ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 3
Effect and safety of probiotics combined early enteral nutrition on severe acute pancreatitis patients
1Department of Social Medicine and Health Management, Xiangya School of Public Health, Central South University, Changsha, Hunan Province, China
2Department of Medical Services, Peace Hospital of Changzhi Medical College, Changzhi, Shaanxi Province, China
3Shaannxi Provincial People’s Hospital, Xian, Shaanxi Province, China
4Department of Medical Affairs, Xiangya Hospital, Central South University, Changzhi, Hunan Province, China
5Department of General Surgery, Xiangya Hospital, Central South University, Changzhi, Hunan Province, China
#These equally contributed to this manuscript
- *Corresponding Author:
- Weijia Sun
Department of General Surgery
Xiangya Hospital
Central South University, China
Accepted date: September 12, 2016
Background: Probiotics and enteral nutrition have been shown to be beneficial in reducing the infection rate in animal experiments and primary clinical trials. The aim of this study was to examine the effects and safety of probiotics combined enteral nutrition in patients with severe acute pancreatitis.
Methods: One hundred and twenty severe acute pancreatitis patients were randomly divided into two groups receiving routine treatment and parenteral nutrition and probiotics combined enteral nutrition. Acute physiology and chronic health evaluation II scores, complications (systemic inflammatory response syndrome, multi-organ failure, and infections), plasma albumin, amylase, symptom disappearance time, average hospitalization time, and rate of infection were evaluated.
Results: The baseline data show balance, and the two groups were comparable. The incidence of infection in treatment and control group was 6.7% and 20.0%, and the incidence of multiple organ dysfunction syndrome in two groups was 11.7% and 26.7%. There were statistical differences between treatment and control group. The incidence of mortality in the two groups was 3.4% and 11.8%, and there was no statistical difference. Compared to control group, the treatment group has higher level amylase and lower albumin (P=0.031, P<0.001). Moreover, the treatment group have shorter duration of abdomen pain and hospitalization.
Conclusion: These findings suggested that probiotics could play a beneficial role in the treatment of Severe Acute Pancreatitis (SAP), and combination therapy can promote the effect of therapeutic.
Keywords
Severe acute pancreatitis, Probiotics, Eternal nutrition, Mortality.
Introduction
Severe acute pancreatitis (SAP) is an acute abdominal disease, characterized by nausea and frequently vomiting, fever, persistent abdomen pain, and elevation of plasma and urine amylase. Patients with severe complications such as systemic inflammatory response syndrome, multi-organ failure, and infections usually showed a higher death risk even if accepted the intensive treatment [1]. Many causes could lead to SAP such as gallstone, alcohol consumption, and hypertriglyceridemia, and so forth. Most of acute pancreatitis is mild types, and 20-30% of these are classified to SAP. The mortality of SAP is still up to 10%-30% [2]. The leading cause of death is attributed to Systemic Inflammatory Response Syndrome (SIRS) caused by infection, followed by Multiple Organ Dysfunction Syndrome (MODS) and secondary infection of pancreatic necrotic tissue [3]. The whole course of acute pancreatitis has three stages: acute reactive phase, overall infection phase, and residual infection phase. The majority of patients could get over acute reactive phase and entered into systemic infection phase thanks to physio pathologic and treatment technical support. During secondary systemic infection period, the primary causes of death for SAP patients are the infection of pancreas necrotic tissue and peripancreatic infected fluid and related complications. Previous studies have found that intestinal dysbacteriosis, intestine barrier functional dysfunction, and bacterial translocation are closely related to pancreas related infections above. Necrotic tissue infection can worsen the prognosis of Severe Acute Pancreatitis (SAP). For a long time, antibiotics are widely used to inhibit bacterial translocation, preventing occurrence of intestinal dysbacteriosis in clinical practice. However, the latest reports from a randomized controlled trial found that prophylactic antibiotics could not protect patients with SAP from secondary infection. The researchers turned their attention to probiotics.
Previous studies have also found early enteral nutrition could alleviate the systemic inflammatory response syndrome and some complications in patients with SAP [4,5].
Probiotics and enteral nutrition have been shown to be beneficial in reducing the infection rate in animal experiments and primary clinical trials. However, the results of some clinical trials have been contradictory due to study population, sample size and degree of disease, and so forth [6,7]. The aim of this study was to examine the effects and safety of probiotics combined enteral nutrition in patients with acute pancreatitis.
