ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 9
Dysregulated miR-98-5p contributes to excessive apoptosis of nucleus pulposus cells by BMP2 in human intervertebral disc degeneration
1Department of Spinal Surgery, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, Shangdong, PR China
2Shandong Medical Image Research Institute, Shandong University, Jinan, Shandong, PR China
- *Corresponding Author:
- Bingyi Tan
Department of Spinal Surgery
Shandong Provincial Hospital affiliated to Shandong University
PR China
Accepted date: January 30, 2017
Background: Dysregulation of microRNAs (miRNAs) has been reported to be associated with Intervertebral Disc Degeneration (IDD), and accumulating evidence suggested that miR-98-5p was involved in pathogenesis of IDD, but the underlying molecular mechanisms remains elusive.
Method: MTT assay and flow cytometry were conducted to examine the viability and apoptosis of pressure-damaged cells, respectively. Computational analysis and luciferase assay were performed to identify the target of miR-98-5p. Western-blot and real-time PCR were utilized to detect miR-98-5p and BMP2 levels in the culture cells.
Results: Compression caused a time-dependent suppression of the growth of Nucleus Pulposus Cells (NPCs), and significant apoptosis was induced in the damaged cells in a time-dependent pattern compared with normal control. Bioinformatics analysis was used to validate Bone Morphogenetic Proteins 2 (BMP2) as a potential target of miR-98-5p, which was further confirmed by luciferase assay results. Experimental cells exhibited a lower level of miR-98-5p, and a higher level of BMP2. The viability of the cultured cells transfected with miR-98-5p were lower than the control, while and cells transfected with miR-98-5p antisense was found to grow faster than the negative control. The increased expression level of miR-98-5p inhibited the expression of BMP2, and knockdown miR-98-5p increased the expression of BMP2.
Conclusion: Taken together, our studies provided evidence that miR-98-5p may serve as a novel therapeutic target for IDD via modulating expression of BMP2.
Keywords
miR-98-5p, Excessive apoptosis, Nucleus pulposus cells, BMP2, Intervertebral disc degeneration
Introduction
Intervertebral Disc Degeneration (IDD) is one of the major causes of Low Back Pain (LBP). Approximately 80% of people in the world develop LBP at certain stage in their entire lives, causing a large economical and medical pressure to society [1]. IDD is featured by Extracellular Matrix (ECM) degradation and associated with elevated expression of pro-inflammatory cytokines [2]. It is known that these inflammation triggers suppress matrix protein synthesis and promote expression and activity of matrix degrading enzymes, such as integrin, metalloprotease with thrombospondin motifs (ADAMTS) and Matrix Metalloproteinases (MMPs), hence worsening the process of IDD [3,4]. The study by Won et al. revealed that the IDDs exhibited elevated fraction of apoptotic NPCs in rats with diabetes [5]. The reduction in the viable cell count may be one of the mediators that initiate disc degeneration [6]. Remarkably, the senescence and apoptosis of IDD cells can cause reduction in viable cell count. Moreover, a lot of studies demonstrated that cell senescence and apoptosis was worsened in the IDD degeneration and correlated to the elevated catabolism of NPCs [7].
IDD can trigger pain of low back. Association and interaction between Bone Morphogenetic Protein (BMP)/Transforming Growth Factor-b (TGF-b) signalling pathway and Wnt/bcatenin pathway have been identified, which are believed to play an important role in the development of IDD [8]. It has been shown that higher expression of b-catenin can be activated by the Wnt/b-catenin activator, Lithium Chloride (LiCl), in NPCs, and the stimulation of the Wnt/b-catenin signals via the inhibition of cyclin-D1 and c-myc suppressed cell proliferation [9]. Representing a member of the Transforming Growth Factor-β (TGF-β) superfamily, Bone Morphogenetic Protein-2 (BMP2) signals via the heterotetrameric complex of receptors of type I and type II serine-threonine kinases [10]. In development of chickens, apoptosis induced by BMP2 has been observed in vivo as well as in vitro, for example, in rhombomeres 3 or 5 and the interdigit field of the limb [11,12]. Moreover, a previous report described that the apoptosis in incubated mouse hybridoma HS-72 cells, the growth of which is independent of IL-6, could be triggered by BMP2 [13].
