ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2018) Volume 29, Issue 16
Delineating antidiabetic proficiency of catechin from Withania somnifera and its Inhibitory action on dipeptidyl peptidase-4 (DPP-4)
Praveen Kumar Kempegowda1, Farhan Zameer2# and Satish Kumar Murari3,4*#
1Department of Biochemistry, Bharathiar University, Coimbatore, Tamil Nadu, India
2Department of Biochemistry, School of Basic and Applied Sciences, Dayananda Sagar University, Shavige Malleshwara Hills, Kumaraswamy Layout, Bangalore, Karnataka, India
3Department of Chemistry, P.E.S. College of Engineering, Mandya, Karnataka, India
4PET Research Foundation, University of Mysore, Mandya, Karnataka, India
#These authors contributed equally to this work
- *Corresponding Author:
- Satish Kumar Murari
Department of Chemistry
P.E.S. College of Engineering, India
Accepted on August 9, 2018
DOI: 10.4066/biomedicalresearch.29-18-922
Visit for more related articles at Biomedical ResearchDipeptidyl peptidase-4 (DPP-4) is a transmembrane serine aminopeptidase which cleaves X-proline dipeptides which degrades incretin hormones in turn stimulating insulin release with reduced GLP-1 secretion in type 2 diabetes. In this study, maximum inhibitory activity of DPP-4 was established with 100% methanol extract of Withania somnifera (WS) roots and the phytobioactives were further characterized. The active compound responsible for the inhibition of DPP-4 was found to be catechin, which was confirmed by phytobioanalytical studies. STC-1 cells exhibited increased GLP-1 concentration which was evident by dosimetry. The active compound responsible for the inhibition of DPP-4 was found to be catechin which was evident from fluorescence spectroscopy. While CD spectra indicated that catechin binds with the α-helix of the enzyme, resulting in structural and conformational changes in secondary structure at 208 and 222 nm. Further, docking studies exhibited binding affinity of catechin with DPP-4 and was found to be -6.601 kcal/mol as compared to standard potent blockers, further the interaction of catechin with other biotargets confirmed its antidiabetic potency. The current study supports synthetic-pharmacological knowledge for innovation in discovery engine for newer medicines implicating the validation DPP-4 as potent drug target for type 2 diabetes mellitus.
Keywords
Secretin tumor cell line (STC-1 cells), Dipeptidyl peptidase-4 (DPP-4), Fluorescence spectroscopy (FS), Circular dichroism (CD), Computational chemistry.
Introduction
Plants have been the major source of drugs for the treatment of many diseases. World ethno botanical information about medicinal plants reports that almost 800 plants could be used to control DM. Medicinal plants are being used currently in India and world for the treatment of diabetes and their efficacy has been proved scientifically by in vitro and in vivo methods. It has been proved that by in vitro methods, some plant extracts inhibited the enzymes which like increase blood glucose levels i.e., -amylase, -glucosidase and DPP-4 enzymes [1-4]. Now a days the incretin therapies adopted a new treatment option for patients with type 2 diabetes mellitus. So incretin therapies focus on the increasing levels of the two incretin hormones, glucogan like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). These incretin hormones triggers insulin secretion and modulate the pancreatic islet hormone secretion and glucose homeostasis [5]. DPP-4 (EC 3.4.14.5) is mainly responsible for inactivation of incretin hormone GLP-1 by proteolytic degradation, hence DPP-4 has emerged as a promising target for type 2 diabetes mellitus. An inhibitor of DPP-4 might lower blood sugar level by increasing the level of active GLP-1 [6]. Patients with type 2 diabetes mellitus frequently require multifactorial pharmacological medicines because of medication associations can be isolated into pharmacokinetic and pharmacodynamics communications. A key process in the development of new drugs is the elucidation of the nature of the interaction between the drug molecule and the target enzyme. Such knowledge then makes it possible to make systematic structure modifications of the drug molecule to optimize the interaction. There are a variety of techniques currently available for obtaining information about drug-protein interactions such as the measurement of kinetics and binding affinities through the analytical interactions studies between drugs and antidiabetic targets by fluorescence spectroscopy (FS), circular dichroism (CD) measurement and molecular docking study [7,8].
Withania somnifera (L.) Dunal (WS), commonly known as Ashwagandha, is a xerophytic plant belonging to the family Solanaceae. It is one of the most recommended plants of the traditional Indian systems of medicines and is used in more than 100 formulations of Ayurveda, Unani and Sidha [9]. WS plant is cultivated for medicinal purpose in the drier region of India, such as Karnataka, Rajasthan, Punjab, Gujarat and other countries like Sri Lanka, Afghanistan and Pakistan [10]. WS is known as winter cherry in English, Aswagandha in Sanskrit, Hire maddinagida in Kannada, Amukkira in Tamil [11,12]. The dried powder of the roots, leaves and berries of WS contains several groups of chemical constituents such as alkaloids, steroidal lactones, flavonoids, tannins, saponins, somniferins etc. [13,14]. Several studies indicated that WS possesses antioxidant, antitumor, antistress, antiinflamatory, antiageing, cardiovascular, antidiabetic, hypocholestrolemic, antidepressive and neurotransmitter properties. WS leaves are used in Ayurvedic and Unani systems for treatment of tumors and tubercular glands and WS roots are prescribed as medicines for bronchitis, rheumatism, dropsy, lung inflammation, skin diseases and sexual disorders in males [15,16]. Udayakumar et al. have reviewed the traditional uses and antidiabetic activities of WS. Results infer that flavonoid compounds present in leaves and roots are responsible for hypoglycaemic and hypolipidaemic activity [4] and high catechin concentrations detected in W. somnifera have been reported [17].
