phate buffer, pH 7.2, at 4°C and the homogenate was centrifuged at 12,000 g for 30 minutes to get rid of unwanted debris. Supernatant was dialyzed against ice-cold water and centrifuged again under the same condition. The supernatant was collected and lyophilized. The freeze-dried material was weighed, dissolved in the same phosphate buffer and used in different experiments needed for this study.
Test animals
Swiss albino mice (male, body weight 25+2 gm) were acclimatized under standard laboratory condition for a fortnight before starting experiments.
Curative effect of PN on NIM-induced oxidative stress
Liver has a unique property of regeneration itself after any drug and toxin injury [13,14]. To ascertain whether the aqueous extract of P. niruri can cause the healing of the liver faster than its natural regeneration, a time course study was conducted where eight mice in each group were injected with the 100 mg of extract for 1, 2, 3, 4 and 5 days after the administration of NIM at a dose of 10 mg/kg body weight twice daily for seven days. Mice were sacrificed after 2nd, 3rd, 4th,5th and 6th day respectively. In another set, eight mice in each group were treated with only NIM and sacrificed after 2nd, 3rd, 4th 5th and 6th day. In addition, eight mice were kept as normal control. For the positive control, a well-known antioxidant agent vi-tamin E was administered to a group of mice at a dose of 200 mg/kg body weight for 5 days post to NIM administration. Another group of mice got BSA at a dose of 5 mg/kg body wt intraperitoneally for 5 days post to NIM administration.
Assay of antioxidant enzymes SOD and CAT
The activity of superoxide dismutase (SOD) was assayed following the method as described by Nishikimi et al [15] and Kakkar et al 16]. Catalase activity was determined by following the method of Bonaventura et al [17].
