ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 4
Early recovery of left ventricular function after revascularization of coronary artery disease detected by myocardial strain
1Departement of Cardiology and Vascular Medicine, Diponegoro University Faculty of Medicine, Dr. Kariadi General Hospital, Semarang, Central Java, Indonesia
2Departement of Cardiovascular Medicine, Tottori University Faculty of Medicine, Tottori University Hospital, Yonago, Japan
3Division of Regenerative Medicine and Therapeutics, Tottori University Graduate School of Medical Science, Yonago, Japan
- *Corresponding Author:
- Udin Bahrudin
Departement of Cardiovascular Medicine
Tottori University, Japan
Accepted on August 05, 2016
Background: Recovering blood flow to a coronary stenosis may improve left ventricular (LV) function in patients with coronary artery disease (CAD). However, the reported data about evaluation of LV function post-percutaneous coronary intervention (PCI) in CAD was limited. Purpose of this study was to compare the LV function measured by ejection fraction (EF) and global longitudinal strain in patients with CAD underwent PCI, and to identify factors affecting the change of LV function.
Methods: Patients with CAD who underwent elective PCI were enrolled. Echocardiographic measurements of LV function by EF as well as by 2D speckle tracking to assess global longitudinal strain were performed in all patients within 24 hours pre- and post-PCI procedure. The LV global longitudinal peak strain average (GLPS-Avg) was calculated from 18 segments measurement.
Results: A total of 40 patients (55.9 ± 7.5 y.o.) were enrolled. Means of GLPS-Avg pre- and post-PCI were -12.41 ± 4.82% and -13.41 ± 4.94%, respectively. Means of EF pre- and post-PCI were 43.2 ± 11.0% and 45.6 ± 11.7%, respectively. The improvement of LV function was more significant statistically when it was measured by GLPS-Avg (p<0.0001) than that of EF (p<0.001). The improvement of GLPSAvg was correlated with target vessel revascularization involving non-left anterior descending artery (p=0.00, coefficient correlation=0.484).
Conclusions: Recovery of left ventricular function could be detected early post-revascularization of coronary artery disease by either ejection fraction or global longitudinal strain measurements; however the latter is more accurate. Improvement of GLPS is correlated moderately with target vessel revascularization involving non-left anterior descending artery.
Keywords
Coronary artery disease, Ejection fraction, Speckle tracking echocardiography.
Introduction
Coronary artery disease (CAD) is a spectrum of heart disease which has the highest mortality in the world [1]. Management of patients with CAD consists of several approaches including percutaneous coronary intervention (PCI). The use of PCI to treat ischemic coronary artery disease (CAD) has expanded dramatically during the past three decades, with the estimated 1,000,000 PCI procedures performed annually in the US [2]. Assessment of the potential benefits of PCI for treating ischemic CAD must address fundamental patient-specific condition including the baseline left ventricular function [3,4]. Clinical evaluation of cardiac function is required both pre and post-PCI. Assessment of cardiac function by measuring left ventricular ejection fraction using echocardiography is the most common in the daily clinical practice. However, this technique has limitation related with its intra- and interobserver variability. A recent technique, 2D speckle tracking for assessing global longitudinal strain, has been introduced to reduce the variability and potentially has a higher accuracy. Speckle tracking is a method which uses two dimensions recording for measuring quantity of movement of myocardium in several segments [5]. Speckle-tracking echocardiography is a current noninvasive ultrasound imaging technique that allows for an objective and quantitative evaluation of global and regional myocardial function independently from the angle of insonation and from cardiac translational movements [2-4,6]. Speckle-tracking echocardiography is based on an analysis of the spatial dislocation (referred to as tracking) of speckles (defined as spots generated by the interaction between the ultrasound beam and myocardial fibers) on routine 2- dimensional sonograms. By tracking the displacement of speckles during the cardiac cycle, speckle-tracking echocardiography allows semi-automated elaboration of myocardial deformation in 3 spatial directions: longitudinal, radial, and circumferential. In addition, speckle-tracking echocardiography offers an evaluation of the occurrence, direction, and velocity of left ventricle (LV) rotation [7]. The semi-automated nature of speckle-tracking echocardiography guarantees good intraobserver and interobserver reproducibility [8]. Nonetheless, although this new technique was introduced for the exclusive analysis of LV function, several studies have recently extended its applicability to other cardiac chambers [9-14].