The intestinal secretin tumor cell line (STC-1 cells) express and secrete a wide range of gut hormones known for their roles in metabolism, feeding and satiety, including glucose dependent insulinotropic polypeptide (GIP), cholecystokinin (CCK), peptide YY (PYY) and the proglucagon derived peptides (glucogon-like peptide-1 (GLP-1), glucogon-like peptide-2 (GLP-2) and oxyntomodulin) [18,19]. These hormones are present throughout the intestinal tract. Therapeutic strategies are currently available involving the GLP-1 pathway in the treatment is associated with enhancing the half-life of GLP-1 by inhibiting the enzyme responsible for its inactivation, the DPP-4 enzyme [20]. Incretin hormones such as glucagon-like peptide-1 (GLP-1, from L-cells) that act on pancreatic β-cells to stimulate the release of insulin. The specific factors that regulate GLP-1 secretion is similar to regulate insulin secretion by β-cells and certain properties of STC-1 cells, including insulin processing, glucose sensitivity and a regulated secretion pathway [21]. To support our above hypothesis and to get insights on the interaction, bioinformatic tools were explored to understand the structure activity relationship (SAR) using molecular docking studies. Patch Dock software package was used to identify the interaction between ligand and the protein. The binding affinity was predicted using atomic contact energy (ACE) or glide values. In Kempegowda et al. confirmed that antidiabetic potency with binding affinity of catechin with DPP-4 was very similar to the standard potent blockers sitoindoside IV, withaferin A, cuscohygrine, withaferin A, scopoletin, tropine [22].
The previous work in our laboratory has shown that catechin, isolated from methanol extract of Withania somnifera (WS) have significant in vitro antidiabetic activity [22]. I e., catechin as a DPP-4 inhibitory agent, also described about enzyme kinetics, phytochemical analysis, RP-HPLC, spectral studies and structure activity relationship by in-silco models. However, this study strongly evidenced the ethnopharmocological effect of WS plant extract and its active compound catechin. Therefore the present study we were extended for catechin from methanolic extract of WS and it’s in vitro DPP-4 inhibition using spectroscopic interaction studies, selective downstream pathway that leads to DPP-4 enzyme mediated GLP-1 secretion and along with their interactions using molecular docking methods on key biotargets (DPP-4 enzyme, glucagon-like peptide-1 (GLP-1), aspartate aminotransferase (AST), glycogen synthase (GS) and lactate dehydrogenase (LDH) and potent inhibitors of sitagliptin, vildagliptin and diprotin-A.
Materials and Methods
Tris-HCl buffer, HPLC grade solvents, dipeptidyl peptidase-4, DMEM media, DMSO, trypsin-EDTA, STC-1 cells, diprotin-A (Ile-Pro-Ile), Gly-pro-p-nitroanilide (GPPN), all other chemicals were of analytical grade.
STC-1 culture and ELISA in vitro assay
STC-1 cells were purchased from ATCC (CRL-3254TM, USA) and cultured in supplemented Dulbecco's Modified Eagle's Medium DMEM (DMEM media contain 4.5 g/L glucose, without sodium pyruvate) medium with 10% FBS (fetal bovine serum), 100 U/ml penicillin, and 100 μg/L streptomycin incubated at 37°C in a humidified atmosphere containing 5% CO2. STC-1 cells were subcultured by trypsinization with 0.25% (w/v) trypsin-EDTA when reaching 70% to 80% of cell confluence was attained in the cells between 15 and 20 passages were used.100 μL of a 5 × 105 cells/ml suspension in DMEM (1 g/L glucose) were incubated overnight in each well of an ELISA 96-well plate. The samples were dissolved in DMEM medium (1 g/L glucose, 0.1% fat free BSA, 2% DMSO). Test sample (catechin) was diluted in dose dependent manner (15, 30, 60 and 120 μg/ml) and standard sample were added to the corresponding cells in order to obtain the desired final concentration of the treatment and was covered with adhesive strips for next 2 h of incubation. The plates were then washed twice and 100 μL of the biotin-conjugated antibody (Thermo Fisher Scientific, Malaysia) was added and incubated for 2 h at room temperature. The plate was then washed twice and 100 μL of streptavidin-horseradish peroxidase (SA-HRP) was added which was followed by incubation for an hour at room temperature. Subsequently, SA-HRP (Thermo Fisher Scientific, Malaysia) was removed by washing the plate twice, and the TMB substrate (3, 3’, 5, 5’-tetramethylbenzidine) was added. After 20 min, the reaction was stopped by the addition of 2 N HCl. The GLP-1 levels in the supernatant absorbance were measured at 450 nm on a microplate reader (Bio-Rad, Hercules, CA, USA) [23].
