ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2019) Volume 30, Issue 4
Effect of Mtb-Ag-activated テδ偲つウテδ偲つエT cells on the expression of CD69
Hongmei Jin1, Jin Han2, Yiren Wang3 and Keqiang Wang4*
1Department of Blood Transfusion, The Affiliated Hospital of Taishan Medical University, China
2Clinical Pathology, Health Science College, Yonsei University, Gangwon-do, Korea
3Medical Laboratory, Medical College, Qingdao University, Shandong, China
4Department of Clinical Laboratory, Affiliated Hospital of Taishan Medical University, Shandong Province, China
- *Corresponding Author:
- Keqiang Wang
Department of Clinical Laboratory
Affiliated Hospital of Taishan Medical University, China
Accepted date: May 14, 2019
DOI: 10.35841/biomedicalresearch.30-19-234
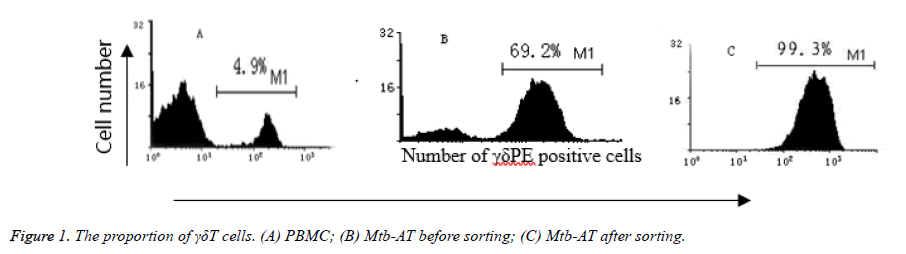
Visit for more related articles at Biomedical ResearchObjective: Activating and amplifying γδΤ cells with Mycobacterium tuberculosis low molecular peptide antigen (Mtb-Ag), in order to investigate the expression of CD69 molecules on γδΤ cellular surface. Methods: Activate health human peripheral blood mononuclear cells (PBMC) separately obtained, PBMCs were stimulated with Mtb-Ag and further isolate positive cells by immuno-magnetic beads selection, measure the proportion of γδΤ cells in the PBMCs by fluorescent monoclonal TCR γδΤ-PE staining and flow cytometry. Then, measure the expression of CD69 molecules in first stimulation and re-stimulation of γδΤ cells by γδ-PE/CD69FITC double staining. Results: The proportion of γδΤ cells were 4.9% in freshly isolated from PBMC, 69.2% after 10 days of Mtb-Ag activation, and 99.3% after immuno-magnetic beads selection. After 24 hours, the expression of CD69 molecules in γδΤ cells with initial Mtb-Ag stimulation arrived at peak at 75.2%. 6 hours later, in the second stimulation, it peaked at 72.0%. Conclusion: Mtb-Ag can specifically stimulate the proliferation of γδΤ cells in the PBMC. Both its initial and the second stimulation can specifically activate γδΤ cells.
Keywords
γδΤ cells, Mtb-Ag, CD69 molecules
Introduction
γδΤ cells are a subgroup of T cells identified in 1986, mainly distributed in mucosa and subcutaneous tissues, such as 10-18% in human intraepithelial lymphocytes (IEL),and 25%-37% in human large intestinal IEL, 50% in mice IEL, accounting for only 0.5% to 5.0% of the total number of lymphocytes in adult peripheral blood [1]. Mucosa and epithelial tissues are the first line of defense against pathogen invasion and are also the frequent occurrence of tumors. The high proportion of γδΤ cells in mucosa and epithelial tissues suggests that γδΤ cells are crucial in resistant to microorganisms and parasites, anti-tumor and immune regulation. Since the recognition of antigen by γδΤ cells is not restricted by major histocompatibility complex (MHC), and antigen-presenting cells are not required to treat and present antigens, so γδΤ cells are more efficient and more extensive than α, β, Τ cells. For this reason, γδΤ cells have received increasing attention [2-5]. In the past, the use of flow cytometry or magnetic cell separation techniques to separate γδΤ cells requires not only a large amount of peripheral blood, but also expensive equipment and complicating operations. The authors used Mtb-Ag to specifically stimulate the proliferation of γδΤ cells. The obtained cells were positively sorted by magnetic beads selection to obtain a large number of high-purity γδΤ cells, and the expression of CD69 molecules in Mtb-Ag primary stimulation and re-stimulation of γδΤ cells was observed. Now introduced as follows [6].