The development and applicability of myocardial speckle tracking has been continuously extended. In this study, we applied the speckle tracking technology for assessing left ventricle function in patients with CAD underwent PCI. Early evaluation of LV function post-PCI by myocardial speckle tracking has never been reported, and we found that recovery of LV function could be detected within 24 hrs postrevascularization of CAD by either EF or GLPS measurements, with the accuracy was achieved higher by GLPS measurement. In addition, improvement of GLPS-Avg was correlated moderately with target vessel revascularization.
Materials and Methods
This prospective single center study was performed at the Kariadi General Hospital, Semarang, Indonesia with the pre-post- test design. A total of 40 patients with stable CAD who underwent elective PCI were enrolled. Subset of CAD were stable angina pectoris, recent acute coronary syndrome, or chronic heart failure due to coronary heart disease. Patients with cardiomyopathy, cancer, or cytostatic treatment were excluded. Anamnesis, physical examination, and laboratory test for routine blood test, electrolyte, and renal function were done for all subjects. An elective PCI was performed using the clinical standard protocol.
A standard echocardiographic study was done using echocardiography machine GE Healthcare Vivid S6 Ultrasound System (Wauwatosa, USA) according to the guideline of American Society of Echocardiography [15]. Measurements of LV function by EF as well as by 2D speckle tracking to assess global longitudinal strain were performed in all patients within 24 hours pre- and post-PCI procedure. The LV global longitudinal peak strain average (GLPS-Avg) was calculated from 18 segments measurement, i.e., vertically were basal, mid, and apical, and horizontally were anterior, anteroseptal, inferoseptal, inferior, posterolateral, dan anterolateral. Anterior and inferior segments were seen from apical long axis (APLAX) view, anterolateral and inferoseptal segments were from apical 4-chambers (4-Ch) view, and anteroseptal and posterolateral from apical 2-chambers (2-Ch) view.
The SPSS statistics software was used for statistical analysis. Paired t-test was used to compare either LV EF or global longitudinal peak strain (GS) between pre- and post-PCI (Figure 1B). Independent t-test was used to compare either delta ejection fraction (EF) or GLPS between target vessel revascularization (TVR) involving left anterior descending artery (LAD) and non-LAD (Figure 2A). Bivariate analysis with Pearson correlation coefficient was used to links factors which may affect GLPS (Table 2). Informed consent for participation in this study was obtained. The investigation was approved by the Institutional Review Board of the Diponegoro University School of Medicine and Dr. Kariadi General Hospital and conformed to the principles outlined in the Declaration of Helsinki [16].