Western blotting
A native polyacrylamide gel electrophoresis (native-PAGE) was prepared as modified method of Chi et al. [24]. The total proteins from the cells were separated on the gel using Withania somnifera catechin (100, 75, 50, 25 μM) and then transferred onto a nitrocellulose membrane (Whatmann) using a semi-dry system as mentioned in our earlier publication [22]. The presence of GLP-1 was detected by incubation of the membrane with a 1:1000 dilution of the primary antibody, a mouse monoclonal antibody against GLP-1 (Abcam), followed by incubation in a 1:10,000 dilution of the secondary antibody, a rabbit polyclonal antibody against mouse IgG conjugated to alkaline phosphatase (Abcam). Finally, proteins were visualized (Alliance 2.7, Uvitec) using a BCIP-NBT kit (Sigma-Aldrich). The 5-bromo-4-chloro-3'-indolyphosphate (BCIP) and the nitro-blue tetrazolium (NBT) react with alkaline phosphatase to produce a purple compound which indicates the presence of protein [25].
Fluorescence spectroscopy
Fluorescence measurement of DPP-4 enzyme was carried out in the presence and absence of catechin, which was isolated from WS using Horiba Jobin Yvon spectrofluorometer-3 at 37°C in 1.0 cm path length quartz cuvette with 20 mM Tris- HCl buffer at pH 8.4 [7]. Briefly the DPP-4 was titrated with successive addition of catechin (0 to 36 μM) at different time intervals (10, 20 and 30 min) at 3 nm excitation and the emission spectra were recorded from 280 to 440 nm. Concentration of catechin compound and DPP-4 enzyme used were same as kinetic method, the intensities of emission maxima was considered for occupation of binding sites from fluorescence spectra.
Circular dichroism (CD) spectroscopy
Structural changes of DPP-4 in presence of WS purified catechin was carried out by CD spectra at 37°C on a Jasco-810 automatic spectropolarimeter with modification [8]. Briefly 0.1 cm cell length at room temperature, the concentration of DPP-4 was 20 mM in Tris-HCl buffer at pH 8.4. Five sets of samples were prepared by adding DPP-4 and WS purified catechin, in the following proportions 1:0, 1:4 and 1:8 respectively. Using quartz cell of 2 mm path length the spectra was recorded in the range of 190 to 300 nm with a scan speed of 50 nm/min and the response time was maintained at 4 s. The mean residual ellipticity (MRE) in deg cm2 dmol-1 can be estimated using equation and each spectrum reported is an average of three successive scans.
In-silico data set preparation and virtual screening studies
Catechin isolated from WS and top three potent inhibitors namely diprotin-A (Pub chem. CID-94701), vildagliptin (Pub chem. CID-6918537) and sitagliptin (Pub chem. CID-4369359) with that of the target accession no DPP-4 enzyme: PDB-1NU6, glucagon-like peptide-1 (GLP-1): PDB-3IOL along with key biomarkers with pivotal function in regulation of diabetes i.e., aspartate aminotransferase (AST): PDB-3WZF, glycogen synthase (GS): DB-1IO9 and lactate dehydrogenase (LDH): PDB-4OJN respectively were identified and selected for computational chemistry study. 3-Dimensional (3D) structures of the target were obtained from Protein Data Bank (PDB) and were analysed and visualized through Py-MOL viewer. Co-crystallized ligands were identified and removed from the target then water molecules removed and H atoms were added to the structure and minimizations were performed using Swiss pdb viewer and gasteiger atomic partial charges were computed. Geometry optimization of all the interactions were performed using chimera for flexible conformations of the ligand during the virtual docking screening [22].
Results
ELISA in vitro assay and Western blotting
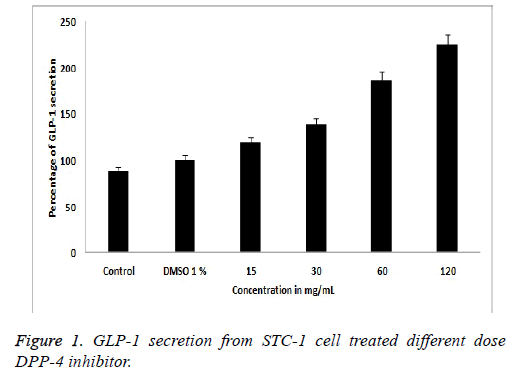
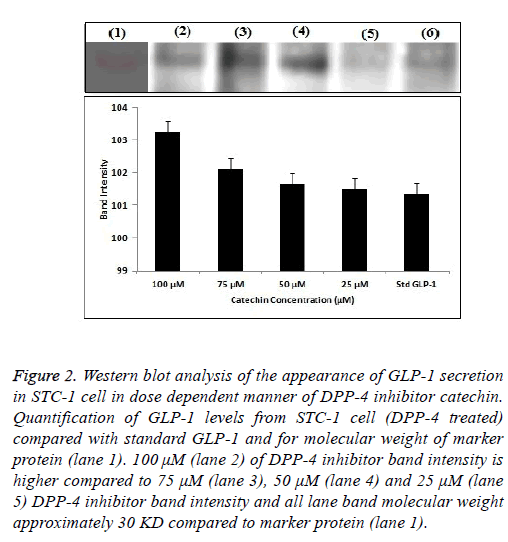
Exposer of STC-1 cells to catechine at the different concentration (15, 30, 60 and 120 μg/ml) caused an increasing the GLP-1 secretion in DPP-4 inhibitor compounds. Results are expressed as a percentage of the GLP-1 content (pM) secreted by the treated cells compared to the basal GLP-1 content (pM) released from the cells treated with DMSO 1%. 120 μg/ml test compounds increased the release of GLP-1 by 119.23% ± 2.5%, 138.18% ± 3.0%, 186.22% ± 3.0%, and 225.1% ± 3.0% respectively, when compared to the control in Figure 1. The total GLP-1 proteins extracted from STC-1 cell were analysed by Western blotting. As observed in Figure 2, different concentration (100, 75, 50, 25 μM) of test samples, standard compound and marker protein confirmed by analysis of the size and structure of the protein secreted [22]. SDSPAGE gel were transferred onto a nitrocellulose membrane, exposer of STC-1 culture to different concentration of Withania somnifera catechin sample were evaluated by quantifying band intensities on dose dependent blots (Figure 2) comparing to molecular protein bands and standard GLP-1 bands it shows GLP-1 secretion is increases slightly with addition of Withania somnifera catechin compound.
