ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2009) Volume 20, Issue 2
Effect of antioxidant therapy on oxidative stress with special reference to hemodialysis
Department of Biochemistry, Dr. V. M. Govt. Medical College, Solapur, India ,yrtsimehcoiB fo tnemtrapeD
*RCSM Govt. Medical College, Shenda Park, Kolhapur, India,
- *Corresponding Author:
- R. B. Bhogade
Department of Biochemistry
Dr. V. M. Govt. Medical College Solapur, India
Accepted February 09 2009
Oxidative damage has been proposed as one of the possible mechanisms involved in the development of dialysis-related complications. Strengthening the defense system by supplementing antioxidants may provide protection against oxidative damage. Therefore, this study was carried out to investigate oxidant and antioxidant status in chronic renal failure patients undergoing hemodialysis and the effect of UbiQ100 supplementation on oxidant and antioxidant status. The blood samples were analyzed for quantitation of MDA as index of lipid peroxide, nitric oxide, vitamin E and erythrocyte superoxide dismutase activity. Significantly increased levels of serum lipid peroxide and decreased levels of nitric oxide, vitamin E, and erythrocyte superoxide dismutase activity were noticed in the patients both before and after hemodialysis, as compared to control subjects. However, significant reduction in lipid peroxide and improvement in nitric oxide, vitamin E and erythrocyte superoxide dismutase activity were observed after supplementary treatment of UbiQ100.
Keywords
Hemodialysis, oxidative stress, antioxidant, coenzyme Q
Introduction
Chronic renal failure (CRF) is the syndrome of persistent renal impairment involving loss of glomeruli, tubular and nephron function [1]. CRF is associated with a complex pathology. Some manifestations such as accelerated aging, atherosclerosis, impaired red blood cell deformability, increased hemolysis and platelet dysfunction may be related to hyperproduction of free radicals [2].
Oxidative stress has been defined as a loss of balance between free radical or reactive oxygen species production and antioxidant systems, with negative effects on carbohydrates, lipids and proteins. Oxidative stress has been proposed to play a role in cardiovascular and infectious diseases, cancer, diabetes, neurodegenerative pathologies etc [3]. The fact that the incidence of these diseases increase in uremia and particularly in dialysis patients, suggests an increased exposure to oxidative stress in this condition [4].
Nitric oxide (NO‘ ) is a potent mediator displaying a broad spectrum of activities, including smooth muscle relaxation, impairment of myocyte proliferation, cytotoxic reactions, and neuronal transmission [5]. Several investigators have focused their attention on a possible role played by nitric oxide in the development of uremic symptoms, but the results were controversial and the matter is still debated [6,7].
Superoxide dismutase (SOD), catalase, glutathione peroxidase, reduced glutathione, vitamin E, vitamin C are the most important antioxidant biomolecules. Coenzyme Q plays a role in cellular energy release, serving as an electron carrier in mitochondrial ATP synthesis. It also serves as an antioxidant by reducing free radicals thereby preventing damage to structural lipids and proteins [8]. A profound imbalance between oxidants and antioxidants has been suggested in uremic patients undergoing hemodialysis [4,9].
With this background, the present study was aimed to evaluate the possible alterations of oxidant/antioxidant status in uremic patients undergoing hemodialysis and effects of Ubi Q100 supplementation.
Material and Methods
This study included twenty chronic renal failure patients in the age group of 35 to 60 years undergoing hemodialysis. Control subjects were twenty healthy individuals matched for age and sex. The patients were diagnosed on the basis of detailed clinical history, clinical examination and other relevant biochemical investigations. All patients
had four hours dialysis sessions with polysulfone dialysis membrane. The patients suffering from other diseases, which may lead to oxidative stress, such as diabetes, inflammatory diseases, hepatic or respiratory diseases as well as smokers and alcoholics were excluded from study. Informed consent was obtained from each participant in the study. The study was cleared by institutional ethical committee.
Blood samples (7 mL each) were collected before and after hemodialysis from each patient. Two mL blood was collected in heparinized bulb and five mL was collected in plain bulb. Plasma and serum were separated from respective bulbs by centrifugation at 3000 rpm for 10 minutes at room temperature. All the samples were analyzed on the same day of collection. Serum creatinine was estimated by using Jaffe’s reaction in which creatinine react with picric acid to form creatinine picrate Serum malondialdehyde (MDA) levels were measured by reacting it with thiobarbituric acid at high temperature to form pink colored complex as in Kei Satoh method [12]. Nitric oxide was determined by Cortas and Wakid method [13], in which nitrate is reduced to nitrite by copper coated cadmium granules. This nitrite produced is determined by diazotization of sulfanilamide and coupling to naphthylethylenediamine to form purple complex. Serum vitamin E was measured by the reduction of ferric to ferrous ion which then forms a red colored complex with α- α’-bipyridyl as in Baker and Frank method Erythrocyte SOD activity was measured by Kajari Das method [15] which is based on the ability of SOD to inhibit nitrite formation.