Materials and Methods
Study population
One hundred and twenty patients with SAP from our hospital were randomly assigned to either a treatment group (n=60) or a control group (n=60). We used the dynamic balancing randomization methods for grouping, and the randomization process was completed by using the software. The diagnostic criteria adopted the guide for diagnosis and treatment of severe acute pancreatitis 2014 drafted by Division of Pancreatic Surgery, Branch of Surgery, Chinese Medical Association [8].
The inclusion criterion is as following: (1) Patients’ age is more than 18 years old and less than 70 years old. (2) Patients should be sent to hospital within 24 hours. (3) Patients did not undergo surgical operation within 2 weeks. (4) Patients are diagnosed according history of disease, clinical symptom, and experimental examination followed by some complications such as organ dysfunction, pancreatic necrosis, pancreatic pseudo cyst and low clinical scores (Ransom ≥ 3 or APACHE II ≥ or Balthazar CT ≥ Stage II). The following patients are excluded: (1) Age: 18< or >70 years old. (2) Patients occurred to pancreatitis after entering into the study. (3) Patients with poor compliance. (4) Patients with malignant tumours. (4) Patients with severe cardiac, lung and renal function insufficiency. (5) Chronic pancreatitis patients. (6) Patients with obstruction of biliary tract need receive surgical operation. The study protocol was approved by the Ethics Committee of Xiangya Hospital. All persons gave their informed consent prior to their inclusion in the study.
Treatment methods
The control group were given conventional treatment, including fasting gastrointestinal decompression, inhibition of pancreatic enzymes and gastric acid secretion, improve microcirculation, acetanilide spasmolysis, maintaining water and electrolyte balance, nutritional support, prevent infection and complications. The treatment received probiotics combined enteral nutrition on the basis of routine treatment, combined with Bifidobacterium quadruple living bacterium within 48-72 hours after the onset of AP (420 mg, Three times a day (TID)). The implementation way of enteral nutrition: To determine the total calorie of enteral nutrition with daily needs of patients, select the appropriate enteral nutrition preparation; calculate the total preparation, calculation of the average speed of infusion: if the patient feed demand is 1500 ml/day, so infusion at a rate of 62.5 ml every hour. If pausing for a few hours of feeding because of clinical examination, only received 400 ml, remaining 9 hours, then in the remaining 9 hours, feeding speed is adjusted to the (1100 ml/9 h) 12 ml/h. The next day feeding speed begins from 62.5 ml/h. Setting the maximum infusion speed is 150 ml/h. Critically ill patients were given optokinetic agents at the start of enteral nutrition: metoclopramide 10 mg Q8H intramuscular injection, improvement of gastric residual threshold to 300 ml. The enteral nutrition will not stop until the abdomen symptoms of patients disappear, the amylase comes back to normal amylase, and inflammatory necrosis is partly absorbed.
For control group, starting of enteral nutrition firstly at the speed of 25 ml/h through a nasogastric tube using enteral nutrition infusion pump, monitor gastric residual every 4 hours, if gastric residual ≤ 200 ml, maintain the original speed of infusion; if the gastric residual ≤ 100 ml, enteral nutrition feeding rate will be increased by 20 ml/h every 4 hours. If the residual gastric volume for 2 or more than 2 times more than 200 ml, it shall temporarily stop infusion or reduce the speed of infusion.
Assessment of outcomes
We used the APACHE II graded computer program version 5.1 for APACHE II scores. Multiple Organ Dysfunction Syndrome (MOD) was defined as two or more organs having persistently ≥ 2 Marshall sub scores for 24 despite aggressive resuscitation and organ support. Patients with SAP received contrast enhanced CT scan when on admission and evaluated the pancreatitis severity. We also recorded the duration of abdomen pain and hospitalization, rate of infection, and prognosis.
Amylase and albumin
Peripheral blood samples from patients were collected for detecting amylase and albumin. Peripheral blood samples were centrifuged to collect serum which was dispensed in 50 mL aliquots and stored at -20°C before use. We detected the content of serum amylase, according to reagent box explanation (Beckman, DXC800, USA).
Statistical analysis
Data were reported as mean ± standard deviation for qualitative variables and as percentages for quantitative variables. Normal distribution of the continuous variables was assessed by the Kolmogorov-Smirnov test. Differences between two groups were tested by Student t test or Chi square test according to different data types. All statistical analysis was performed using SPSS version 19.0 (SPSS Inc., Chicago, Illinois, USA), figure was obtained through Grappad 6.0 and the statistical significance level was defined as P<0.05.