Figure 2: Western blot analysis of the appearance of GLP-1 secretion in STC-1 cell in dose dependent manner of DPP-4 inhibitor catechin. Quantification of GLP-1 levels from STC-1 cell (DPP-4 treated) compared with standard GLP-1 and for molecular weight of marker protein (lane 1). 100 μM (lane 2) of DPP-4 inhibitor band intensity is higher compared to 75 μM (lane 3), 50 μM (lane 4) and 25 μM (lane 5) DPP-4 inhibitor band intensity and all lane band molecular weight approximately 30 KD compared to marker protein (lane 1).
Fluorescence spectroscopy
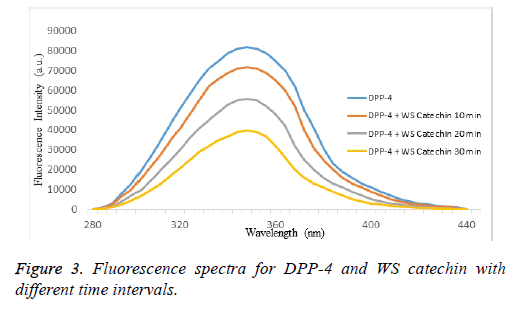
The fluorescence emission spectra of DPP-4 in absence and presence of WS purified catechin compound. The fluorescence intensity of DPP-4 decreases with successive addition of WS purified catechin in 20 mM Tris-HCl buffer at pH 8.4 indicating that WS purified catechin can quench the intrinsic fluorescence of DPP-4 enzyme. Generally, strong intrinsic UVfluorescence from DPP-4 is attributed to the presence of aromatic amino acid residue at various domains like catalytic site and calcium binding site of the enzyme (Figure 3). Thus quenching of intrinsic fluorescence signifies the direct interaction of WS purified catechin compound with aromatic amino acid residues present in DPP-4 domains. Figure 3 showed decay in the fluorescence intensity of DPP-4 enzyme in the presence of WS purified catechin compound at different time intervals (10, 20 and 30 min). DPP-4 and catechin binding activity were shown in the Figure 4 at 30 min incubated sample showed maximum binding activity when compared to 10th and 20th min. This study reveals that the presence of catechin inhibited the activity of DPP-4 at aromatic amino acid residues.
Circular dichroism spectroscopy
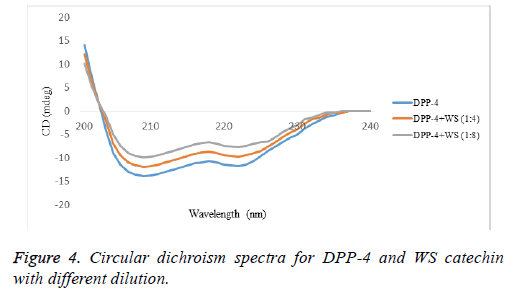
CD studies identified the nature of interaction of DPP-4 with catechin (Figure 4). Detailed information about the secondary structure of enzyme in presence and in absence of catechin, two peaks of DPP-4 at 208 and 222 nm, are attributed to a high α-helical content of enzyme. Alteration in the conformational changes of DPP-4 has reflected in CD spectra, diminished minimum. The diminish in negative humped peaks at 208 nm and 222 nm in presence of catechin compound in the respective proportions 1:0, 1:4 and 1:8, were observed when compared with the control (Figure 4). This observation exhibited a possible binding interaction of catechin with the α-helix of DPP-4 enzyme, resulting in the conformational change in secondary structure of the enzyme.