Determination of GSH level and lipid peroxidation
Hepatic GSH level was determined by the method of Ellman [18] and lipid peroxidation was assessed by the method of Esterbauer and Cheeseman [19].
Measurement of protein concentration
Protein concentrations in the liver homogenates were determined according to the method of Bradford [20].
Liver histopathology
Liver specimens from all the experimental groups were fixed in 10% buffered formalin and were processed for paraffin sectioning. Sections of about 5 μm thickness were stained with haematoxylin and eosin to study the general structure of the liver.
Statistical Analysis
The results have been expressed as mean + S.D. Student’s ‘t’ test was employed for all statistical comparisons. Any value of P< 0.05 was regarded as significant.
Results
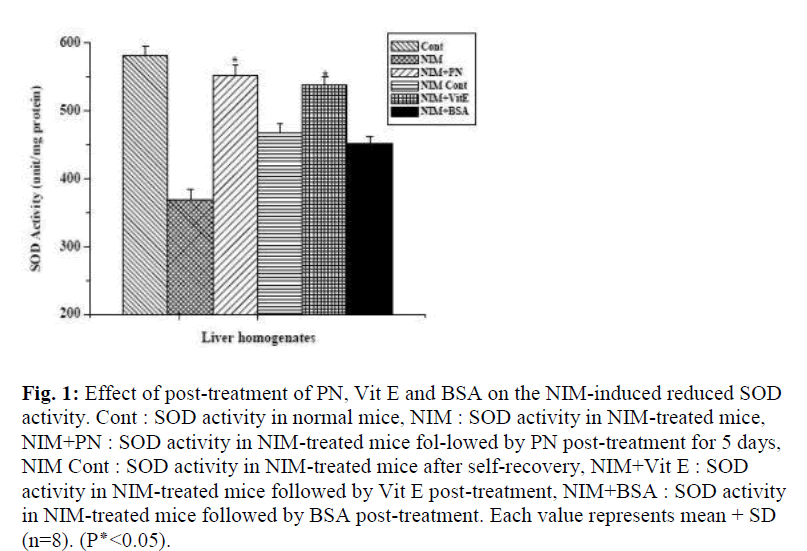
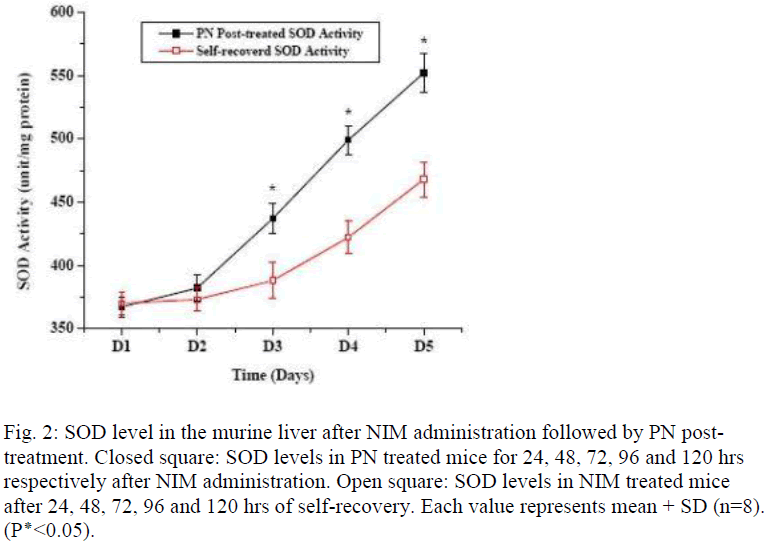
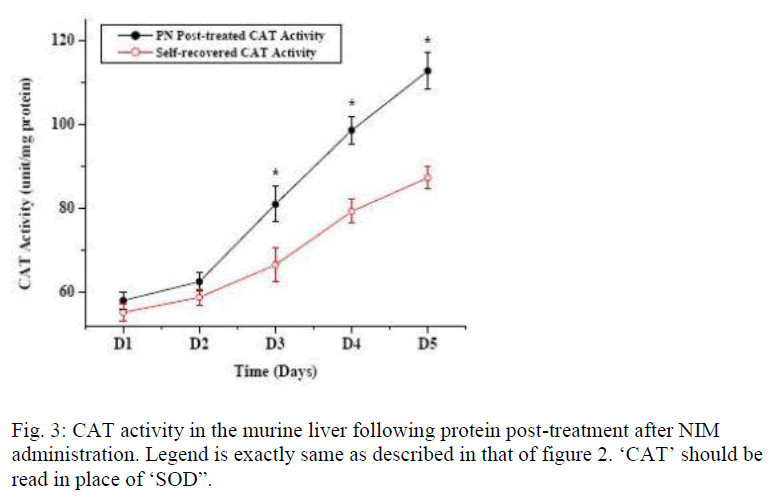
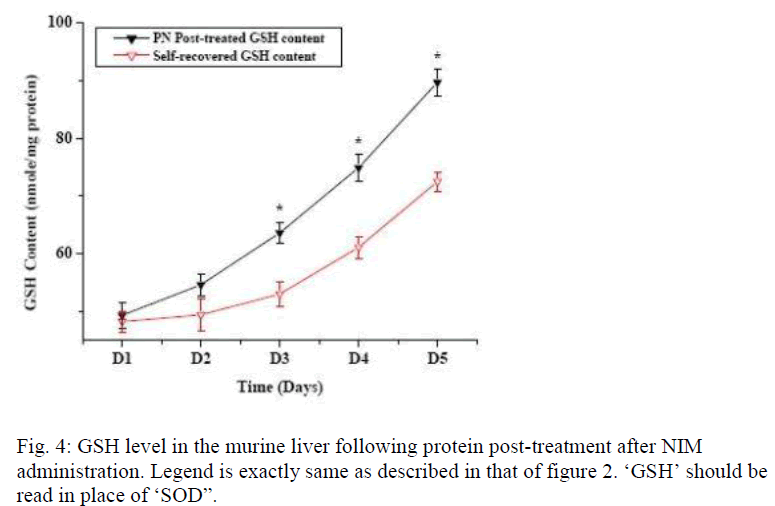
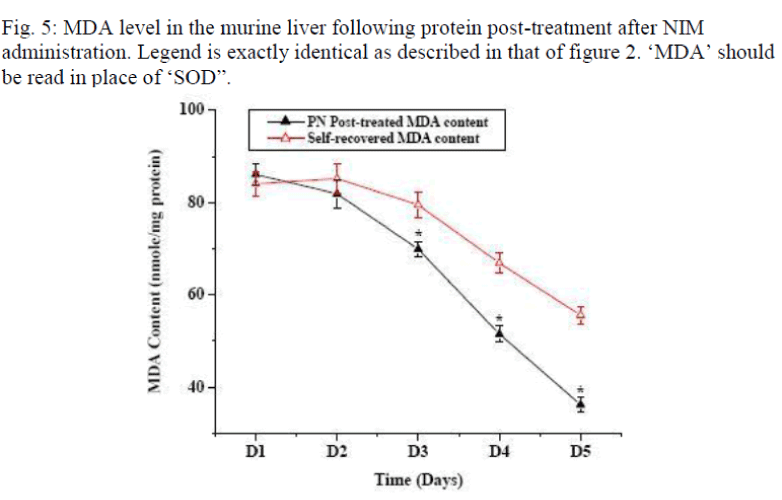
Figure 1 shows the effect of post- treatment of PN, Vit E and BSA on the reduced SOD activity resulted due to NIM induced oxidative stress. Five days treatment of PN post to NIM administration for 7 days significantly enha-nced the SOD level. Similar result was obtained with the antioxidant Vit E although BSA could not show any hepa-to-stimulating effect. As liver possesses the property of regeneration by itself after any drug or toxin-induced damage, we conducted a time dependent study to evaluate the curative effect of PN on NIM induced oxidative stress. Figure 2 shows the SOD activity after NIM in-duced oxidative stress followed by the post-treatment with PN. NIM treatment caused reduction of the SOD activity (369+14.9 unit/mg total protein) compared to the normal (582+13.0 unit/mg total protein) controls. Post-treatment with PN