ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2011) Volume 22, Issue 4
Deficiency of Micronutrient Status in Pulmonary Tuberculosis Patients in North India
Irfan Ahmad1, VK Srivastava1, R Prasad2, Mohd. Yusuf3, Safia3, M Saleem1, Wahid Ali3*
1Department of Hospital Administration, CSM Medical University UP, Lucknow, India
2Department of Pulmonary Medicine, CSM Medical University UP, Lucknow, India
3Department of Pathology, CSM Medical University UP, Lucknow, India
- *Corresponding Author:
- Wahid Ali
Department of Pathology/Biochemistry
Chhatrapati Shahuji Maharaj Medical University
(Earlier King Georg’s Medical University)
Lucknow, Uttar Pradesh, India
Accepted date: June 27 2011
Malnutrition is observed frequently in patients with pulmonary tuberculosis (TB), but their micronutrient status, especially of Vitamin A and Zinc, is still poorly documented. The ob-jective of this study was to investigate the micronutrient status of patients with active pul-monary tuberculosis, admitted in the Department of Pulmonary Medicine, CSM Medical University UP, Lucknow. In this case-control study, 43 patients aged 18–55 year with active pulmonary TB were enrolled and blood sample was taken. Cases had clinical and radio-graphic abnormalities consistent with pulmonary TB and at least two sputum specimens showing acid-fast bacilli. Micronutrient status data were collected. Compared with healthy control cases, TB patients had significantly lower concentrations of blood haemoglobin, WBC count, serum albumin, serum retinol and zinc, whereas the concentration of free erythrocyte zinc protoporphyrin concentration, was greater. In conclusion, the micronutri-ent status of patients with active pulmonary TB was poor compared with healthy subjects. Low concentrations of haemoglobin and of serum retinol and zinc were more pronounced in malnourished TB patients.
Key Words
Malnutrition, Micronutrient, Tuberculosis, Vitamin A, Zinc.
Introduction
India is tuberculosis burden country of the world. Tuberculosis is a major barrier to social and economic development and it is one of the most important causes of death in developing countries. Vitamin A deficiency has been found to be associated with many infectious diseases. A high prevalence of vitamin A deficiency has been observed in patient with pulmonary tuberculosis, which is more pronounced in those coinfected with HIV and this indicates an association between vitamin A deficiency and tuberculosis [1].
Hanekom et al found a low plasma Vitamin A levels to be associated with more extensive or severe disease, and low levels of retinol binding protein, prealbumin and albumin [2]. They also found that a high dose of vitamin A supplementation had no effect on the out come of disease. Ramachandran et. al, also found a lower serum vitamin A level in patient with pulmonary tuberculosis [3]. Koyanagi et al, found a lower serum concentration of retinol and zinc in patients with pulmonary tuberculosis as compared with healthy volunteers [4].
These studies have shown that in developing countries patients with tuberculosis have low serum Vitamin A levels. This could be because patients with Vitamin A deficiency have an increased risk of developing tuberculosis or because of development of active tuberculosis which may decrease the plasma Vitamin A levels Getz et al, [5].
Karyadi et. al, in their study in Indonesia observed that the Vitamin A and Zinc supplementation improves the effect of tuberculosis medication which is seen usually after 2 months of antitubercular treatment and results in earlier sputum smear conversion [6]. Vitamin A and zinc supplements given together with antitubercular drugs would increase the efficacy of the anti tubercular treatment. As there is no evidence available on this issue from India, the present study is planned to investigate the micronutrient status.