MicroRNAs (miRNAs) is a new class of small (19-25 nucleotides (nt)) noncoding RNA molecules, that completely or partially bind to the 3‘-untranslated region (3’UTR) of target mRNAs to regulate biological functions by modulating the expression of the target gene [14]. Moreover, miRNAs are pivotal in the modulation of stress responses, cell proliferation, differentiation, migration, apoptosis and developmental processes. Moreover, deregulated expression of miRNA directly links to the development and pathophysiologic progression of various human diseases [15]. What should be noted is that miRNAs have been associated with collagen loss, and the control of proliferation and apoptosis of NPCs [16]. Earlier study showed that dysregulation of miR-98-5p and some other miRNAs have been detected in NPCs from IDD patients compared with the controls. In addition, BMP2, known as a tumor suppressor, might be a virtual target of miR-98-5p, and has been demonstrated to enhance the apoptosis of NPCs, thereby possibly involved in the pathology of IDD [17-19]. In this study, we investigated the association between the BMP2 and miR-98-5p and also explored the mechanism that underlies the occurrence of IDD on molecular level.
Materials and Methods
Isolation and primary culture of human nucleus pulposus cells
The NPCs were isolated from tissue samples derived from subject (Male, 59 years old, height: 169 cm, and weight: 65 kg), forceps were utilized to mince the tissue samples, and 4 mg/ml dispase (Sigma-Aldrich, St Louis, MO) was utilized to digest the tissue pieces firstly for 30 min at 37ºC, and subjected to an additional incubation for another 5 h. 40 μm cell strainer (BD Falcon, Bedford, MA, USA) was utilized to filter the cell suspension dissociated. And we conducted a centrifugation for 15 min at 800 rpm to yield the cells. RPMI-1640 medium (Gibco, Grand Island, NY, USA) with 10% FBS (Fetal Bovine Serum) (Sijiqing, Hangzhou, China) and 1% streptomycin-penicillin was utilized to culture the cells with 5% CO2 at 37ºC. We changed the medium at 2-day intervals until the cells cloned. The cells at passage 3 were used in our research. The research protocol was approved by the research ethnic committee in Shandong Provincial Hospital affiliated to Shandong University.
Establishment of a model of compression induced apoptosis in nucleus pulposus cells
Primary NPCs were subdivided into two groups, the control group was maintained under an atmosphere with 1.0 MPa, and while the experimental group was maintained in pressurized cabin with artificial compressed air (99.5% compressed air +0.5% CO2).
RNA isolation and real-time PCR
To analyse the expression levels of miR-98-5p and BMP2 mRNA, TRIzol reagent (Invitrogen, CA, US) was utilized to isolate total RNA from NPCs in accordance with the manufacturer’s recommendation. AMV First-Strand cDNA Synthesis kit (Fermentas, Pittsburgh, PA, USA) with a random primer for BMP2 mRNA and a specific stem loop primer for miR-98-5p was utilized to reverse transcribe 1 μg total RNA following the instruction by the suppler. ABI SYBR-Green PCR Master mix (Applied Biosystems, Bedford, MA, USA) on a Light Cycler 480 (Roche, Indianapolis, IN, USA) was utilized to carry out the real-time PCR after RT reaction. The small nuclear RNA U6 and β-actin were chosen as internal control. The 2-Delta Delta Ct (cycle threshold) value shown as the relative expression level was utilized to analyze the RT-qPCR results. Three tests were performed.
Cell proliferation assay
NPCs were maintained into 48-well plates at a final concentration of 2 × 103 cells/well in 120 μL medium for 1, 2, 3, 4, 5, 6, and 7 days. 20 μL MTT were added in each well at a final concentration of 0.5 mg/ml for another 4 h at 37ºC, then medium were removed, and DMSO was utilized to dissolve precipitated formazan, and shook for 15 min, and Quant Universal Microplate Spectrophotometer (Biotek Instruments) was utilized to detect the cell viability based on the absorbance at 570 nm following the instruction by the supplier. Three independent tests were carried out.