for 1 and 2 days after NIM administra-tion practically did not alter the SOD level. After that, SOD activity started increasing compared to their respec-tive NIM controls. After 5 days of PN treatment, SOD activity came back to almost normal (552.3+15.2 unit/mg total protein) whereas it remained low (468+13.9 unit/mg total protein) in its NIM control group. As shown in figure 3, CAT activity in normal mice liver (124.0+4.0 unit/ mg total protein) was reduced when treated with NIM (56.5+2.9 unit/mg total protein). The CAT activity signi-ficantly elevated when the animals were post-treated with PN for 3 days compared to their respective NIM controls. After 5 days of post-treatment with the PN, the CAT ac-tivity was almost normal (112.7±4.3 unit/mg total protein) whereas for the NIM controls, the activity was still lower (8.73±2.7 unit//mg total protein). Effect of PN on GSH level post to NIM administration has been shown in Fig. 4. NIM caused significant depletion of GSH level (48.8±1.1 unit/mg total protein for NIM treatment vs. 96.9±3.3 unit/mg total protein for normal control). Like SOD and CAT, post- treatment with PN could not enhance the reduced level of GSH for treatment up to 2 days. After that, the enhancement was significant. The normal recovery was; however, lower in all the NIM controls. Effect of PN post-treatment on hepatic lipid peroxidation has been shown in figure 5. MDA level in NIM treated mice liver (85.5+2.4 nmoles/g of tissue) increased almost 2.5 fold than the normal mice liver (35.8+1.3 nmoles/g of tissue). On day 3, the MDA level in PN treated mice liver was lower compared to that level in NIM treated mice liver. Post- treatment with PN for 5 days reduced the MDA level to normal (36.2+1.6 nmoles/g tissue), however, MDA level in NIM treated control liver remained elevated (55.6+1.8 nmoles/g tissue).