Subjects and Methods
Subjects
Cases were outpatients with untreated active pulmonary TB admitted to the Department of Pulmonary Medicine, CSM Medical University UP, Lucknow, which is a tertiary care hospital located in north India. Controls were healthy subjects with no history of pulmonary TB, matched with cases for sex and age, and selected randomly from nonfamily neighbours of the patients. One person was selected at random as a control from the list of 3–7 persons proposed. Selection of cases was based on the following criteria: age 18–55 y; at least two sputum specimens positive for acidfast bacilli by microscopy; and clinical and radiographic abnormalities consistent with pulmonary TB. Exclusion criteria for cases and controls were as follows: previous anti-TB treatment, pregnancy, lactation, use of corticosteroids or supplements containing vitamin A, zinc or iron during the previous month, moderate to severe injury or surgery during the last month and diseases such as abnormal liver function as measured by elevated serum levels of aspartate amino transferase (ASAT) and alanine amino transferase (ALAT), diabetes mellitus as measured by elevated fasting blood glucose levels, neoplasm as determined by clinical examination, chronic renal failure as determined by elevated serum levels of urea and creatinine, and congestive heart failure.
Study design
The study was designed as a casecontrol study. The sample size was based on the ability to determine a difference with α = 0.05 and 1-β = 0.95 using a one-tailed test for concentrations of serum retinol and zinc and of blood haemoglobin. Because serum zinc concentration was the variable requiring the largest sample size, we calculated that with a sample size of 35 in each group, a betweengroup difference of 0.46 μmol/L in Zn could be detected [7]. We recruited 43 subjects for each group because we assumed that 25% of patients might not meet the inclusion criteria.
Blood samples (5 ml) were collected from fasting subjects via vein puncture to determine haemoglobin, white blood cell count, erythrocyte sedimentation rate (ESR), serum albumin, serum retinol and zinc concentration. All bio-chemical tests above were carried out on the same day. Haemoglobin concentration and white blood cells were measured directly using an automatic analyzer (Sysmex Microdilutor F-800, Kobe, Japan). ESR was determined directly using the Westergreen technique [8]. Albumin was determined by the bromcresol green method [9]. Serum retinol was measured using RBP4 (human) ELISA Kit (Cat. No. AG-45A-0011EK-KI01) and zinc concentration was measured using simple colorimetric method [10].
Ethical considerations
The study was approved by the Institutional Ethics Committee of CSM Medical University UP, Lucknow, India. Informed consent was obtained from each subject before the start of the study.
Statistical analysis
A one-sample Kolmogorov-Smirnov test was used to check whether data were normally distributed. Mean and standard deviation (SD) are used for reporting normally distributed data, and median and 25th–75th percentiles are used for reporting non-normally distributed data. An independent sample t-test was used to assess the differences between patients and controls for normally distributed parameters, whereas differences in non-normally distributed parameters were tested using the Mann-Whitney test. A multiple stepwise regression analysis was performed to predict concentrations of serum retinol and zinc by using age, sex, body weight, presence of pulmonary cavity, white blood cell count, ESR and serum albumin as independent variables. Differences in prevalence were tested with a chisquare test. The SPSS software was used for all statistical analyses and a P-value 0.05 was considered significant.