Materials and Methods
Main instruments and reagents
Ordinary optical microscope (Olympus BH, model BH2- MA-2, Japan); inverted microscope (Dawning WJ12-50, XSB-14, China); CO2 incubator (Harris hw0301T-VBA, USA); flow Cytometry (Coulter EPICSR XL-MCL, Beckmancounter, USA); cell culture plates (Falcon, USA); fully automated microplate reader (SLT-II, Austria); magnetic cell sorter (Miltenyi Biotec, Midi) -MACS, Germany). Mtb-Ag (a gift from Dr. Henry Boom, Department of Medicine, Case Western Reserve University, USA); lymphocyte separation solution (Institute of Hematology, Chinese Academy of Medical Sciences, batch number: 20000408); RPMIMedia1640 (RPMI1640) cell culture medium (Gibico, US). mouse antihuman fluorescent monoclonal antibody TCRγδ-PE (Becton Dickinson, USA, product number: 3437907); activationinducing molecule CD69 antibody (anti-CD69FITC, Ancell, USA, product number: 819010); recombinant human leukocytes Recombinant Human Interleukin-2 (rhIL-2, PTK, Korea); TCR γδ magnetic bead kit (Miltenyi Biotec, USA).
Methods
Cell preparation: Taking out the peripheral venous blood of 5 healthy adults and make heparin anticoagulated. PBMC was isolated by routine separation with lymphocyte separation solution, and the cell concentration was adjusted to 1.5 × 106/ml with RPMI1640 complete culture solution [5] for spare.
Mtb-Ag activated γδΤ cell proliferation and γδΤ cell isolation and purification: Activated γδΤ cells by Mtb-Ag, then separates and purifies the γδΤ cells. Take 1.5 × 106/ml PBMC suspension in 24-well culture plate, 1ml/well, add Mtb- Ag 5 μg/well for culture. Add the rIL-2 50 μ once each three days to keep cells growing. After 10 days, collect the γδΤ cells activated by Mtb-Ag. The γδΤ cells were sorted by immunomagnetic beads positive sorting method. Strictly follow instructions, and by PI single staining way, measure the freshly isolated PBMC, before sorting after culturing cells and after sorting cells proportion of γδΤ cells in the PBMCs with flow cytometry.
Detection of CD69 expression in γδΤ cells stimulated by Mtb-Ag for the first time: Draw 24 well cell culture plates, add the above prepared PBMC suspension to 24 wells in culture plate, 1 ml/well, and add Mtb-Ag (5 μg/hole). Then, cells were harvested at 37°C, 5% CO2 incubator for 0 h, 6 h, 12 h, 24 h, 48 h and 72 h. Use the direct immunofluorescence staining measure the expression of the CD69 in γδΤ cells by CD3PE/CD69FITC, γδPE/CD69FITC cell double staining. On the flow cytometer (Coulter EPICS XL), the argon ion laser wavelength was 488 nm as the excitation light, and the FSC/SSC (three-color flow cytometry) two-dimensional dot pattern was set. The gates were tested in lymphocyte populations and the resulting data files were analyzed using MinMDI 2.8 software.
Mtb-Ag re-stimulation of Mtb-AT cells induced reexpression of CD69 molecules in γδΤ cells: PBMC was stimulated by Mtb-Ag stimulation and cultured for 10 days, then Mtb-Ag (5 μg/well) was added again. The cells were harvested at 37°C, 5% CO2 incubator for 0 h, 6 h, 12 h, 24 h, 48 h and 72 h. The detection method was the same as before.
Results
Mtb-Ag-induced lymphocyte expansion
The proliferation of lymphocytes was observed by active quantitative method. After stimulation with Mtb-Ag, PBMC was cultured with rIL-2. The cells proliferated slowly in the first few days. After about 4 days, the proliferation accelerated, reaching a peak at 12 days, and the number of cells increased by nearly 40 times.
Proportion of γδΤ cells in lymphocytes
Freshly isolated PBMCs and PBMCs cultured for 10 days after stimulation with Mtb-Ag were detected by flow cytometry. As a result, γδΤ cells in freshly isolated PBMCs accounted for only 4.9%, while Mtb After 10 days of Ag-stimulated culture, the proportion of γδΤ cells can be as high as 69.2%, and then by immunomagnetic beads positive sorting, the proportion of γδΤ cells can be as high as 99.3%, as shown in Figure 1.