All patients were given two months supply of commercially available tablet Ubi Q100 (Ubidecarenone (Coenzyme Q10) – 100mg, Arginine–100mg, Alpha tocopheryl acetate–25 IU, Selenium–100mcg) under medical supervision. Blood samples were collected after first and second month and processed identically. The values were expressed as mean±SD. Student’s ‘t’ test was done for comparison of data.
Results
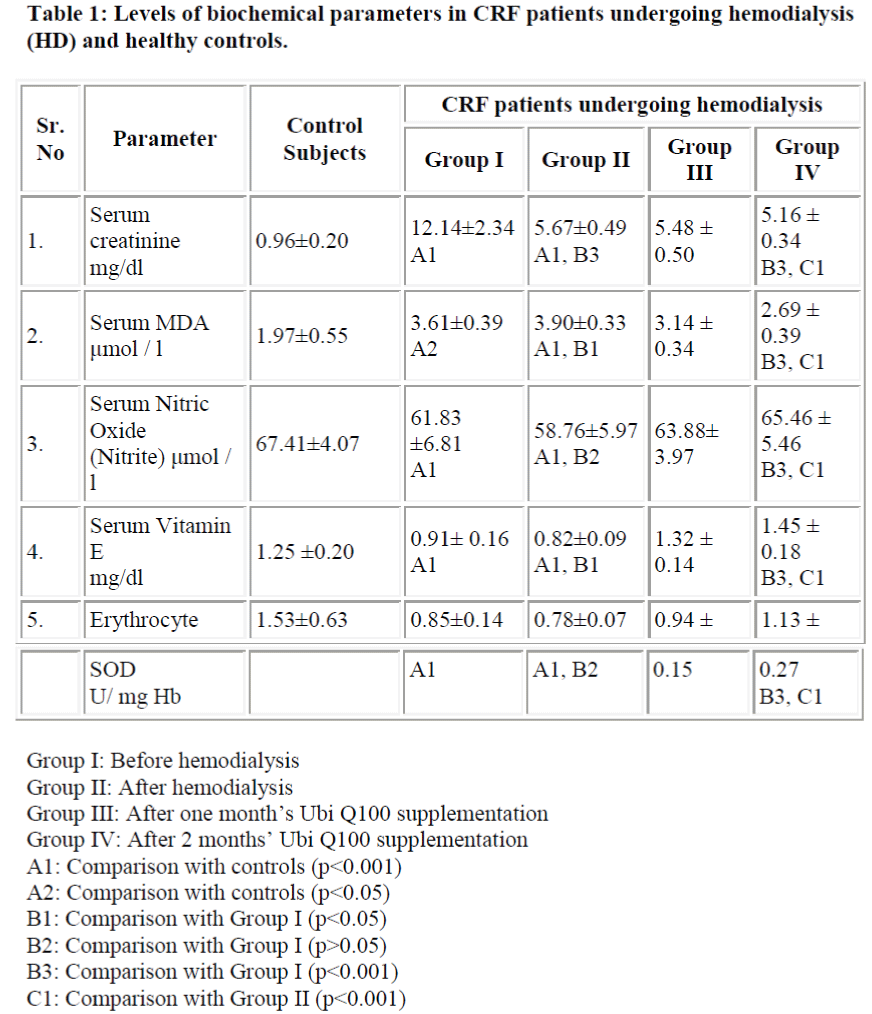
Table 1-shows levels of serum MDA, serum nitric oxide, serum vitamin E and erythrocyte superoxide dismutase activity in hemodialysis patients and healthy subjects. Prehemodialytic samples showed a significant increase in serum creatinine (p<0.001) and serum total lipid peroxide (MDA) (p<0.001) and significant decrease in serum nitric oxide (p<0.05), serum vitamin E (p<0.001), and erythrocyte SOD activity (p<0.001) as compared to control subjects.
There was significant increase in serum total lipid peroxide (MDA) after hemodialysis as compared to prehemodialysis level. The posthemodialytic levels of creatinine were significantly decreased as compared to those of prehemodialysis. The posthemodialytic levels of vitamin E were significantly decreased as compared to those of prehemodialysis and control subjects. There was slight but not significant decrease in erythrocyte SOD activity and serum nitric oxide level after hemodialysis as compared to prehemodialytic level. It was observed that levels of serum creatinine and serum total lipid peroxide (MDA) were significantly decreased whereas, activity of erythrocyte SOD, levels of serum vitamin E and serum nitric oxide were significantly increased in hemodialysed patients after two months’ Ubi Q100 supplementation.