In-silico data set preparation and virtual screening studies
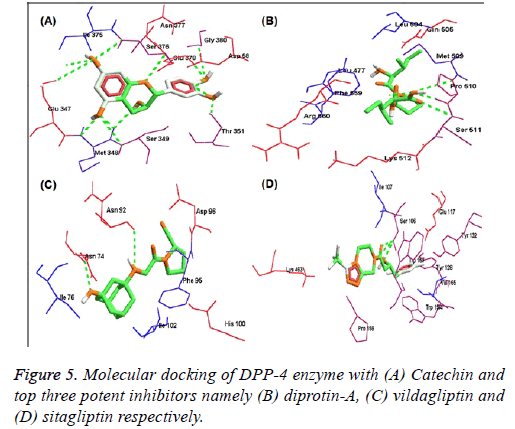
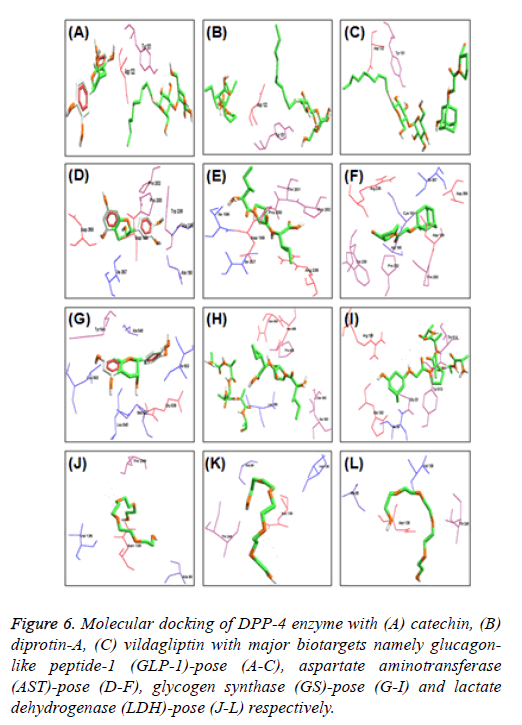
Further, molecular docking of DPP-4 with catechin and potent inhibitors revealed the efficacy of binding is high when compared to sitagliptin and is moderate when compared to diprotin-A and vildagliptin respectively as indicated with the docking pose (Figure 5) and their hydrogen bonding with atomic contact energy gliding values as indicated (Table 1) along with their respective docking domain sites. Catechin from WS was found to be an active inhibitor of DPP-4 protein with binding energy -6.60 kcal/mol. Total 13 hydrogen bond interactions, among them 6 interactions were strongly bound at amino acid residues of Ser 349, Met 348, Glu 347, Asn 377 Ile 375 and Asp 588. However, the same residues were also seen in the standard inhibitor ligands Met and Ser in diprotin-A with binding energy -8.504 kcal/mol and marketly available drugs contain Glu and Ser in sitagliptin and Asn, Ile, Asp vildagliptin binding energy -4.456 and -8.570 kcal/mol respectively (Table 1). Further, to extrapolate the hypothesis, molecular interaction studies were undertaken on key enzymes involved in glucose homeostasis namely glucagon-like peptide-1 (GLP-1), aspartate aminotransferase (AST), glycogen synthase (GS) and lactate dehydrogenase (LDH). The results are indicated with the docking pose (Figure 6) and their hydrogen bonding with atomic contact energy gliding values as indicated (Table 2) along with their respective docking domain sites.
Figure 6: Molecular docking of DPP-4 enzyme with (A) catechin, (B) diprotin-A, (C) vildagliptin with major biotargets namely glucagonlike peptide-1 (GLP-1)-pose (A-C), aspartate aminotransferase (AST)-pose (D-F), glycogen synthase (GS)-pose (G-I) and lactate dehydrogenase (LDH)-pose (J-L) respectively.
| Name of the biomarker (receptor) | Name of the compound (inhibitor) | Details of H-bond interaction | Glide score (kcal/mol) | Amino acid residues of docked domains | ||
|---|---|---|---|---|---|---|
| No. of bonds | Bond energy | Bond length | ||||
| DPP-4 enzyme | Catechin | 13 | -2.5 | 3.01254 | -6.601* | Glu 347, Met 348, Ser 349, Thr 351, Ile 375, Ser 376, Asn 377, Glu 378, Gly 380 and Asp 588. |
| 2.2513 | 2.92854 | |||||
| 3.95106 | 2.64807 | |||||
| 0.657809 | 3.19146 | |||||
| -1.28608 | 3.34278 | |||||
| -0.03535 | 3.32651 | |||||
| -0.5643 | 3.48714 | |||||
| -0.24522 | 3.48392 | |||||
| -0.92637 | 2.41116 | |||||
| -1.28438 | 3.34312 | |||||
| -2.5 | 2.99273 | |||||
| 2.67393 | 1.9925 | |||||
| -2.5 | 3.09999 | |||||
| Diprotin-A | 4 | -2.62746 | 1.86647 | -8.504** | Leu 477, Leu 504, Gln 505, Met 509, Pro 510, Ser 511, Lys 512, Phe 559, Arg 560. | |
| -0.02668 | 2.59162 | |||||
| -1.14789 | 2.1106 | |||||
| -1.05361 | 1.42643 | |||||
| Vildagliptin | 2 | -0.70421 | 2.33067 | -8.750*** | Asn 74, Ile 76, Asn 92, Phe 95, Asp 96, His 100, Ile 102. | |
| -2.5 | 1.68007 | |||||
| Sitagliptin | 3 | -1.00925 | 2.39815 | -4.456 | Ser 106, Ile 107, Glu 117, Tyr 128, Tyr 132, Trp 154, Val 155, Trp 157, Pro 159, Lys 463. | |
| 3.44894 | 1.78837 | |||||
| -0.685 | 2.463 | |||||
Note: *indicates level of significance in its ascending order.