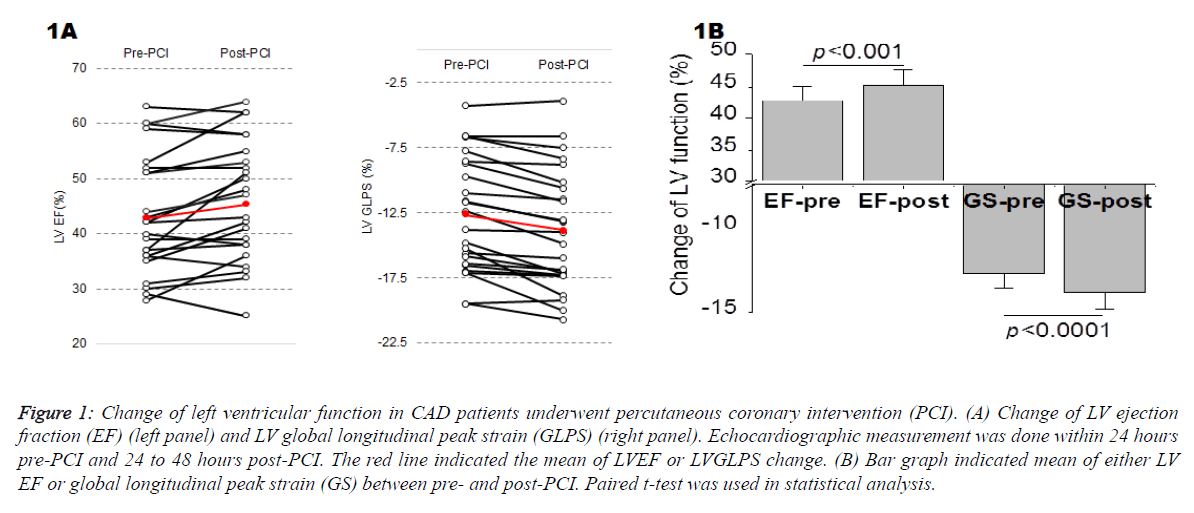
Figure 1: Change of left ventricular function in CAD patients underwent percutaneous coronary intervention (PCI). (A) Change of LV ejection fraction (EF) (left panel) and LV global longitudinal peak strain (GLPS) (right panel). Echocardiographic measurement was done within 24 hours pre-PCI and 24 to 48 hours post-PCI. The red line indicated the mean of LVEF or LVGLPS change. (B) Bar graph indicated mean of either LV EF or global longitudinal peak strain (GS) between pre- and post-PCI. Paired t-test was used in statistical analysis.
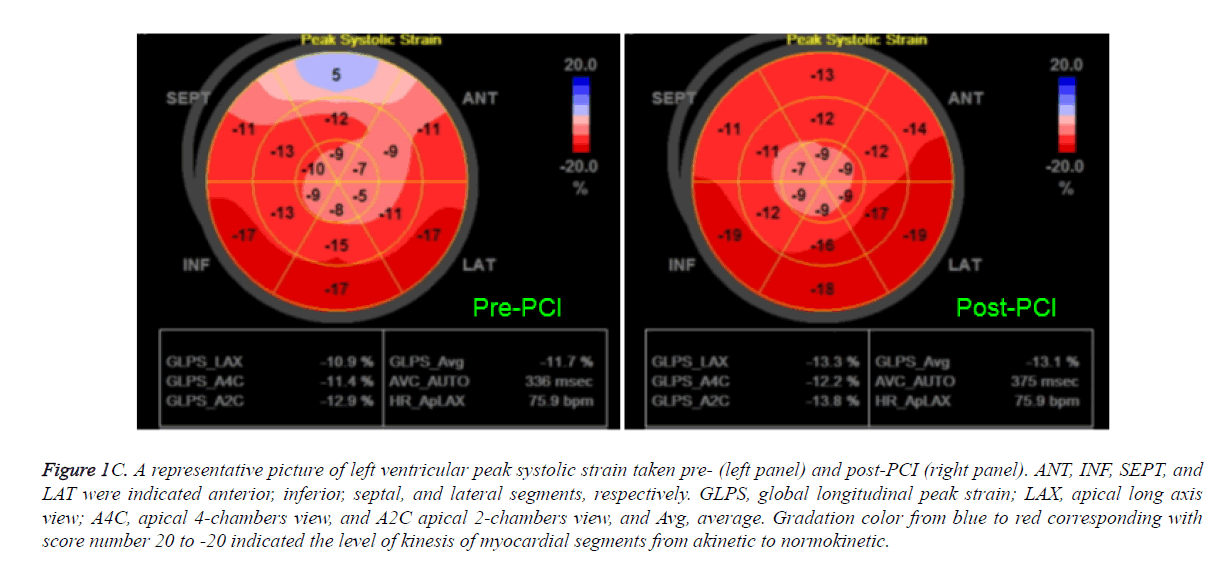
Figure 1C. A representative picture of left ventricular peak systolic strain taken pre- (left panel) and post-PCI (right panel). ANT, INF, SEPT, and LAT were indicated anterior, inferior, septal, and lateral segments, respectively. GLPS, global longitudinal peak strain; LAX, apical long axis view; A4C, apical 4-chambers view, and A2C apical 2-chambers view, and Avg, average. Gradation color from blue to red corresponding with score number 20 to -20 indicated the level of kinesis of myocardial segments from akinetic to normokinetic.