Mtb-Ag firstly stimulated the expression of CD69 in γδΤ cells
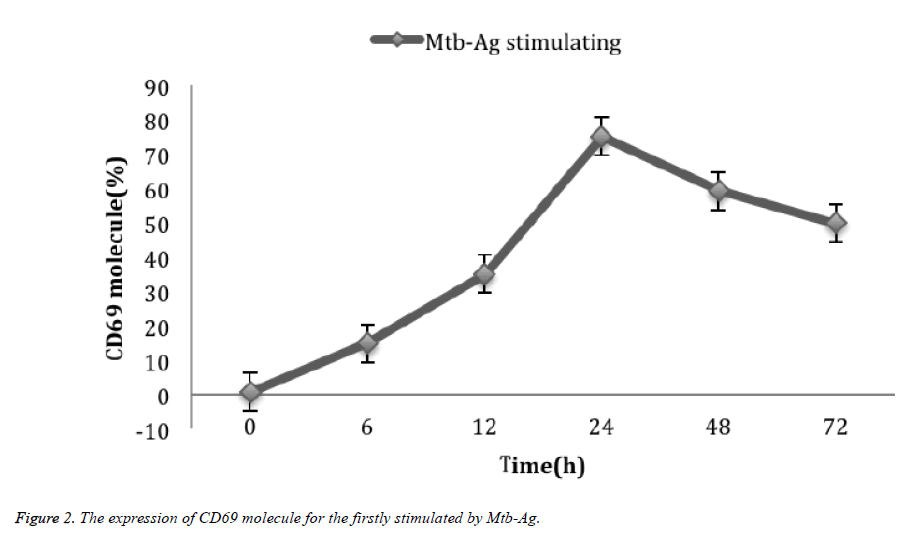
After 0 h, 6 h, 12 h, 24 h, 48 h and 72 h, the expression of CD69 molecules in Mtb-Ag was stimulated by 0.9 ± 0.22%, 15.1 ± 2.59, 35.2 ± 3.12%, 75.2 ± 6.29%, 59.4 ± 5.51%, and 50 ± 4.97% (Figure 2).
Mtb-Ag re-stimulated Mtb-AT cells induced reexpression of CD69 molecules in γδΤ cells
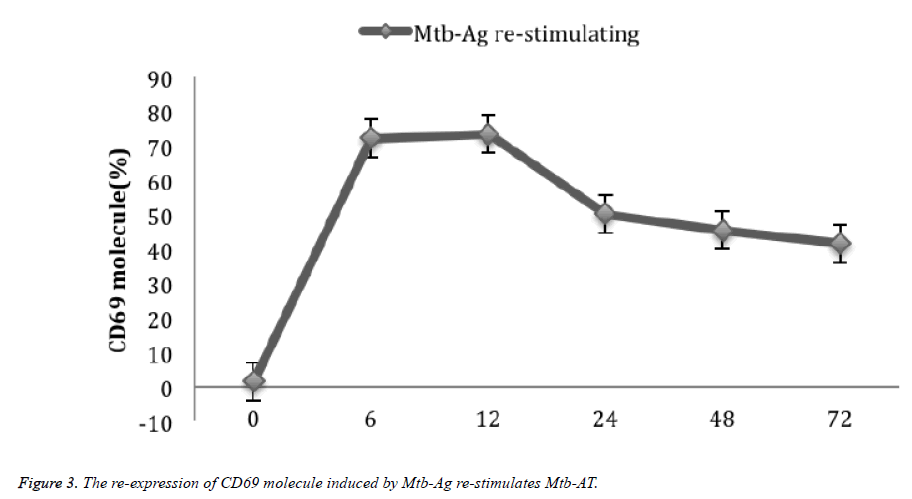
PBMC was stimulated by Mtb-Ag stimulation and cultured for 10 days, then stimulated with Mtb-Ag for 0 h, 6 h, 12 h, After 24 h, 48 h and 72 h, the expression of CD69 in γδΤ cells was 1.7 ± 0.46%, 72.3 ± 6.12%, 73.5 ± 6.45%, 50.3 ± 5.11%, 45.6 ± 4.84%, and 41.7 ± 4.49% respectively (Figure 3).