Discussion
Under normal conditions, there is a steady state of balance between the production of oxygen free radicals and their neutralization by the cellular antioxidant systems. The oxygen free radicals, which accumulate via an imbalance between generation and scavenging, are believed to induce many disease states [2,16].
In the present study, we confirmed that oxidative stress is enhanced in hemodialysis patients as demonstrated by significant augmentation in pre and posthemodialytic serum MDA levels. Although haemodialysis leads to improvement in blood pressure and several biochemical parameters like creatinine and urea levels, it can also evoke several harmful atherogenic effects due to bioincompatibility of dialyzer components, accumulation of polyvinyl chloride, aluminium and acetate. Generation of reactive oxygen species could be an important harmful outcome of hemodialysis [17]. Oxidants induced by dialysis procedure mainly derive from oxidative burst of activated neutrophils and the subsequent superoxide anion (O2‘) generation [18].
Reactive oxygen species react with and inactivate nitric oxide and in the process produce highly reactive and cytotoxic products such as peroxynitrite and peroxynitrous acid. Peroxynitrite in turn reacts with and modifies various molecules such as lipids, DNA and protein. In addition to this, the oxidation of nitric oxide by reactive oxygen species inevitably results in functional nitric oxide deficiency [19]. In the present study we found significantly decreased serum nitric oxide levels in prehemodialytic samples, which further decreased slightly but not significantly after hemodialysis. It is known that oxidative stress may increase the synthesis of asymmetric dimethyl arginine (ADMA), which is an endogenous inhibitor of endothelial nitric oxide synthase [20].
Vitamin E is the most powerful important lipophilic anti-oxidant in humans. It contributes to membrane stability and protects critical cell structures against harm from oxygen free radicals and reactive lipoperoxides. In the present study, we found significantly decreased serum vitamin E levels both before and after hemodialysis. The diminished vitamin E level could partly be due to its overconsumption as an antioxidant subsequent to abnormal production of free radicals, while some reports also demonstrate low dietary intake of vitamin E [21].
There are conflicting results regarding the status of enzymatic antioxidant systems in patients with CRF. Several authors have reported reduced erythrocyte SOD activity in hemodialysis patients [1,16], whereas some studies described increased erythrocyte SOD activity in chronic uremic patients [22]. We further procure evidence supporting diminished antioxidant enzymes in hemodialysis patients. This could be due to a possible direct inactivation of the enzyme by its product hydrogen peroxide, or by superoxide anion itself [23,24], it could also be related to trace element deficiencies in hemodialysed patients [2].
The use of antioxidant therapy in hemodialysis opens a promising field in dialysis quality and prevention of oxidative stress related pathologies in renal patients. Coenzyme Q10 (CoQ10) is an essential co-factor in the mitochondrial electron transport chain and is found in all cell membranes. It is present in the body in both reduced and oxidized forms; the reduced form (CoQ10H2) is having antioxidant properties [8].
It has been observed that CoQ10 administration to experimental animals resulted in an increase not only in the CoQ content of mitochondria, but also in the mitochondrial level of α-tocopherol, which in turn was inversely correlated with a decrease in the rate of O2‘ generation. In homogeneous solutions [25] and membranes [26] CoQ has been shown to react with tocopheroxyl radicals to regenerate α-tocopherol, thus it has sparing effect on vitamin E. Significantly decreased serum MDA levels and increased antioxidant levels after Ubi Q100 supplementation in hemodialysis patients reported in present study suggests a protective effect of coenzyme Q and vitamin E against lipid peroxidation.
Our data led us to conclude that oxidative stress is enhanced in hemodialysis patients which may contribute to the development of dialysis-related complications such as cardiovascular disease, anemia etc. The study further reports beneficial effects of antioxidant supplementation-coenzyme Q, on oxidative stress in hemodialysis patients. Treatment with coenzyme Q improves renal function in these patients and may prolong the need for dialysis. Long-term follow-up in a large number of patients would be necessary to confirm these results.
References
- Ghoeshi Z, Jagtap PE, Ahaley SK, Gandhi R. Oxidant-antioxidant status in acute and chronic renal failure. Ind J Med Sci 2000; 54: 131-35.
- Hernandez de Rojas A, Martin Mateo MC. Superoxide dismutase and catalase activities in patients undergoing hemodialysis and continuous ambulatory peritoneal dialysis. Renal Failure 1996; 18: 937-946.