Results
A total of 40 patients with established coronary artery diseases by angiography were enrolled. Clinical characteristics of subjects were shown in Table 1. The average of age was 55.9 ± 7.5 years old, with 71.1% of them were 45 to 60 years old. The most patients (92.5%) were male. Thirty four percent patients had diabetes and more than half of them were diagnosed as chronic heart failure with underlying disease was ischemic CAD. Half of the patients had complex coronary lesion with 3 vessels disease, 60% of them were accompanied with coronary chronic total occlusion, and 66% of them had lesion in their left anterior descending artery. PCI was done in all patients with 37% complete revascularization by deploying one or two stents in the most of patients (79%).
| No | Variable | Category | n | Percentage (%) |
|---|---|---|---|---|
| 1 | Age | <45 y.o. | 3 | 7.5 |
| 45-60 | 19 | 47.5 | ||
| >60 y.o. | 18 | 45 | ||
| 2 | Sex | Male | 37 | 92.5 |
| Female | 3 | 7.5 | ||
| 3 | Diabetes mellitus (DM) | DM | 15 | 37.5 |
| Non DM | 25 | 62.5 | ||
| 4 | Diagnosis | SAP | 9 | 22.5 |
| Recent STEMI | 10 | 25 | ||
| CHF e.c. IHD | 21 | 52.5 | ||
| 5 | Number of vessel disease | 1 vessel | 11 | 27.5 |
| 2 vessels | 10 | 25 | ||
| 3 vessels | 19 | 47.5 | ||
| 6 | Chronic total occlusion (CTO) | CTO | 24 | 60 |
| Non CTO | 16 | 40 | ||
| 7 | Target vessel revascularization | LAD (+) | 26 | 65 |
| LAD (-) | 14 | 35 | ||
| 8 | Completeness of revascularization | Complete | 15 | 37.5 |
| Non complete | 25 | 62.5 | ||
| 9 | Number of implanted stent | 0 stent | 1 | 2.5 |
| 1 stent | 14 | 35 | ||
| 2 stents | 18 | 45 | ||
| 3 stents | 5 | 12.5 | ||
| 4 stents | 2 | 5 | ||
| CAD: Coronary Artery Disease; PCI: Percutaneous Coronary Intervention; n: frequency; y. o: years old; SAP: Stable Angina Pectoris; STEMI: ST Elevation Myocardial Infarction; CHF: Chronic Heart Failure; IHD: Ischemic Heart Disease; LAD: Left Anterior Descending Artery. | ||||
Table 1. Clinical characteristic of the 40 subjects with CAD underwent PCI.
Figure 1 showed the change of left ventricular function in CAD patients underwent PCI. Means of global longitudinal peak strain average (GLPS-Avg) pre- and post-PCI were 12.41 ± 4.82% and -13.41 ± 4.94%, respectively, while means of EF pre- and post-PCI were 43.2 ± 11.0% and 45.6 ± 11.7%, respectively. The improvement of LV function was more significant statistically when it was measured by GLPS-Avg (p<0.0001) than that of EF (p<0.001).