Luciferase assay
The 3’-UTR (untranslated region) of BMP2 containing the binding site of miR-98-5p was amplified through PCR and cloned into the firefly luciferase reporter vector (Promega, Madison, WI, USA). Meanwhile, the mutagenesis was performed for the same site and introduced to the control vector (Ambion, Cambridgeshire, UK). Using Lipofecamine 2000 (Invitrogen, CA, USA), the cells transfected miR-98-5p mimics encoding plasmid , NC (negative control) and firefly luciferase reporter vector with wild-type or mutant 3’UTR of BMP2, respectively following standard protocol from supplier. And pRL-TK plasmid (Promega, Madison, WI, USA) expressing Renilla luciferase was introduced to each group to monitor transfection efficiency. The cells were washed twice 48 hours post-transfection, and 1×Passive lysis buffer (Promega, Madison, WI, USA) was utilized to suspend the cells, Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) with a GloMax 40/40 luminomete r (Promega, Madison, WI, USA) were utilized to measure the luciferase activity of firefly and Renilla in accordance with manufacturer’s instruction. All data are presented as the mean ±SD, and each experiment was performed at least three times.
Western blot analysis
The NPCs were harvested 48 h after transfection, and pre-chilled PBS was utilized to wash the cell, and whole-cell ice-cold lysis buffer (1% NP-40, 0.1% SDS (Sodium Dodecyl Sulfate), 10 ml/tablet supplemented with proteinase inhibitor (Roche, Indianapolis, IN, USA), 150 mmol/L NaCl and 50 mmol/L Tris-HCl, pH 7.4) was utilized to lyse the cells in accordance with the manufacturer’s protocol. DC protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA) was utilized to quantify the concentration of proteins extracted from NPCs and tissue samples, and hypotonic buffer was utilized to dilute the protein sample to an equal concentration. 4-20% SDS-PAGE was utilized to isolate the protein, and then followed by transbloting electrically on to nitrocellulose membranes (Invitrogen, CA, USA). TBST containing 5% non-fat dry milk was utilized to block the membranes for 60 min. The primary antibody against BMP2 at a dilution of 1:5000 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and β-actin (1:15,000; Abcam, Shanghai, China) were utilized to hybridize the membrane at 4ºC for 12 hours. TBST was utilized to wash the membranes and HRP (horseradish peroxidase)-conjugated anti-rabbit or mouse secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was utilized to treat the membrane for at 37ºC for 2 hours. TBST was utilized to wash the membrane, an enhanced chemiluminescence reagents, Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific, Waltham, MA, USA) was utilized to detect the specific protein in accordance with manufacturer’s instruction. Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA) was utilized to quantify the optical density of the immunoblots. All tests were performed three times.
Apoptosis analysis
NPCs were harvested after transfection, and PBS was utilized to wash the cells. FITC-Annexin V/propidium iodide Apoptosis Detection Kit (BestBio, Shanghai, China) was utilized to double stain the cells following standard protocol by supplier, and flow cytometry (BD FACSCanto II, BD Biosciences, San Jose, USA) was utilized to analyze the cell stained. All experiments were carried out three times.
Statistical analysis
SPSS 16.0 software (IBM, Chicago, US) was utilized to conduct all statistical analyses. All Results were shown as mean ± Standard Deviation (SD). Student’s t-test was utilized to compare the variable, and paired t-test was utilized to compare data from different transfections, and others were utilized two-group-test. Pearson’s correlation coefficient was utilized to determine the correlations. The value of P less than 0.05 was considered significant.
Results
Compression induces apoptosis of NPCs
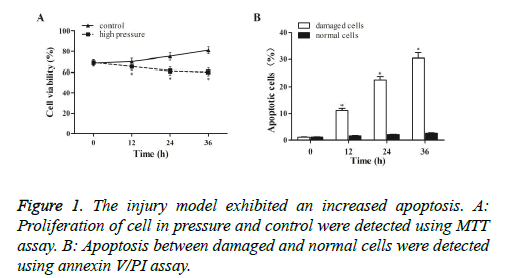
To explore the impact of compression on cell proliferation, MTT assay was conducted to examine cell survival under normal and high pressure. As shown in Figure 1A, compression caused a time-dependent suppression of NPCs viability in the pressure groups compared with the control. To further study whether the inhibited cell proliferation was caused by apoptosis, Annexin V/PI assay was performed to detect apoptosis. As shown in Figure 1B, apoptosis was evidently induced in the damaged cells in a time-dependent pattern, indicating that compression reduced cells viability though induce apoptosis.