Results
Comparison of baseline data
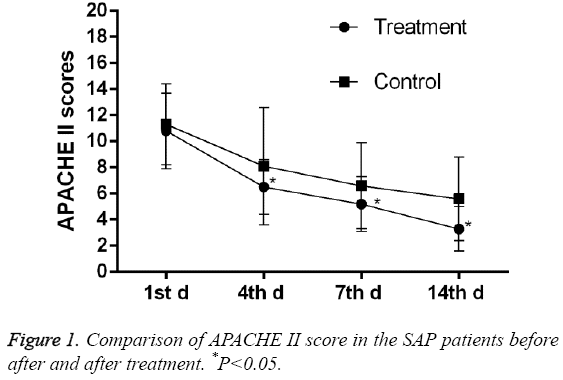
As shown in Table 1, the baseline information of the patients, such as gender, age, rate of alcohol, gallstone, and hyperlipidaemia was similar between the treatment and control group (P>0.05). The level of plasmas albumin and amylase are close (24.3 ± 1.98 vs. 23.5 ± 2.4; 1123.0 ± 217.0 vs. 1064.0 ± 236.0), and no significant difference were observed between two groups (P=0.050, 0.157). As shown in Table 2, the differences in APACHE II, Ranson score, STSI, and Marshall Score were not significant between two groups. The baseline data show balance, and the two groups were comparable. As shown in Figure 1, APACHE II scores decreased in control group on the 4th day until the 14th day and were further lowered in the treatment group.
| Parameter | Treatment group | Control group | t/c2 | P-value |
|---|---|---|---|---|
| Age(y) | 42.7 ± 11.5 | 42.6 ± 13.6 | 0.043 | 0.965 |
| Gender(Male/Female) | 34/26 | 32/28 | 0.135 | 0.714 |
| Alcohol(yes/no) | 13/47 | 12/48 | 0.050 | 0.822 |
| Gallstone(yes/no) | 25/35 | 23/37 | 0.139 | 0.709 |
| Hyperlipidaemia(yes/no) | 11/49 | 14/46 | 0.454 | 0.500 |
| Albumin(g/L) | 24.3 ± 1.9 | 23.5 ± 2.3 | 1.984 | 0.050 |
| Amylase(u/L) | 1123.0 ± 217.0 | 1064.0 ± 236.0 | 1.425 | 0.157 |
Table 1: Comparisons of baseline data between treatment and control group.
| Parameter | Treatment group | Control group | t/c2 | P-value |
|---|---|---|---|---|
| APACHE II | 10.8 ± 2.9 | 11.3 ± 3.1 | -0.852 | 0.395 |
| Ranson score | 3.8 ± 1.3 | 3.9 ± 1.6 | -0.376 | 0.708 |
| CTSI | 6.6 ± 1.7 | 6.7 ± 2.2 | -0.279 | 0.781 |
| Marshall score | 4.9 ± 1.6 | 4.7 ± 1.8 | 0.643 | 0.521 |
Table 2: Comparisons of clinical scores between two groups.
Comparison of clinical outcomes
The incidence of infection in treatment and control group was 6.7% and 20.0%, and the incidence of MODS in two groups was 11.7% and 26.7%. There were statistical differences between treatment and control group. The incidence of mortality in the two groups was 3.4% and 11.8%, and there was no statistical difference. Compared to control group, the treatment group has higher level amylase and lower albumin (P=0.031, P<0.001). Moreover, the treatment group have shorter duration of abdomen pain and hospitalization (21.2 ± 7.5 vs. 32.2 ± 8.4; 29.7 ± 12.3 vs. 40.5 ± 15.7). The results were shown in Table 3.
| Parameter | Treatment group | Control group | c2 | P-value |
|---|---|---|---|---|
| Albumin(g/L) | 32.6 ± 2.2 | 27.7 ± 2.2 | 12.199 | <0.001 |
| Amylase(u/L) | 226.0 ± 68.0 | 255.0 ± 77.0 | -2.187 | 0.031 |
| Duration of abdomen pain | 21.2 ± 7.5 | 32.2 ± 8.4 | -7.566 | <0.001 |
| Hospitalization | 29.7 ± 12.3 | 40.5 ± 15.7 | -4.194 | <0.001 |
| Rate of infection | 4/56 | 12/48 | 4.615 | 0.031 |
| MODS(%) | 7/53 | 16/44 | 4.356 | 0.037 |
| Mortality(%) | 2/58 | 6/51 | 2.374 | 0.123 |
Table 3: Comparison of the rates of morbidity of albumin, amylase, pancreatic sepsis, MODs and mortality between two groups after 14 days.