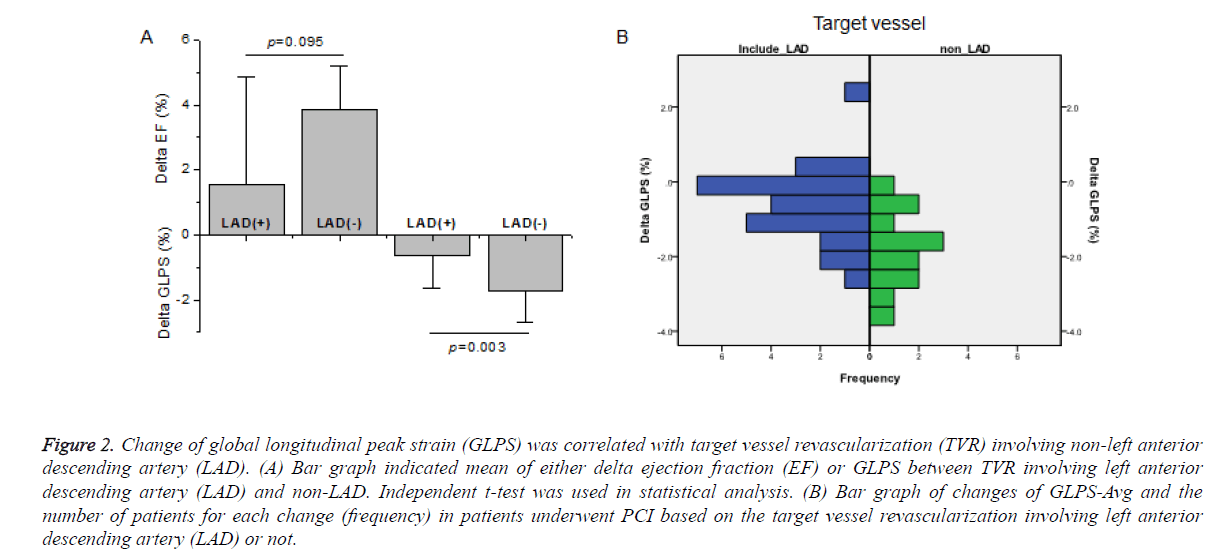
Figure 2A showed that while the delta LVEF pre-and post-PCI was not different significantly between target vessel revascularization (TVR) involving left anterior descending artery (LAD) and non-LAD (p=0.095), the delta GLPS was higher significantly in non-LAD TVR than that of in LAD (p=0.003). Bivariate analysis showed that the improvement of GLPS-Avg was correlated with TVR involving non-LAD (p=0.003, coefficient correlation=0.471) (Table 2). The distribution of patients for each changes of GLPS-Avg based on the involvement of LAD or not was shown in Figure 2B.
Figure 2. Change of global longitudinal peak strain (GLPS) was correlated with target vessel revascularization (TVR) involving non-left anterior descending artery (LAD). (A) Bar graph indicated mean of either delta ejection fraction (EF) or GLPS between TVR involving left anterior descending artery (LAD) and non-LAD. Independent t-test was used in statistical analysis. (B) Bar graph of changes of GLPS-Avg and the number of patients for each change (frequency) in patients underwent PCI based on the target vessel revascularization involving left anterior descending artery (LAD) or not.
| Variables | Delta GLPS-Avg | |
|---|---|---|
| Pearson correlation coefficient |
p | |
| Delta EF | -0.268 | 0.1 |
| Age | -0.037 | 0.8 |
| Diagnosis | -0.237 | 0.1 |
| Diabetes or none | 0.017 | 0.9 |
| Vessel disease | -0.212 | 0.2 |
| CTO or none | 0.092 | 0.5 |
| Target vessel revascularization | -0.471 | 0.003 |
| Completeness of revascularization | -0.128 | 0.4 |
| GLPS-Avg: Global Longitudinal Peak Strain Average; EF: Ejection Fraction; CTO: Chronic Total Occlusion | ||
Table 2. Bivariate analysis of factors which were correlated with change of GLPS.