BMP2 was direct downstream target of miR-98-5p
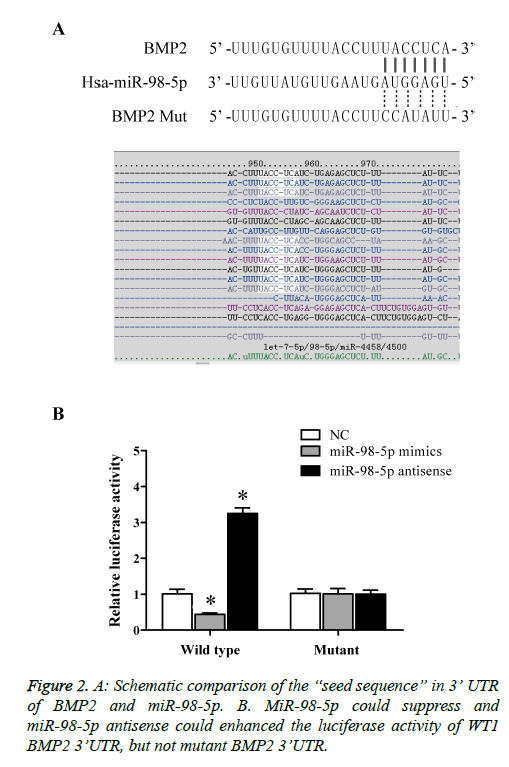
To understand further explore the role of miR-98-5p in IDD; we performed computational analysis to identify the target of the miRNA. As predicted by miRTarG (http://mirtar.mbc.nctu.edu.tw), miRanda (http://www.microrna.org/), TargetScan (http://www.targetscan.org/), there is a potential binding site in 3’-UTR of BMP2 (Figure 2A). To confirm the bioinformatics prediction, luciferase reporter assays were conducted. Briefly, NPCs introduction of vectors contained wild-type BMP2 3’UTR with putative binding sites of miR-98-5p or mutant BMP2 3’UTR. Furthermore, introduction NPCs with miR-98-5p mimic achieved up-regulated of miR-98-5p, while introduction NPCs with miR-98-5p antisense achieved knockdown of miR-98. As shown in Figure 2B, over-expressed of miR-98-5p remarkably diminished luciferase activity of wild-type BMP2 3’UTR, and miR-98-5p antisense strikingly enhanced the luciferase activity of wild-type BMP2 3’UTR compared with control, while mutation with binding site miR-98-5p in BMP2 3’UTR notably abrogated the suppression and improvement of miR-98-5p and miR-98-5p antisense, respectively. The results demonstrated that miR-98-5p directly regulated the expression of BMP2 via binding to the 3’UTR of BMP2.
Differential expression of miR-98-5p and BMP2 between damaged and normal cells
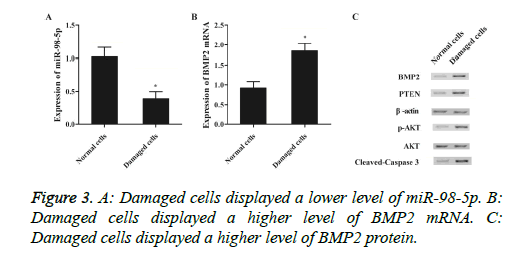
To further identify that miR-98-5p was involved in IDD via targeting BMP2, the expression level of miR-98-5p and BMP2 between damaged and normal cells were determined using real-time qPCR and western-blot. As shown in Figure 3, miR-98-5p level (Figure 3A) of damaged cells was apparently down-regulated than normal control, and both mRNA (Figure 3B) and protein (Figure 3C) levels of BMP2 in damaged cells was apparently up-regulated compared with normal control, validated that down-regulated of miR-98-5p induced IDD via improved expression of BMP2.