Discussion
The present study found that patients with SAP can benefit from probiotics combined enteral nutrition better than enteral nutrition alone. Patients receiving combination therapy have lower incidence of infection and shorter hospitalization and abdomen time.
Generally speaking, patients with SAP are called to restrict diet and decompress gastrointestinal, which means gastro enteric function is in a stasis status. The dysbacteriosis followed by gut mucosal barrier dysfunction would happen if this disease condition were not improved. More seriously, the barrier dysfunction of intestine makes some poisoned materials such as bacteria, endotoxin enter into blood circulation more easily, and finally cause intestinal infection, SIRS and increased mortality [9-11]. Early internal nutrition is the primary treatment for patients with SAP besides implementing acid suppression, fluid infusion, gastrointestinal decompression and pancreatic excretion inhibition, correcting electrolyte imbalances, and anti-infection [12]. It is reported that the intestinal probiotics could effectively improve and adjust the balance of intestinal micro ecology through increase the level of probiotics, inhibit pathogenic growth. However, some studies found that probiotic could become pathogenic and cause infection when human immunodeficiency occurs. Besselink et al. reported that probiotics combined enteral nutrition could increase the risk of mortality in patients with SAP [13]. This result is totally different from our study. In our results, all outcomes indictors but mortality have obvious improvement after 14 days’ treatment. The incidence of infection and hospitalization and abdomen time are lower than control. The mortality did not show significant difference, and this finding agrees with previous studies. However, this study had been proved to have major defects in study design and selection bias, and the conclusion is not reliable. The main issue is that Sand and Nor-back believed there are millions of bacteria and only small part of them could have benefits for the human organism. The selection of the probiotics based on experience and safety makes the results unreliable [14]. Besselink’s finding is undependable. Olah et al. reported that internal nutrition alone could reduce the mortality, rate of infection and MODS within 48-72 hours when patients are admitted to hospital. These results did no support the effective role of probiotics [15-17]. Capurso thought by-product generated during treatment of probiotics on SAP could exert an influence on infectious complication, but the specific products are not clear [18]. Summing up the above discussion, we notice that much work has been done about the role of probiotics in the SAP treatment, and the results still remain controversial. What we know is that function of probiotics cannot be ignored.
The present study is based on expending experiment. Early internal nutrition offer energy support for organism, does not stimulate the exocrine pancreas, and help to maintain the integrity of the structure and function of intestinal mucosa cells that will prevent bacteria and endotoxin from entering circulation. These series process would reduce the incidence of SIRS and MODS. The shorten of abdomen pain and hospitalization suggested probiotics combined internal nutrition have important adjuvant effect, and maintain the human immune response and gut integrity. The present study still has some limitations. First, our study only consisted of some evaluation indicators. More outcomes are needed such as plasma cytokine levels or endotoxin levels because it is reported that early endotoxin translocation from the gut lumen into the intestinal wall occurred and led to consequent access of gut-derived endotoxin to the mesenteric lymph node, liver, and lung [19]. Second, though there are no obvious difference in mortality, the difference of mortality between two groups are obvious, which means the combined treatment is at least no worse than the routine treatment. The present follow-up period is not enough to get certain outcomes. Our results were based on the effect of two weeks’ treatment, and longer follow-up is needed. Third, the sample size of our study is relatively smaller, but the power of our study arrives up to enough degree after calculating. Study with larger sample size is needed.
Conclusion
In the present study, we found that probiotics combined enteral nutrition could decrease the APACHE II score, incidence of infection, MODS, and reduce the duration of abdomen and hospitalization even more than enteral nutrition alone. The probiotics combined enteral nutrition improves the plasma albumin and amylase level. These findings suggested that probiotics could play a beneficial role in the treatment of SAP, and combination therapy can promote the effect of therapeutic. The future should focus on the mechanism of beneficial effects.
References
- Hoogenboom SA, Lekkerkerker SJ, Fockens P, Boermeester MA, van Hooft JE. Systematic review and meta-analysis on the prevalence of vitamin D deficiency in patients with chronic pancreatitis. Pancreatology 2016.