Discussion
Recovering blood flow to a coronary stenosis may improve left ventricular (LV) function in patients with coronary artery disease (CAD). This study compared the LV function measured by ejection fraction (EF) and global longitudinal strain (GLS) in patients with CAD underwent PCI and found that either EF or GLS measurement could detect an early (within 24 hours) recovery of LV function post-PCI, and the latter is more accurate. This study demonstrates that abnormal longitudinal strain is frequently present in ischemic CAD and improved to a greater degree than abnormal systolic wall motion abnormality in patients with CAD treated by PCI. This finding contributes to the understanding of how myocardium recovers following a reperfusion therapy in ischemic CAD patients. This is supported by a report that longitudinal strain correlates with the global and regional extent of scar tissue as evaluated by contrast-enhanced MRI [17,18]. Others reported that a radial peak strain cutoff value of 17.2% predicts LV functional recovery after revascularization with accuracy similar to that of a cutoff value of 43% hyperenhancement on MRI [17]. Furthermore, when measured immediately after reperfusion therapy, longitudinal strain is an excellent predictor of LV remodeling and adverse events, such as congestive heart failure and death [19].
Subset of patients in this study were stable angina pectoris, recent acute coronary syndrome, or chronic heart failure due to coronary heart disease. As shown in Table 1, the different subset of CAD in this study did not affect the degree of early improvement of LV function measured by GLPS-Avg. However, this measurement has limitation when it is used in patients with a low value of longitudinal strain. It has been reported that a lower longitudinal strain value is a strong predictor of stable ischemic cardiopathy in asymptomatic patients without wall motion abnormalities [20]. Moreover, a cutoff value of −4.5% for regional longitudinal strain discriminates between segments with a viable myocardium and those with transmural scar tissue on contrast-enhanced MRI [21]. Thus, in this study, we excluded patients with cardiomyopathy, cancer, or cytostatic treatment.
Strain and strain rate measured by tissue Doppler or speckle tracking are now actively being studied for their potential use as prognostic tools in CAD patients, since they have unique ability to detect layer-specific changes in mechanical function [22]. Global LV longitudinal strain is correlated with myocardial viability and also predicts recovery of LV function after acute MI [23]. Vartdal et al. showed that global LV strain by tissue Doppler imaging was inversely related to infarct size after acute anterior wall MI as determined by gadolinium-enhanced MRI [24]. Another study by Zhang et al. similarly performed on patients with acute MI who underwent strain rate quantification by tissue Doppler imaging as well as contrast-enhanced MRI found that the peak systolic strain rate of transmurally infarcted segments was significantly lower than with normal myocardium or with non-transmurally infarcted segments, [25] thus supporting the ability of strain rate imaging to determine the degree of transmurality of scar tissue following MI [23,26]. Park et al. examined patients with acute MI who underwent reperfusion by either PCI or thrombolysis and found that longitudinal strain by both tissue Doppler and speckle tracking imaging predicted LV dilatation with increased LV end diastolic volume during 18 months of follow-up [19]. Strain also independently predicted death and congestive heart failure in the study [19]. The present study is additive to previous findings in that specifically, longitudinal shortening mechanics were correlated with the target vessel revascularization involving non-left anterior descending artery. It means that, in this study population, patients with TVR non- LAD get more beneficial effect from PCI than those of with LAD. Recovery of LV function in CAD patients depends on the presence of stunned or hibernating myocardium [27]. The hibernating myocardium restores the contractile function when revascularization is acquired, resulting in improvement of LV function [28]. The better improvements of GLPS in patients with TVR non-LAD observed in the current study might be a result of the bigger area or better recovery of hibernating myocardium in those patients than those with TVR LAD. Another possibility is that since the vascular coverage of LAD in myocardium is wider than that of right coronary artery as well as left circumflex artery, the remodeling area of LAD-covered myocardial cells is wider when LAD get ischemia or infarct. This is warrant for further study.