Up-regulated of miR-98-5p promoted NPCs apoptosis
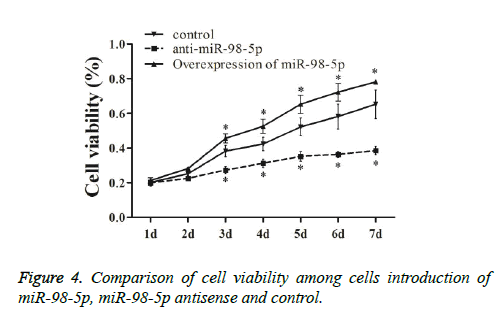
To understand the biological function of miR-98-5p in NPCs, miR-98-5p was up-regulated and down-regulate by transfecting miR-98-5p mimics or antisense in NPCs. As shown in Figure 4, cells introduction of miR-98-5p were explored to grow more rapidly three days post-transfection, and cells introduction of miR-98-5p antisense were explored to grow more slowly three days after transfection compared to that in the negative control, indicated that miR-98-5p reduced cell proliferation via induced apoptosis.
MiR-98-3p down-regulated endogenous BMP2 expression in NPCs via destabilizing BMP2 mRNA
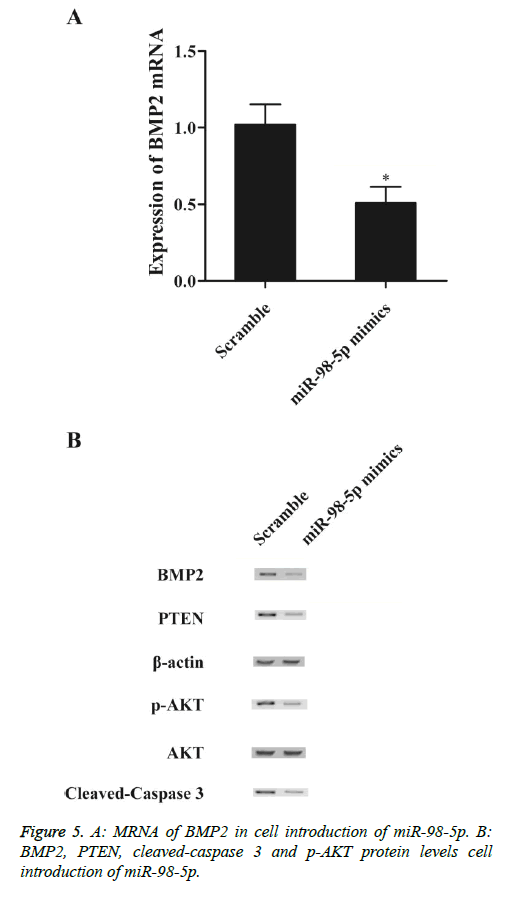
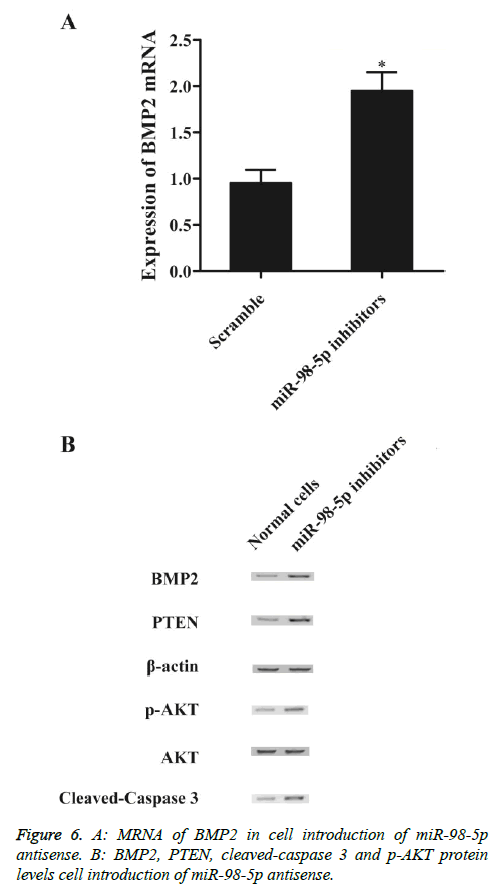
To explore the regulatory relationship between miR-98-5p and BMP2 in vitro, we also investigated the expression levels of BMP2 mRNA and protein under miR-98-5p and miR-98-5p antisense treatments. Compared with the scramble controls, substantial under-expression of BMP2 mRNA (Figure 5A) and protein (Figure 5B), PTEN (Figure 5B) and cleaved-caspase 3 (Figure 5B) expression levels was detected in cells introduction of miR-98-5p mimics, while evident over-expression of p-Akt protein (Figure 5B). Subsequently, knockdown miR-98-5p significantly up-regulated the expression of BMP2 mRNA (Figure 6A) and protein (Figure 6A), PTEN (Figure 6B) and cleaved-caspase 3 (Figure 6B), while p-Akt protein level was down-regulated than control, demonstrated that miR-98-5p negatively regulated BMP2 expression.