- Villa-Caballero L, Nava-Ocampo AA, Frati-Munari AC, Ponce-Monter H. Oxidative stress. Should it be measured in the diabetic patient? Gac Med Mex 2000; 36: 249-256.
- Galli F, Canestrari F, Buoncristiani U. Biological effects of oxidant stress in haemodialysis: the possible roles of vitamin E. Blood Purif 1999; 17: 79-94.
- Amero A, Bonaudo R, Ghigo D, Arese M, Costamagna C, Cirina P, et al. Enhanced production of nitric oxide by blood – dialysis membrane interaction. J Am Soc Nephrol 1995; 6: 1278-283.
- Sarkar SR, Kaitwatcharachai C, Levin NW. Nitric oxide and hemodialysis. Semin Dial 2004; 17: 224-228.
- Lin SH, Chu P, Yu FC, Diang LK, Lin YF. Increased nitric production in hypotensive hemodialysis patients. ASAIO J 1996; 42: M895-899.
- Yorihiro Yamamoto. Coenzyme Q10 as a frontline antioxidant against oxidative stress. J Clin Biochem Nutr 2005; 36: 29-35.
- Roselaar SE, Nazhat NB, Winyard PG, Jones P, Cunningham J, Blake DR. Detection of oxidants in uremic plasma by electron spin resonance spectroscopy. Kidney Int 1995; 48: 199-206.
- Brod J and Sirota JH. Determination of creatinine in blood. J Clin Invest 1948; 27: 645.
- Bonsnes RW, Taussky HH. Determination of creatinine in urine. Varley’s Practical Clinical Biochemistry 4th edition : 197 – 8
- Satoh K. Serum lipid peroxide in cerebrovascular disorder determined by a new colorimetric method. Clin Chim Acta 1978; 90: 37-43.
- Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium reduction method. Clin Chem 1990; 36: 1440- 43.
- Baker and Frank. Determination of serum tocopherol by colorimetric method. In: Gowenlock AH ed. Varley’s practical clinical biochemistry, 6th edition: Heinemann professional publishing;1988.p.902-3.
- Kajari Das. A modified spectrophotometric assay of superoxide dismutase using nitrate formation by superoxide radical. Ind J Biochem Biophy 2000; 7:201-204.
- Loughrey CM, Young IS, Lightbody JH, McMaster D. Oxidative stress in hemodialysis. Q J Med 1994; 87: 679-683.
- Dakshinamurty KV, Srinivas Rao PVLN, Saibaba KSS, Sheela RB, Venkataramana G, Shyam C, Sreekrishna V. Antioxidant status in patients on maintenance hemodialysis. Ind J Nephrol 2002; 12: 77-80.
- Galle J. Oxidative stress in chronic renal failure. Nephrol Dial Transplant 2001;16:2135-37.
- Nosratola DV, Zhenmin Ni, Fariba O, Kaihul L, Raj P. Enhanced nitric Oxide inactivation and protein nitration by reactive oxygen species in renal insufficiency. Hypertension 2002; 39: 135-141.
- Saran R, Novak JE, Desai A, Abdhulhayoglu E, Warren JS, Bustami R. Impact of vitamin E on plasma asymmetric dimethylarginine (ADMA) in chronic kidney disease (CKD): a pilot study. Nephrol Dial Transplant 2003; 18: 2415-2420.
- Leena OA, Sompong OA, Kanya T, Sanga N. Reduced free radical scavengers and chronic renal failure. J Med Assoc Thia 1997; 80: 101-108.
- Cavdar C, Camsari T, Semen I, Gonene S, Acikgoz O. Lipid peroxidation and antioxidant activity in chronic hemodialysis patients treated with recombinant human erythropoietin. Scand J Urol Nephrol 1997; 31: 371-375.
- Salo DC, Lin SW, Pacifici RE, Davies KJ. Superoxide dismutase is preferentially degraded by a proteolytic system from red blood cells following oxidative modification by hydrogen peroxide. Free Radic Biol Med 1998. 5: 335-339
- Sinet PM, Garber P. Inactivation of the human Cu-Zn superoxide dismutase during exposure to O2 and H2O2 Arch Biochem Biophys 1981; 212: 411-416.
- Stoyanovsky DA, Osipov AN, Quinn PJ, Kagan VE. Ubiquinone-dependent recycling of vitamin E radicals by superoxide. Arch Biochem Biophys 1995; 323: 343-351.
- Lass A, Sohal RS. Electron transport-linked ubiquinone dependent recycling of α- tocopherol inhibits autooxidation of mitochondrial membranes. Arch. Biochem Biophys 1998; 352: 229-236.