- Servin Torres E, Velazquez Garcia JA, Delgadillo Teyer G, Galindo Mendoza L, Bevia Perez F, Rivera Bennet F. Severe acute pancreatitis: surgical management in a third-level hospital. Cir Cir 2009; 77: 407-410.
- Apte MV, Pirola RC, Wilson JS. Mechanisms of alcoholic pancreatitis. J Gastroenterol Hepatol 2010; 25: 1816-1826.
- Zhou H, Gao J, Wu W, Liu L, Wei L, Zou D, Li Z. Octreotide ameliorates intestinal dysmotility by interstitial cells of Cajal protection in a rat acute necrotizing pancreatitis model. Pancreas 2011; 40: 1226-1233.
- Horst NL, Marques RG, Diestel CF, Matzke BD, Caetano CE, Simoes FC, Andrade AF, Lobao WI, Vaz LC, Portela MC, Braga JU, Melo PA. Effects of probiotic supplementation on markers of acute pancreatitis in rats. Curr Ther Res Clin Exp 2009; 70: 136-148.
- Jafri NS, Mahid SS, Idstein SR, Hornung CA, Galandiuk S. Antibiotic prophylaxis is not protective in severe acute pancreatitis: a systematic review and meta-analysis. Am J Surg 2009; 197: 806-813.
- Dellinger EP, Tellado JM, Soto NE, Ashley SW, Barie PS, Dugernier T, Imrie CW, Johnson CD, Knaebel HP, Laterre PF, Maravi-Poma E, Kissler JJ, Sanchez-Garcia M, Utzolino S. Early antibiotic treatment for severe acute necrotizing pancreatitis: a randomized, double-blind, placebo-controlled study. Ann Surg 2007; 245: 674-683.
- Division of Pancreatic Surgery, Branch of Surgery, Chinese Medical Association. Guide for diagnosis and treatment of severe acute pancreatitis 2014. Chin J Surg 2015; 01: 50-53.
- Shen X, Sun J, Zhang J, Ke L, Tong Z, Li G, Jiang W, Li W, Li J. Risk factors and outcome for massive intra-abdominal bleeding among patients with infected necrotizing pancreatitis. Medicine (Baltimore) 2015; 94: e1172.
- Kim IB, Prowle J, Baldwin I, Bellomo R. Incidence, risk factors and outcome associations of intra-abdominal hypertension in critically ill patients. Anaesth Intensive Care 2012; 40: 79-89.
- Kang CI, Chung DR, Ko KS, Peck KR, Song JH. Risk factors for mortality and impact of broad-spectrum cephalosporin resistance on outcome in bacteraemic intra-abdominal infections caused by gram-negative bacilli. Scand J Infect Dis 2011; 43: 202-208.
- Ke L, Ni HB, Sun JK, Tong ZH, Li WQ, Li N, Li JS. Risk factors and outcome of intra-abdominal hypertension in patients with severe acute pancreatitis. World J Surg 2012; 36: 171-178.
- Sharma B, Srivastava S, Singh N, Sachdev V, Kapur S, Saraya A. Role of probiotics on gut permeability and endotoxemia in patients with acute pancreatitis: a double-blind randomized controlled trial. J Clin Gastroenterol 2011; 45: 442-448.
- Sand J, Nordback I. Probiotics in severe acute pancreatitis. Lancet 2008; 371(9613): 634-635.
- Olah A, Romics LJ. Enteral nutrition in acute pancreatitis: A review of the current evidence. World J Gastroenterol 2014; 20: 16123-16131.
- Olah A, Belagyi T, Issekutz A, Gamal ME, Bengmark S. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg 2002; 89: 1103-1107.
- Olah A, Belagyi T, Poto L, Romics LJ, Bengmark S. Symbiotic control of inflammation and infection in severe acute pancreatitis: a prospective, randomized, double blind study. Hepatogastroenterology 2007; 54: 590-594.
- Capurso G, Marignani M, Piciucchi M, Merola E, Delle FG. Probiotics and severe acute pancreatitis. J Clin Gastroenterol 2008; 1: S148-S151.
- Qin HL, Su ZD, Gao Q, Lin QT. Early intrajejunal nutrition: bacterial translocation and gut barrier function of severe acute pancreatitis in dogs. Hepatobiliary Pancreat Dis Int 2002; 1: 150-154.