Table 1. Structure-activity relationship (SAR) and binding affinity of DPP-4 enzyme with catechin and top three potent inhibitors namely diprotin- A, vildagliptin and sitagliptin respectively.
| Name of the biomarker (receptor) | Name of the compound (inhibitor) | Details of H-bond interaction | Glide score (kcal/mol) | Amino acid residues of docked domains | ||
|---|---|---|---|---|---|---|
| No. of bonds | Bond energy | Bond length | ||||
| Glucagon-like peptide-1 (GLP-1) | Catechin | 1 | -2.5 | 2.81381 | -3.3846 | Tyr 101, Asp 122 |
| Diprotin A | 1 | -2.5 | 2.81381 | -2.684 | Tyr 101, Asp 122 | |
| Vildagliptin | 1 | -2.5 | 2.81381 | -4.826 | Tyr 101, Asp 122 | |
| Aspartate aminotransferase (AST) | Catechin | 4 | -2.0127 | 2.06853 | -4.1264 | Ala 190, Asp 199, Pro 200, Pro 202, Trp 238, Ala 239, Asp 355, Ile 357 |
| -0.5956 | 3.07753 | |||||
| -1.9742 | 3.20515 | |||||
| -0.0851 | 3.58286 | |||||
| Diprotin A | 2 | -1.5113 | 3.13999 | -4.5594 | Ile 198, Asp 199, Pro 200, Thr 201, Pro 202, Arg 235, Ile 357 | |
| -0.8462 | 2.78558 | |||||
| Vildagliptin | 0 | 0 | 0 | -5.1108 | Ala 190, Cys 191, Asp 199, Pro 200, Pro 202, Arg 235, Trp 238, Asp 355, Ile 357 | |
| Glycogen synthase (GS) | Catechin | 3 | -0.6757 | 3.40795 | -5.2198 | Met 537, Glu 538, Leu 540, Ala 546, Tyr 549, Ile 553, Leu 593 |
| 0.18052 | 2.27924 | |||||
| -0.189 | 3.39112 | |||||
| Diprotin A | 3 | -2.5 | 3.03104 | -3.094 | Ser 363, Leu 369, Pro 486, Ser 363, Asn 484, Asn 485 | |
| -0.2905 | 2.75579 | |||||
| -1.9904 | 2.53885 | |||||
| Vildagliptin | 5 | -2.5 | 2.76233 | -3.208 | Gly 23, Ile 24, His 193, Arg 199, Tyr 513, Thr 514 | |
| -2.1796 | 3.16407 | |||||
| -2.0134 | 3.19731 | |||||
| 1.5062 | 2.12678 | |||||
| -2.5 | 2.7413 | |||||
| Lactate dehydrogenase (LDH) | Catechin | 3 | -1.2086 | 3.35827 | -1.515 | Ala 98, Val 136, Asn 138, Thr 248 |
| -0.5485 | 3.4903 | |||||
| -0.2609 | 3.54782 | |||||
| Diprotin A | 3 | -1.2086 | 3.35867 | -2.7126 | Ala 98, Val 136, Asn 138, Thr 248 | |
| -0.5485 | 3.4903 | |||||
| -0.2609 | 3.54782 | |||||
| Vildagliptin | 3 | -1.2086 | 3.35867 | -2.5734 | Ala 98, Val 136, Asn 138, Thr 248 | |
| -0.5485 | 3.4903 | |||||
| -0.2609 | 3.54782 | |||||
Table 2. Structure-activity relationship (SAR) and binding affinity of glucagon-like peptide-1 (GLP-1), aspartate aminotransferase (AST), glycogen synthase (GS) and lactate dehydrogenase (LDH) with catechin, diprotin-A and vildagliptin respectively.
Discussion
The flavonoid rich WS root methanol extract resulted in the significant reduction of peak level of sugar and increase insulin level within a certain time this fact further strengthens the antihyperglycemic potentiality of WS flavonoids as reported by Udaykumar et al. [4]. Modern studies have found that catechin is responsible for antioxidant activity, antimicrobial activity, anti-ageing and anti-diabetic properties [26,27].
STC-1 cells are routinely used in screening platforms to identifying a specific target molecule for compound to reveal a selective downstream pathway that leads to DPP-4 enzyme mediated GLP-1 secretion. GLP-1 is an incretin hormone that potentiates insulin secretion in a glucose-dependent manner through the inhibition of DPP-4 enzyme would be one of the potential therapeutic targets for type 2 diabetes. In this study, our result suggests that DPP-4 inhibitor WS root catechin stimulating the GLP-1 secretion from STC-1 cell line based on ELISA assay and western blotting. Figure 1 showed that DPP-4 inhibitor catechin compound acts an antidiabetic property through activation of GLP-1 peptide. ELISA assay confirmed that DPP-4 inhibitor catechin compound stimulate the GLP-1 secretion from STC-1 cell at the percentage of 225.1% ± 3.0% when compared to the other dilution of DPP-4 inhibitor. We were able to correlate DPP-4 inhibitor catechin compound and STC-1 cell to reveal the effects of GLP-1 production detected by western blot analysis. Figure 2 showed that STC-1 cell, DPP-4 inhibitor catechin treatment could be regulated by GLP-1 protein expression compared to the control group and standard marker protein without DPP-4 inhibitor treatment at 100 μM compound (Figure 2-lane 2) led to the overexpression secretion of GLP-1 protein when compared to the other doses respectively. Henceforth, we suggest the catechin compound acts as a DPP-4 inhibitor and downstream cascading pathway also increased the GLP-1 protein secretion.