Discussion
The serum miRNAs obtained from 50 IDD patients and 50 NC subjects were used in a variant small RNA-Seq study carried out on HiSeq 2000, revealing that IDD has lower expressions of miR-885-5p, miR-483-3p, miR-342-3p, miR-191-5p, miR-98-5p and let-7d-5p compared with NC [20]. Downregulated let-7g-5p and miR-98-5p and upregulated miR-26b-3p have been confirmed in AD blood [21]. Growing data indicate that miRNAs are related to the progression and prognosis of an array of disorders and may be targeted by gene therapy [22]. Studies have started to investigate the function of miRNA in human degenerative disorder, and few studies have identified the role of miRNA in degeneration of disc. It has been identified that degenerative NP tissues had substantially reduced expression of miR-98, which was closely correlated with the grade of degenerative disc disease [23]. The clear biochemical characteristic of IDD is degradative ECM due to the loss of collagens and proteoglycans caused by an imbalance in homeostasis between catabolism and anabolism. Available data indicate that lack of type II collagen is regarded as an indicator of early stage of IDD [24]. Over-expressed level of miR-98 substantially elevated expression of aggrecan and type II collagen in NPCs, while suppression of miR-98 elevated type I collagen and reduced type II collagen level [23,25]. Moreover, NPCs proliferation and apoptosis can be impacted by the miR-98 analogue and suppressor [23]. These results showed that the development of IDD might be associated with the downregulated expression of miR-98 [23]. In this study, we performed bioinformatics target prediction, finding that BMP2 was the direct target of miR-98-5p, and luciferase activity were detected, and revealed that miR-98-5p can inhibited wild-type BMP2 3’UTR luciferase activity, but not mutant BMP2 3’UTR, whereas miR-98-5p antisense enhanced wild-type BMP2 3’UTR luciferase activity, but not mutant BMP2 3’UTR.
In the present study, we found that compression caused a time-related inhibition the NPCs proliferation in pressure groups, and increase in apoptosis of cell in damaged groups was observed. Furthermore, found that miR-98-5p was evidently up-regulated in damaged cells, and BMP2 was substantially down-regulated in damaged cells compared with normal control. BMPs modulate developmental and adult processes such as cell differentiation and growth, and apoptosis, and they trigger formation of cartilage and bone either during embryonic development or post-natally [26-28]. Regulation of apoptosis during development assures appropriate morphogenesis of tissue. For instance, BMP2 triggers apoptosis in the apical ectodermal ridge by regulation of signalling of fibroblast growth factor during limb formation [29]. There are expressions of BMP2 and BMP4 in the anterior, interdigital and posterior necrotic zones before the onset of apoptosis in the chick limb bud [12].