Results
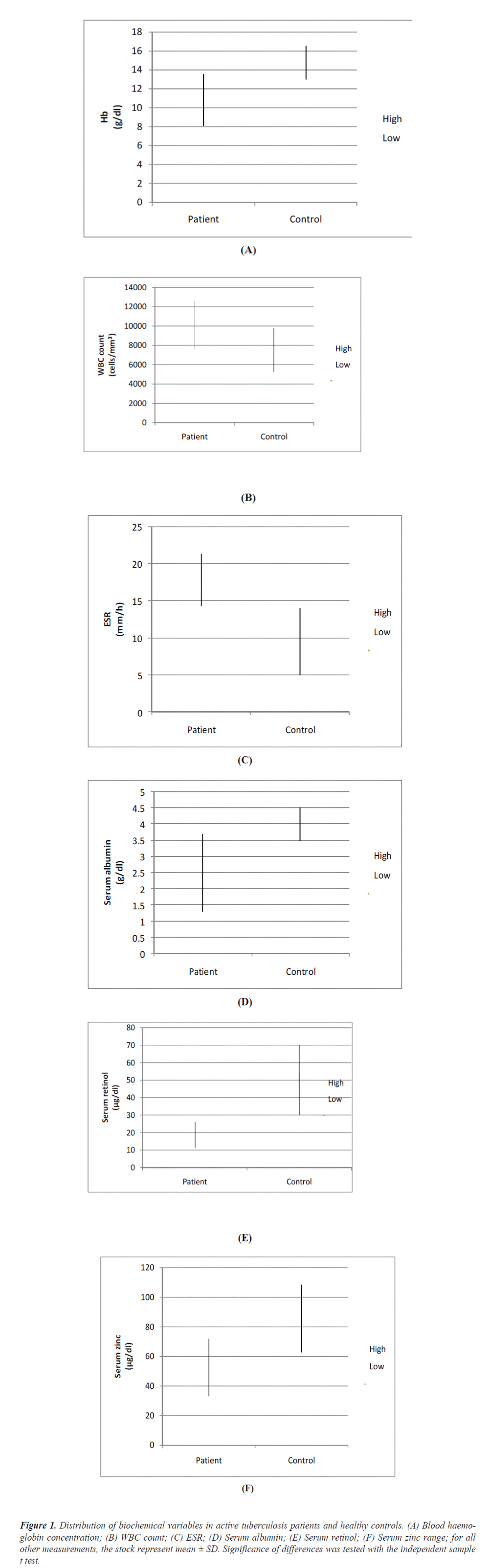
Total 43 pulmonary tuberculosis (27 men & 16 women) age 18 to 55 enrolled for study. All the patients have fever, cough, haemoptysis, chest pain and loss of appetite. Of these cases 59% patients had three sputum smear positive and 41% patients had two sputum smear positive. Concentration of haemoglobin, serum albumin, serum retinol and serum zinc was significantly lower in pulmonary tuberculosis patients rather than in control. Erythrocyte sedimentation rate and WBC count was higher in pulmonary tuberculosis patients rather in control (Table 1).
Figure 1: Distribution of biochemical variables in active tuberculosis patients and healthy controls. (A) Blood haemo-globin concentration; (B) WBC count; (C) ESR; (D) Serum albumin; (E) Serum retinol; (F) Serum zinc range; for all other measurements, the stock represent mean ± SD. Significance of differences was tested with the independent sample t test.
Discussion
In this study we demonstrated that, concentrations of selected micronutrients tested in TB patients were significantly lower than in controls. Low concentrations of haemoglobin and serum retinol and zinc in malnourished patients were more pronounced than in healthy controls and wellnourished patients. Furthermore, the prevalence of low concentrations of vitamin A and zinc was higher in patients than in controls. Low concentrations of serum retinol can be due to a number of factors, including reduced intake or reduced absorption of fat. In addition, the infection itself can compromise vitamin A status in a number of ways. It can increase urinary excretion of vitamin A as has been shown in patients with fever, e.g., due to pneumonia and shigellosis [11, 12]. Finally, low serum retinol levels can also result from increased utilization of retinol by tissues [13]. It is likely that a combination of mechanisms is operative in TB patients.
TB patients had significantly lower weight and serum albumin concentration than healthy controls. As a result, serum albumin concentration in malnourished patients was lower than that in wellnourished healthy controls, wellnourished patients and malnourished healthy controls. The poorer nutritional status of patients with pulmonary TB may be due to anorexia [14], impaired absorption of nutrients or increased catabolism. On the other hand, patients and controls may have similar food habits and food intakes because their socioeconomic background and living conditions are similar. Thus, infectious disease such as TB may led to impaired absorption and increased rates of metabolism [15, 16]. The disease induced production of cytokines such as interleukin-6 and tumour necrosis factor-a may induce fever, hepatic synthesis of acute phase reactant proteins, inhibit production of serum albumin and cause dramatic shifts in plasma concentration of certain essential micronutrients [17].
Conclusion
This study shows that the nutritional status of patients with active pulmonary TB was poor compared with healthy controls. The prevalence of low concentrations of serum retinol and zinc was significantly higher in patients than in controls. The low concentrations of haemoglobin and serum retinol and zinc were more pronounced in malnourished TB patients. Further studies are required to establish the role of these low concentrations in host defence against TB.