Limitations of this study was a relatively small number of ischemic CAD underwent PCI who received 2-D echocardiography and speckle tracking analysis performed within 24 hours post-PCI. Long term clinical outcomes including subsequent major adverse cardiac events and mortality were not measured and will be important to examine for future speckle tracking imaging investigations in this population.
In conclusions, recovery of left ventricular function could be detected early post-revascularization of coronary artery disease by either ejection fraction or global longitudinal strain measurements, however the latter is more accurate. Improvement of GLPS-Avg is correlated moderately with target vessel revascularization involving left anterior descending artery.
Abbreviations
SAP: Stable Angina Pectoris, STEMI: ST Elevation Myocardial Infarction; LV: Left Ventricular; EF: Ejection Fraction; GLPS-Avg: Global Longitudinal Peak Strain; PCI: Percutaneous Coronary Intervention; STEMI: ST-Elevation Myocardial Infarction; WMSI: Wall Motion Scoring Index.
Acknowledgements
This study was supported by a research grant from the Indonesian Government (Ditlitabmas Ditjen Dikti) No. DIPA-023.04.1.67345312015 to UB.
Competing Interests
The authors declare that they have no competing interests.
References
- Gouvinhas C, Severo M, Azevedo A, Lunet N. Worldwide patterns of ischemic heart disease mortality from 1980 to 2010. Int J Cardiol 2014; 170: 309-314.
- Perk G, Tunick PA, Kronzon I. Non-Doppler two-dimensional strain imaging by echocardiography--from technical considerations to clinical applications. J Am Soc Echocardiogr 2007; 20: 234-243.
- Dandel M, Lehmkuhl H, Knosalla C, Suramelashvili N, Hetzer R. Strain and strain rate imaging by echocardiography - basic concepts and clinical applicability. Curr Cardiol Rev 2009; 5: 133-148.
- Blessberger H, Binder T. Non-invasive imaging: two dimensional speckle tracking echocardiography-basic principles. Heart 2010; 96: 716–772.
- Helle-Valle T, Crosby J, Edvardsen T, Lyseggen E, Amundsen BH. New noninvasive method for assessment of left ventricular rotation: speckle tracking echocardiography. Circulation 2005; 112: 3149-3156.
- Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr 2010; 23: 351-69.
- Teske AJ, De Boeck BW, Melman PG, Sieswerda GT, Doevendans PA, Cramer MJ. Echocardiographic quantification of myocardial function using tissue deformation imaging, a guide to image acquisition and analysis using tissue Doppler and speckle tracking. Cardiovasc Ultrasound 2007; 5: 27.
- van Dalen BM, Soliman OI, Vletter WB, Kauer F, van der Zwaan HB, ten Cate FJ, Geleijnse ML. Feasibility and reproducibility of left ventricular rotation parameters measured by speckle tracking echocardiography. Eur J Echocardiogr 2009; 10: 669-676.
- Vianna-Pinton R, Moreno CA, Baxter CM, Lee KS, Tsang TS, Appleton CP. Two-dimensional speckle-tracking echocardiography of the left atrium: feasibility and regional contraction and relaxation differences in normal subjects. J Am Soc Echocardiogr 2009; 22: 299-305.
- Sirbu C, Herbots L, D'hooge J, Claus P, Marciniak A. Feasibility of strain and strain rate imaging for the assessment of regional left atrial deformation: a study in normal subjects. Eur J Echocardiogr 2006; 7: 199-208.
- Okamatsu K, Takeuchi M, Otsuji Y. Effects of aging on left atrial function assessed by two-dimensional speckle tracking echocardiography. J Am Soc Echocardiogr 2009; 22:537-538.
- D'Andrea A, Caso P, Romano S, Scarafile R, Riegler L. Different effects of cardiac resynchronization therapy on left atrial function in patients with either idiopathic or ischaemic dilated cardiomyopathy: a two-dimensional speckle strain study. Eur Heart J 2007; 28: 2738-2748.