Jang et al. demonstrated that BMP2 enhanced transcription of Activating Transcription Factor 6 (ATF6) by promotion of the direct binding of RUNX2 to the osteoblast-specific cis-acting element 2 (OSE2) motif of the region of ATF6 promoter [30]. It has been demonstrated that BMP2 activates the IRE1α-XBP1 pathway and triggers ER stress during chondrocyte differentiation. BMP2 has multiple significant effects in various cellular functions including embryogenesis, cell differentiation and growth, bone development and bone fracture repair [31]. BMP2 has been known to induce activation of UPR signalling molecules, including PKR-like ER-resistant Kinase (PERK), BiP and IRE1α [32]. Murakami et al. showed that stimulation of BMP2 upregulated the expression levels of the ER stress markers and the UPR of ER stress triggered another BMP2 signalling pathway in osteoblasts [33]. Despite of promising results observed in single-gene transfection of intervertebral disc cells, a large number of viral vectors is required, which elevates the risk of the mediation of an immune response and cytotoxicity [34]. Wallach et al. integrated insulin-like growth factor 1, Bone Morphogenetic Protein (BMP)-2, and TGF-b1, to transfect intervertebral disc cells [35]. It has been shown AAV-mediated SOX9-double gene and BMP-2 for in vitro co-transfection of nucleus pulposus cells of the human intervertebral disc degeneration applying BMP-2 to increase the expression function of SOX9 and promote the synthesis of marrow stromal cell components and SOX9 to enhance matrix expression function [36]. Hunziker and Shintani investigated the modulatory role of BMP-2 on chondrogenesis and discovered that BMP-2 had dose-dependently positive regulatory role in the expression of the major type II collagen gene and chondrogenesis factor SOX9 in intervertebral disc cells [37]. Similar with our study, Ji et al. reported a similar role of miR-98-5p in the disc degeneration disease by showing that miR-98 is abnormally suppressed, and elevation of il-6/STAT3 expression which is physiologically inhibited by miR-98. In the study by Ji et al. a rescue test was performed, and even though introduction of IL6 significantly “rescued” the effect of miR-98, but it is not a 100% restore, indicating there are some other factors possibly involved in this process, and the data from our study provided one explanation for the observation by Ji et al. [23].
Conclusion
Taken together, our studies provided evidence that miR-98-5p may serve as a novel therapeutic target for IDD via mediating expression of BMP2.
Conflict of Interest
None
References
- Luoma K, Riihimaki H, Luukkonen R, Raininko R, Viikari-Juntura E. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 2000; 25: 487-492.
- Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA. Expenditures and health status among adults with back and neck problems. JAMA 2008; 299: 656-664.
- Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol 2014; 10: 44-56.
- Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther 2005; 7: R732-745.
- Won HY, Park JB, Park EY, Riew KD. Effect of hyperglycemia on apoptosis of notochordal cells and intervertebral disc degeneration in diabetic rats. J Neurosurg Spine 2009; 11: 741-748.
- Antoniou J. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest 1996; 98: 996-1003.
- Roberts S, Evans EH, Kletsas D, Jaffray DC, Eisenstein SM. Senescence in human intervertebral discs. Eur Spine J 2006; 15: 312-316.
- Hiyama A, Sakai D, Tanaka M, Arai F, Nakajima D. The relationship between the Wnt/β-catenin and TGF-β/BMP signals in the intervertebral disc cell. J Cell Physiol 2011; 226: 1139-1148.
- Ghoneum MFN, Nwaogu OM, Fayanju IY, Jeffe JA, Margenthaler DB. Clinical trials in surgical oncology. Asian Pac J Surg Oncol 2015; 1: 73-82.
- Massague J, Weis-Garcia F. Serine/threonine kinase receptors: mediators of transforming growth factor beta family signals. Cancer Surv 1996; 27: 41-64.
- Graham A. The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature 1994; 372: 684-686.
- Yokouchi Y, Sakiyama J, Kameda T, Iba H, Suzuki A. BMP-2/-4 mediate programmed cell death in chicken limb buds. Development 1996; 122: 3725-3734.
- Ishisaki A. Differential inhibition of Smad6 and Smad7 on bone morphogenetic protein- and activin-mediated growth arrest and apoptosis in B cells. J Biol Chem 1999; 274: 13637-13642.
- Papaioannou G. MicroRNA-140 provides robustness to the regulation of hypertrophic chondrocyte differentiation by the PTHrP-HDAC4 pathway. J Bone Miner Res 2015; 30: 1044-1052.
- Maurer B, Stanczyk J, Jungel A, Akhmetshina A, Trenkmann M. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum 2010; 62: 1733-1743.