Fig. 1: Effect of post-treatment of PN, Vit E and BSA on the NIM-induced reduced SOD activity. Cont : SOD activity in normal mice, NIM : SOD activity in NIM-treated mice, NIM+PN : SOD activity in NIM-treated mice fol-lowed by PN post-treatment for 5 days, NIM Cont : SOD activity in NIM-treated mice after self-recovery, NIM+Vit E : SOD activity in NIM-treated mice followed by Vit E post-treatment, NIM+BSA : SOD activity in NIM-treated mice followed by BSA post-treatment. Each value represents mean + SD (n=8). (P٭<0.05).
Fig. 2: SOD level in the murine liver after NIM administration followed by PN post-treatment. Closed square: SOD levels in PN treated mice for 24, 48, 72, 96 and 120 hrs respectively after NIM administration. Open square: SOD levels in NIM treated mice after 24, 48, 72, 96 and 120 hrs of self-recovery. Each value represents mean + SD (n=8). (P٭<0.05).
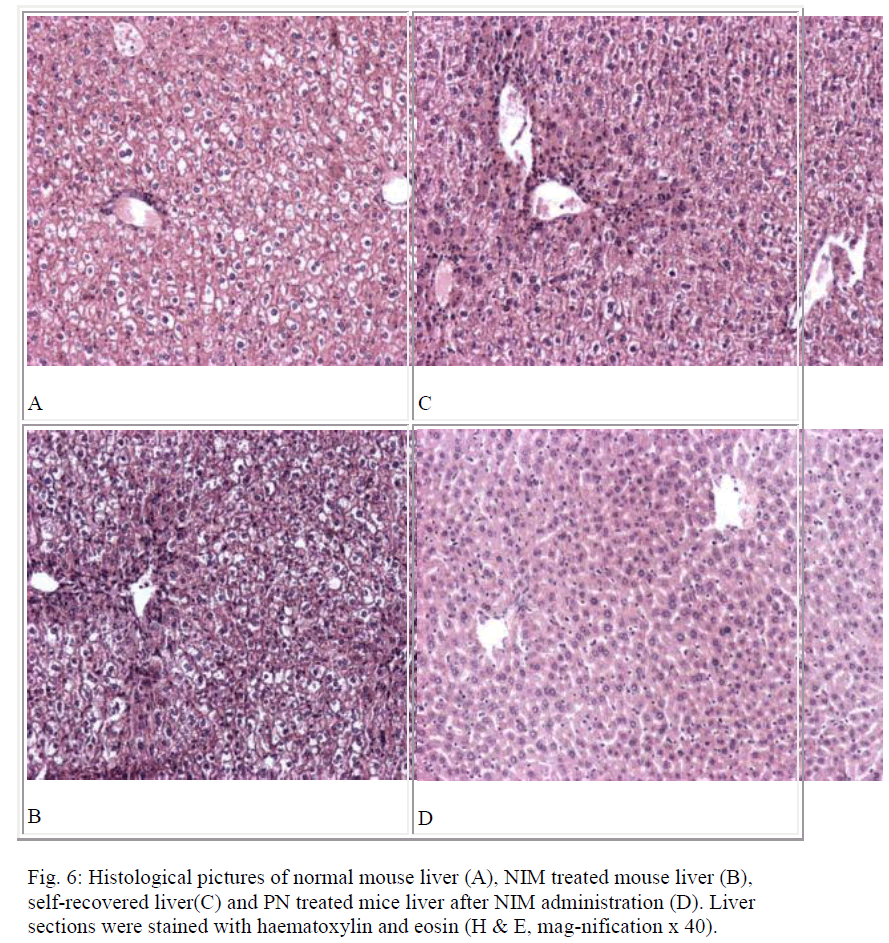
Histological assessment of all the liver sections in the study groups have been represented in figure 6. Figure 6A shows the normal histology of liver. Prominent changes including centrilobular necrosis, bile duct proliferation, disorganization of normal radiating pattern of cell plates around central vein etc. are observed in the hepatic tissues treated with NIM (6B). Qualitatively, the inflammations in PN treated mice liver post to NIM administration were less severe (6D) compared to that obtained after the self-recovery (6C).