- Di Salvo G, Pacileo G, Castaldi B, Gala S, Morelli C, D’Andrea A, Limongelli G, Del Gaizo F, Merlino E, Russo MG, Calabro R. Two-dimensional strain and atrial function: a study on patients after percutaneous closure of atrial septal defect. Eur J Echocardiogr 2009; 10: 256-259.
- Cameli M, Caputo M, Mondillo S, Ballo P, Palmerini E, Lisi M, Marino E, Galderisi M. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc Ultrasound 2009; 7:6.
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16: 233-270.
- [No authors listed]. World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. Cardiovasc Res 1997; 35: 2-3.
- Becker M, Lenzen A, Ocklenburg C, Stempel K, Kühl H, Neizel M, Katoh M, Kramann R, Wildberger J, Kelm M, Hoffmann R. Myocardial deformation imaging based on ultrasonic pixel tracking to identify reversible myocardial dysfunction. J Am Coll Cardiol. 2008; 51: 1473-1481.
- Roes SD, Mollema SA, Lamb HJ, van der Wall EE, de Roos A. Validation of echocardiographic two-dimensional speckle tracking longitudinal strain imaging for viability assessment in patients with chronic ischemic left ventricular dysfunction and comparison with contrast-enhanced magnetic resonance imaging. Am J Cardiol 2009; 104: 312-317.
- Park YH, Kang SJ, Song JK, Lee EY, Song JM, Kang DH, Kim YH, Lee CW, Hong MK, Kim JJ, Park SW, Park SJ. Prognostic value of longitudinal strain after primary reperfusion therapy in patients with anterior-wall acute myocardial infarction. J Am Soc Echocardiogr. 2008; 21: 262-267.
- Choi JO, Cho SW, Song YB, Cho SJ, Song BG, Lee SC, Park SW. Longitudinal 2D strain at rest predicts the presence of left main and three vessel coronary artery disease in patients without regional wall motion abnormality. Eur J Echocardiogr. 2009; 10: 695-701.
- Voigt JU, Nixdorff U, Bogdan R, Exner B, Schmiedehausen K, Platsch G, Kuwert T, Daniel WG, Flachskampf FA. Comparison of deformation imaging and velocity imaging for detecting regional inducible ischaemia during dobutamine stress echocardiography. Eur Heart J. 2004; 25: 1517-25.
- Takeuchi M, Nakai H, Kokumai M, Nishikage T, Otani S. Age-related changes in left ventricular twist assessed by two-dimensional speckle-tracking imaging. J Am Soc Echocardiogr 2006; 19: 1077-1084.
- Horton KD, Meece RW, Hill JC. Assessment of the right ventricle by echocardiography: a primer for cardiac sonographers. J Am Soc Echocardiogr 2009; 22: 776-792.
- Hui L, Pemberton J, Hickey E, Li XK, Lysyansky P, Ashraf M, Niemann PS, Sahn DJ. The contribution of left ventricular muscle bands to left ventricular rotation: assessment by a 2-dimensional speckle tracking method. J Am Soc Echocardiogr 2007; 20: 486-491.
- Zhang Y, Chan AK, Yu CM, Yip GW, Fung JW, et al.. Strain rate imaging differentiates transmural from non-transmural myocardial infarction: a validation study using delayed-enhancement magnetic resonance imaging. J Am Coll Cardiol 2005; 46: 864-871.
- Buckberg G, Hoffman JIE, Mahajan A, Saleh S, Coghlan C. Cardiac mechanics revisited: the relationship of cardiac architecture to ventricular function. Circulation 2008; 118:2571–87.
- Wijns W, Vatner SF, Camici PG. Hibernating myocardium. N Engl J Med 1998; 339: 173-181.
- Bax JJ, Visser FC, Poldermans D, Elhendy A, Cornel JH. Relationship between preoperative viability and postoperative improvement in LVEF and heart failure symptoms. J Nucl Med 2001; 42: 79-86.