- Wang HQ, Yu XD, Liu ZH, Cheng X, Samartzis D. Deregulated miR-155 promotes Fas-mediated apoptosis in human intervertebral disc degeneration by targeting FADD and caspase-3. J Pathol 2011; 225: 232-242.
- Xiao H. BAG3 regulates epithelial-mesenchymal transition and angiogenesis in human hepatocellular carcinoma. Lab Invest 2014; 94: 252-261.
- Yang X, Tian Z, Gou WF, Takahashi H, Yu M. Bag-3 expression is involved in pathogenesis and progression of colorectal carcinomas. Histol Histopathol 2013; 28: 1147-1156.
- Peng F, Xiao X, Jiang Y, Luo K, Tian Y. HBx down-regulated Gld2 plays a critical role in HBV-related dysregulation of miR-122. PLoS One 2014; 9: e92998.
- Tan L, Yu JT, Tan MS, Liu QY, Wang HF. Genome-wide serum microRNA expression profiling identifies serum biomarkers for Alzheimers disease. J Alzheimers Dis 2014; 40: 1017-1027.
- Satoh J, Kino Y, Niida S. MicroRNA-Seq data analysis pipeline to identify blood biomarkers for Alzheimer’s disease from public data. Biomark Insights 2015; 10: 21-31.
- Carlsen AL. Circulating microRNA expression profiles associated with systemic lupus erythematosus. Arthritis Rheum 2013; 65: 1324-1334.
- Ji ML, Lu J, Shi PL, Zhang XJ, Wang SZ. Dysregulated miR-98 contributes to extracellular matrix degradation by targeting IL-6/STAT3 signaling pathway in human intervertebral disc degeneration. J Bone Miner Res 2016; 31: 900-909.
- Williams FM, Bansal AT, van Meurs JB, Bell JT, Meulenbelt I. Novel genetic variants associated with lumbar disc degeneration in northern Europeans: a meta-analysis of 4600 subjects. Ann Rheum Dis 2013; 72: 1141-1148.
- Rutges JP, Kummer JA, Oner FC, Verbout AJ, Castelein RJ. Increased MMP-2 activity during intervertebral disc degeneration is correlated to MMP-14 levels. J Pathol 2008; 214: 523-530.
- Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev 1996; 10: 1580-1594.
- Hay E. Bone morphogenetic protein-2 promotes osteoblast apoptosis through a Smad-independent, protein kinase C-dependent signalling pathway. J Biol Chem 2001; 276: 29028-29036.
- Cao X, Chen D. The BMP signaling and in vivo bone formation. Gene 2005; 357: 1-8.
- Pajni-Underwood S. BMP signals control limb bud interdigital programmed cell death by regulating FGF signaling. Development 2007; 134: 2359-2368.
- Jang WG, Kim EJ, Kim DK, Ryoo HM, Lee KB. BMP2 protein regulates osteocalcin expression via Runx2-mediated Atf6 gene transcription. J Biol Chem 2012; 287: 905-915.
- Liu Y, Zhou J, Zhao W, Li X, Jiang R. XBP1S associates with RUNX2 and regulates chondrocyte hypertrophy. J Biol Chem 2015; 290: 10643.
- Peng Y. Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. J Biol Chem 2004; 279: 32941-32949.
- Murakami T, Saito A, Hino S, Kondo S, Kanemoto S. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat Cell Biol 2009; 11: 1205-1211.
- Lohr F. Systemic vector leakage and transgene expression by intratumorally injected recombinant adenovirus vectors. Clin Cancer Res 2001; 7: 3625-3268.
- Wallach CJ, Gilbertson LG, Kang JD. Gene therapy applications for intervertebral disc degeneration. Spine (Phila Pa 1976) 2003; 28: 93-98.
- Ren XF. Adeno-associated virus-mediated BMP-7 and SOX9 in vitro co-transfection of human degenerative intervertebral disc cells. Genet Mol Res 2015; 14: 3736-3744.
- Shintani N, Hunziker EB. Chondrogenic differentiation of bovine synovium: bone morphogenetic proteins 2 and 7 and transforming growth factor beta1 induce the formation of different types of cartilaginous tissue. Arthritis Rheum 2007; 56: 1869-1879.