Fluorescence spectrometry has been widely used to study interactions between polyphenols and proteins. The fluorescence emission spectral characteristics change when their tryptophan, tyrosine and phenylalanine aromatic residues are exposed to different solvent conditions. From the past decades, studies of polyphenol-protein interactions were performed by fluorescence quenching methods [28]. In the present study, we carried DPP-4 interacted with successive addition of catechin in 20 mM Tris-HCl with different incubation time i.e., 10, 20 and 30 min. Among them 30 min incubation sample were shown maximum binding activity when compared to the 10th and 20th min of incubation. These results indicated that the catechin isolated from WS can quench the intrinsic fluorescence of DPP-4 enzyme. CD spectroscopy is an important tool to which alteration in the native conformation of α-helix, β-sheet, especially for showing changes in the conformation of compounds arises from the difference in adsorption of left and right circularly polarized light [29]. Among many examples in the literature which studied on polyphenol-protein binding using CD spectroscopy showed two negative bands [30]. In our study the CD spectrum of DPP-4 enzyme exhibited two negative peaks in the region at 208 nm and 222 nm which are characteristics of secondary structure of the protein, α-helix and the interaction between 1:4 and 1:8 dilution of DPP-4 with catechin caused a decrease in band intensity at all wavelengths without any significant shift in the peak position. This study exhibits the binding interactions of catechin and DPP-4 was confirmed by spectral
The SAR data infers that, catechin was found to be possessing higher affinity than compared to diprotin-A, but lesser than vildagliptin and interaction of catechin was elevated against glucose metabolizing key biotargets namely glucagon-like peptide-1 (GLP-1), aspartate aminotransferase (AST), glycogen synthase (GS) and lactate dehydrogenase (LDH) for confirming its anti-diabetic potency.
Finally, in this study, catechin was identified to be very promising candidate for blocking DPP-4 protein hence to be very beneficial in the treatment of diabetes mellitus, which is evident from the results of in vitro and in-silico models, together with its remarkable activity make catechin an interesting molecule for further studies. We have shown in our previous report that, compounds from WS could act as novel inflammatory inhibitors with high selectivity and precision [23]. Studies are currently underway to confirm the role of this active compound(s) and to characterize the precise mechanism involved at a molecular level to trace the pathway of action.
The accurately known geometries may allow us to draw some conclusions on the enzyme mechanism and suggest a possible scenario to hamper many life style disorders specifically diabetes.
Conclusion
Based on the DPP-4 inhibition with strong evidenced by the interaction and in-silico studies the present study concluded that catechin, a flavonoid present in 100% methanolic extract of WS matured roots has potent antidiabetic effects. However, further work can be developed for in vivo method for determining the pharmacological and clinical parameters through the evaluation of antidiabetic activity.
Conflict of Interest
Authors have no conflict of interest.
Acknowledgment
All authors thank PET Research Foundation, Mandya, The authors gratefully acknowledge Prof. Dr. K.S. Rangappa, Department of Chemistry, University of Mysore. FZ sincerely acknowledge University Grants Commission (UGC), Govt. of India, New Delhi, for awarding Sir CV Raman Post-Doctoral Fellowship 2014-15 to USA (Ref No. F.5-97/2014 (IC) FD dairy no: 6725). FZ would like to place on record the deepest gratitude towards the management of Dayananda Sagar University (DSU), Bangalore and special thanks to the Dean, School of Basic and Applied Sciences, DSU, Bangalore for their constant motivation and support. Author Praveen Kumar Kempegowda specially thanks to Kalegowda Papegowda (Teacher), Kestur Koppal, Kantharajegowda Kempegowda (Teacher), Kestur Koppal, Nagarajegowda Sannaswamygowda (Superident), Malali, K R Nagar, Mysore for their constant helping to plant collection and field analysis.
References
- Bae EJ. DPP-4 inhibitors in diabetic complications: role of DPP-4 beyond glucose control. Arch Pharm Res 2016; 39: 1114-1128.
- Are PC, Adidala RRR, Puchchakayala G. Hypoglycemic and antidiabetic activity of Glochidion velutinum on streptozotocin-nicotinamide induced type 2 diabetic rats. Eur J Biol Sci 2011; 3: 126-130.
- Das H, Chakraborty U. Anti-hyperglycemic effect of Scoparia dulcis in streptozotocin induced diabetes. Res J Pharm Biol Chem Sci 2011; 2: 334-342.
- Udayakumar R, Kasthurirengan S, Mariashibu TS, Rajesh M, Anbazhagan VR, Kim SC, Ganapathi A, Choi CW. Hypoglycaemic and hypolipidaemic effects of Withania somnifera root and leaf extracts on alloxan-induced diabetic rats. Int J Mol Sci 2009; 10: 2367-2382.