References
- Wiid I, Seaman T, Hoal EG, Benade AJ, Van helden PD. Total antioxidant levels are low during active TB and rise with anti-tuberculosis therapy. IUBMB Life. 2004; 56: 101-106.
- Hanekom WA, Potgieter S, Hughes EJ, Malan H, Kessow G, Hussey GD. Vitamin A status and theraphy in childhood pulmonary tuberculosis. J Pediater. 1997; 131: 1130-1133.
- Ramachandran G, Santha T, Garg R, Baskaran D, lliayas SA, Venkatesan P. Vitamin A levels in sputumpositive pulmonary tuberculosis patients in comparison with household contacts and healthy ‘normals’. Int J Tberc Lung Dis 2004; 8: 1130-1133.
- Koyanagi A, Kuffo D, Gresely L, Shenkin A, Cuevas
- LE. Relationships between serum concentrations of
- C-reactive protein and micronutrients, in patients with tuberculosis. Ann Trop Med Parasitol. 2004; 98: 391-399.
- Getz HR, Long ER, Hendrson HJ. A study of the relation of nutrition to the development of tuberculosis: In-fluence of ascorbic acid and vitamin A. Am Rev tuberc. 1951; 64: 381-393.
- Karyadi et al; A double-blind, placebo-controlled study of vitamin A and zinc supplementation in persons with tuberculosis in Indonesia: effects on clinical response and nutritional status. Am J Clin Nutr 2002; 75: 720-727.
- Narang A.P.S., Whig J. And Mahajan R. Serum copper and zinc levels in patients with pulmonary tuberculosis. Trace Elem. Electrolytes 1995; 12: 74-75. 8.
- Kohli RN, Singh S, Singh M. Studies on erythrocyte sedimentation rate in buffaloes. I. Evaluation of various techniques. Indian Vet J 1975; 52:12, 915-918.
- Dumas BT, Watson WA, Biggs HG. Albumin stan-dards and the measurement of serum albumin with bromcresol green. Clin Chim Acta 1997; 25: 821-830.
- Arroyave G, Chichester CO, Flores H. Biochemical methodology for the assessment of vitamin A status: a report of the International Vitamin A Consultative Group. Washington, DC: Nutrition Foundation, 1982.
- Mitra AK, Alvarez JO, Guay WL, Fuchs GJ,Wahed MA, Stephensen CB. Urinary retinol excretion and kidney function in children with shigellosis. Am J Clin Nutr 1998; 68: 1095-1003.
- Stephensen CB, Alvarez JO, Kohatsu J, Hardmeier R, Kennedy JI, Gammon RB. Vitamin A is excreated in the urine during acute infection. Am J Clin Nutr. 1994; 60: 388-392.
- Fleck A, Myers MA. Diagnostic and prognostic signifi-cance of the acute phase proteins. In: The Acute Phase Response to Injury and Infection. Elsevier, Amsterdam, The Netherlands. (Gordon, A. H. & Koj, A., eds.), pp. 1985; 249-271.
- Hopewell, P. C. Overview of clinical tuberculosis. In: Tuberculosis: Pathogenesis, Protection and Control (Bloom, B.R., ed.), ASM Press, Washington, DC. 1994; pp. 25–36.
- Ginzburg V. S. and Dadamukhamedov A. A. Absorption of nutrients in patients with pulmonary tuberculosis. Probl. Tuberk 1990; 0: 44-46 (abs.).
- Ulijaszek S. Transdisciplinarity in the study of under-nutrition-infection interactions. Coll. Antropol. 1997; 21: 3-15.
- Beisel, W. R. Metabolic responses of the host to infec-tion. In: Textbook of Pediatric Diseases (Feigin R.D. & Cherry J.D., eds.). Saunders, Philadelphia, PA.1998; pp. 54-69. W. B.