Discussion
The aim of this study was to evaluate whether PN possesses any curative role against NIM-induced oxidative stress. We observed that NIM administration at a dose of 10 mg/kg/twice daily for one week significantly reduced the levels of two antioxidant enzymes, SOD and CAT and the non-protein thiol, GSH accompanied by the enhance-ment of lipid peroxidation. Administration of PN (100 mg/kg body weight) to the experimental animals, post to NIM treatment, altered the levels of antioxidant enzymes and GSH near to those present in normal liver along with significant reduction of lipid peroxidation. Histological studies support this result. Administration of BSA had no effect on this oxidative insult although similar hepatoprotective results were obtained when the animals were treated with the antioxidant, vitamin E, post to NIM administration.
Liver is the first organ to metabolize all foreign compounds and hence its injury can be induced by the exposure of various toxicants, and also by a number of drugs when taken very frequently or beyond therapeutic doses. These toxicants mainly damage liver by producing reac-tive oxygen species, ROS [21-22]. To encounter the oxi-dative stress, antioxidant defense mechanism operates in our body to detoxify or scavenge ROS. The antioxidant system comprises different types of functional compo-nents including different antioxidant enzymes, (like SOD and CAT) together with the substances that are capable of reducing ROS or preventing their formation. Both these enzymes work in a coordinated manner to quench the oxygen free radicals produced in our body [23-24]. GSH serves as a scavenger of different free radicals and is one of the major defenses against oxidative stress [25]. Besides, different antioxidants like ascorbic acid, vitamin E etc. are also known scavengers of these ROS [26-27]. Results of our studies indicate that NIM induces oxidative insult and suggest that the antioxidant defense of the body has been suppressed effectively by NIM. Post-treatment studies showed that treatment with PN for two days prac-tically could not alter the levels of CAT, SOD, GSH etc. However, post-treatment for 3, 4 and 5 days significantly reduced the increased MDA level and elevated the decreased levels of the antioxidant enzymes and GSH. PN acted in a time dependent manner and post-treatment for 5 days had the most significant curative effect. Although with the help of its self-recovery mechanism, liver recovered to some extent by its own after NIM -induced oxida-tive stress, but the recovery process was rapid when the mice were post-treated with PN. Liver histology of NIM treated mice showed considerable necrosis along the central vein. The sizes of the hepatocytes were also larger and balloon like compared to those in the normal mice liver. The necrosis was mostly centrilobular and extend-ing through the whole liver lobule (6B). A little improvement has been observed due to self-recovery of liver (6C). On the other hand, the inflammations in the livers of post-treated mice were less necrotic (6D). Combining all, the data suggest that PN possesses curative role against NIM induced oxidative stress in mice.
In conclusion, we would like to mention that the curative role of PN against NIM-induced hepatic disorder is probably due to its antioxidant properties. It is likely that the active ingredients of the herb might be able to subside the side effects of this anti-inflammatory drug. To have a clear picture, further investigation is needed to identify and fully characterize the active principle(s) and is currently in progress.
Acknowledgements
The study was supported in part by the Council of Scientific and Industrial Research, Government of India (Project sanction number : 01/1788/02/EMR-II to P.C.S). We are grateful to Mr. Prasanta Pal for his excellent technical assistance.
References
- de Mejia EG, Ramirez-Mares MV. Leaf extract from Ardisia compressa protects against 1-nitropyrene-induced cytotoxicity and its antioxidant defense disrup-tion in cultured rat hepatocytes. Toxicology 2002; 179: 151-162.
- Iwu MM, Jackson JE, Schuster BG. Medicinal plants in the fight against leishmaniasis. Parasitol. Today 1994; 10: 65-68.
- Chopra RN, Nayar SL, Chopra IC: In: Glossary of Indian Medicinal Plants, Publication and Information Directorate, CSIR, New Delhi, 1986; p. 44.
- Tona L, Ngimbi NP, Tsakala M, Mesia K, Cimanga K, Apers S, De Bruyne T, Pieters L, Totte J, Vlietinck AJ. Antimalarial activity of 20 crude extracts from nine African medicinal plants used in Kinshasa, Congo. J Ethnopharmacol 1999; 68: 193-203.