- Pratley RE, Salsali A. Inhibition of DPP-4: a new therapeutic approach for the treatment of type 2 diabetes. CMRO J 2007; 23: 919-931.
- Joseph AD, Tulasi VK, Abhijit R, Bhatnagar P. Detection of dipeptidyl peptidase activity with Dppiv-Glo™ assay. Signal 2009; 50: 25.
- Lakowicz JR, Masters BR. Principles of fluorescence spectroscopy. J Biomed Optic 2008; 13: 29901.
- Chen YH, Yang JT, Martinez HM. Determination of the secondary structures of proteins by circular dichroism and optical rotary dispersion. Biochemistry 1972; 11: 4120-4131.
- Sangwan RS, Chaurasiya ND, Misra LN, Lal P, Uniyal GC, Sharma R, Sangwan NS, Suri KA, Qazi GN, Tuli R. Phytochemical variability in commercial herbal products and preparations of Withania somnifera Ashwagandha. Curr Sci 2004; 86: 461-464.
- Uddin Q, Samiulla L, Singh VK, Jamil SS. Phytochemical and pharmacological profile of Withania somnifera Dunal: a review. J App Pharm Sci 2012; 2: 170.
- Shiddamallayya N, Yasmeen A, Gopakumar K. Hundred common forest medicinal plants of Karnataka in primary healthcare. Ind J Trad knowl 2010; 9: 90-95.
- Verma SK, Kumar A. Therapeutic uses of Withania somnifera Ashwagandha; with a note on withanolides and its pharmacological actions. Asian J Pharm Clin Res 2011; 4: 1-4.
- Mirjalili MH, Moyano E, Bonfill M, Cusido RM, Alazon J. Steroidal lactones from Withania somnifera an ancient plant for novel medicine. Molecules. 2009; 14: 2373-2393.
- Tiwari R, Chakraborty S, Saminathan M, Dhama K, Singh SV. Ashwagandha Withania somnifera role in safeguarding health immunomodulatory effects combating infections and therapeutic applications: a review. Int J Biol Sci 2014; 14: 77.
- Andallu B, Radhika B. Hypoglycemic diuretic and hypocholesterolemic effect of winter cherry Withania somnifera Dunal root. Indian J Exp Biol 2000; 38: 607.
- Singh G, Sharma PK, Dudhe R, Singh S. Biological activities of Withania somnifera. Ann Biol Res 2010; 1: 56-63.
- Alam N, Hossain M, Khalil MI, Moniruzzaman M, Sulaiman SA, Gan SH. High catechin concentrations detected in Withania somnifera Ashwagandha; by high performance liquid chromatography analysis. BMC Complement Altern Med 2011; 11: 65.
- Hand K, Bruen C, OHalloran F, Panwar H, Calderwood D, Giblin L, Green B. Examining acute and chronic effects of short- and long-chain fatty acids on peptide YY PYY; gene expression cellular storage and secretion in STC-1 cells. Eur J Nutr 2013; 52: 1303-1313.
- Rindi G, Grant SG, Yiangou Y, Ghatei MA, Bloom SR, Bautch VL, Solcia E, Polak JM. Development of neuroendocrine tumors in the gastrointestinal tract of transgenic mice Heterogeneity of hormone expression. Am J Pathol 1990; 136: 1349-1363.
- Schwanstecher M. Diabetes-perspectives in drug therapy. Springer 2011.
- Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM. Human duodenal enteroendocrine cells: source of both incretin peptides GLP-1 and GIP. Am J Physiol Endocrinol Metab 2006; 290: 550-559.
- Kempegowda PK, Zameer F, Narasimashetty CK, Kollur SP, Murari SK. Inhibitory potency of Withania somnifera extracts against DPP-4: an in vitro evaluation. Afr J Tradit Complement Altern Med 2018; 15: 11-25.
- Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 2005; 329: 386-390.
- Chi Q, Huang K. Polyacrylamide gel electrophoresis of insulin. Anal Lett 2007; 40: 95-102.
- Katkar GD, Sundaram MS, NaveenKumar SK, Swethakumar B, Sharma RD, Paul M, Vishalakshi GJ, Devaraja S, Girish KS, Kemparaju K. Netosis and lack of DNAse activity are key factors in Echis carinatus venom-induced tissue destruction. Nat Comm 2016; 7: 11361-11374.
- Lambert JD, Yang CS. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat Res 2003; 523: 201-208.
- Liu J, Lu JF, Kan J, Wen XY, Jin CH. Synthesis characterization and in vitro anti-diabetic activity of catechin grafted inulin. Int J Biol Macromo 2014; 64: 76-83.
- Peng X, Qi W, Huang R, Su R, He Z. Elucidating the influence of gold nanoparticles on the binding of salvianolic acid B and rosmarinic acid to bovine serum albumin. PloS One 2015; 10: 0118274.
- Greenfield NJ. Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc 2006; 1: 2876-2890.
- Ulrih NP. Analytical techniques for the study of polyphenol-protein interactions. Crit Rev Food Sci Nutr 2015.