- Odetola AA, Akojenu SM. Anti-diarrhoeal and gastro-intestinal potentials of the aqueous extract of Phyllanthus amarus (Euphorbiaceae). Afr J Med Sci 2000; 29: 119-122.
- Thyarajan SP, Jayaram S, Villiammai T, Madana-gopalan N, Pal VG, Jayaraman K. Phyllanthus amarus and hepatitis B virus. Lancet 1990; 336: 949-950.
- Thyarajan SP, Subramanian S, Thirunalasundari, T, Venkateswaran, PS, Blumberg BS. Effect of Phyllanthus amarus on chronic carriers of hepatitis B virus. Lancet 1998; 2: 764-766.
- Asha VV, Akhila S, Wills PJ, Subramoniam A. Further studies on the antihepatotoxic activity of Phyllanthus maderaspatensis Linn. J Ethnopharmacol 2004; 92: 67-70.
- Padma P, Setty OH. Protective effect of Phyllanthus against CCl4-induced mitochondrial dysfunction. Life. Sci 1999; 64: 2411-2417.
- Davis R, Brogden RN. Nimesulide. An update of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 1994; 48: 431-454.
- Sbeit W, Krivoy N, Shiller M, Farah R, Cohen HI, Struminger L, Reshef R. Nimesulide-induced acute hepatitis. Ann Pharmacother 2001; 35: 1049-1052.
- Schattner A, Sokolovskaya N, Cohen J. Fatal hepatitis and renal failure during treatment with nimesulide. J Intern Med 2000; 247: 153-155.
- Soni MG, Raniah SK, Mumtaz MM, Clewell H, Mehendale HM. Toxicant inflicted injury and stimulated tissue repair are opposing toxicodynamic forces in predictive toxicology. Reg Pharmacol Toxicol 1999; 19: 165-174.
- Thakore KN, Mehendale HM. Effect of Phenobarbital and mirex pretreatments on carbon tetrachloride auto protection. Toxicologic pathology 1994; 22: 291-299.
- Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 1972; 46: 849-854.
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophys 1984; 21: 130-132.
- Bonaventura J, Schroeder WA, Fang S. Human erythrocyte catalase: an improved method of isolation and a revalution of reported properties. Arch Biochem Biophys 1972; 150: 606-617.
- Ellman GL. Tissue sulphydryl group. Arch Biochem Biophy 1959; 82: 70-77.
- Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol 1990; 186: 407-421.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248-254.
- Slater TF. Free radical mechanism in tissue injury. Biochem J 1984; 222 1-15.
- Kyle ME, Miccadei S, Nakae D, Farber JL. Superoxide dismutase and catalase protect cultured hepatocytes from the cytotoxicity of acetaminophen. Biochim Bio-phys Res Commun 1987; 149: 889-896.
- MacMillan-Crow LA, Crow JP, Thompson JA. Peroxy-nitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry 1998; 37: 1613-1622.
- Munhoz DC, Netto LE. Cytosolic thioredoxin peroxi-dase I and II are important defenses of yeast against organic hydroperoxide insult: catalases and peroxiredox-ins cooperate in the decomposition of H2O2 by yeast. J Biol Chem 2004; 279: 35219-35227.
- Halliwell B, Gutteridge JMC, Free radicals in biology and Medicine, 3rd ed. (Oxford University Press, Oxford) (1999) 237-248.
- Sheweita SA, Abd El-Gabar M. Carbon tetrachloride–induced changes in the activity of phase II drug-metabolizing enzyme in the liver of male rats: role of antioxidants. Toxicology 2001; 165: 217-224.
- Liu SL, Degli Esposti S, Yao T, Diehl AM, Zern MA. Vitamin E therapy of acute CCl4-induced hepatic injury in mice is associated with inhibition of nuclear factor kappa B binding. Hepatology 1995; 22: 1474-1481.