Discussion
The results of this experiment showed that the ratio of γδΤ cells in freshly isolated peripheral PBMC was 4.9 ± 1.85%, and that Mtb-Ag stimulated PBMC up to 69.2 ± 6.57% after 10 days of culture; It is the same as the results of previous experiments in our laboratory and other researchers [7,8], indicating that Mtb-Ag has the characteristics of preferentially activating and amplifying γδΤ cells. Therefore, Mtb-Ag stimulates PBMC, interleukin-2 (IL-2) maintains cell proliferation and culture, and then positively sorted by immunomagnetic beads, a large number of γδΤ cells can be obtained, which can be used as a simple Rapid γδΤ cell amplification acquisition method. The method has the advantages of low blood volume, high specificity, short cycle, no special equipment, and the like, and can provide a source for the research on the biological characteristics of γδΤ cells and the immunotherapy of clinical diseases. Of course, domestic stimulants have also been used to amplify γδΤ cells, such as Han et al. [9] After stimulating PBMC with zoledronic acid for 10 days, γδΤ cells increased from 4.21% to 70.35% before amplification; After Gui [10] sheep culturing human PBMC with isopentenyl pyrophosphate and rhIL-2 for 10 days, γδΤ cells increased from 4.34% to 55.65% before amplification; Xi et al. found that isopentenyl pyrophosphate (IPP) and ammonia Disodium hydroxy diphosphate (PAM) has similar effects on stimulating γδΤ cells at 14 days of action [11]; heat extracted by anti-γδΤ cell receptor, IL-2, human hepatoma cell SMMC-7721, etc. Shock protein 70 (HSP70) and their different combinations induced the production of γδΤ cells in human peripheral blood. As a result, γδΤ cell receptor (0.4 μg/ml) induced a large amount of γδΤ cells, reaching 61.5%, at 0.8. When it was increased at μg/ml, it reached 75.7% [12]. When it was combined with IL-2, the yield did not change significantly. When used in combination with HSP70, the yield increased significantly, reaching 71.1% and 85.6%, respectively. HSP70 and IL-2 combined use can also produce a large number of γδΤ cells, up to 75.6%; Ding [13] and other use of Mycobacterium tuberculosis heat-resistant antigen (Mtb- HAg) and butenyl diphosphate stimulated human PBMC, while IL-2 (50 μ/ml) was administered to maintain cell proliferation, and a control group supplemented with IL-2 was also established. As a result, after 10 days of culture, the rhIL-2 group proliferated. The ratio of γδΤ cells was (13.61 ± 4.14%), the ratio of γδΤ cells in the Mtb-HAg-stimulated group was (50.71 ± 7.49%), and the ratio of γδΤ cells in the butenyl diphosphate-stimulated group was (67.39 ± 6.40%). These stimulation methods can also obtain a large number of γδΤ cells, but no immunomagnetic beads positive sorting method is used to obtain higher purity γδΤ cells.
For the two stimulations of Mtb-Ag, the activation of γδΤ cells was very different. The expression of CD69 molecules in the first stimulation of γδΤ cells reached a peak at about 24 h (75.2%), then decreased rapidly, and decreased to 1.7% on the 10th day. about. At this time, Mtb-Ag re-stimulation can reexpress CD69 molecules in γδΤ cells in Mtb-AT. Unlike the initial stimulation, the number of CD69-positive cells reached the peak (72%) after 6 h of Mtb-Ag stimulation. By 12 h (73%), it decreased to 24 h (50%), and decreased to 41% at 72 h. When the polypeptide purified from Mtb-Ag (C-main peptide) reported by Chen [14] stimulated γδΤ cells again, it can significantly express CD69 molecules, and same as the results of γδΤ have significant proliferative activity. It lays a methodological basis for seeking the rapid activation of γδΤ cells and the rapid expression of CD69 molecules, crucial to further exploring the signaling pathways involved in γδΤ cells activation.
Conclusion
Mtb-Ag can specifically stimulate the proliferation of γδΤ cells in the PBMC. Both its initial and the second stimulation can specifically activate γδΤ cells.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding
The research was supported by the National Natural Science Foundation of China (No.81473687), Shandong Provincial Medical, Health Science and Technology Development Plan (No.2015WS0095) and Tai’an Science and Technology Plan (No. 2018NS0116).
Ethical Approval and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards. The study was performed according to the Declaration of Helsinki and was approved by the ethics committee of the affiliated hospital of Taishan University. Written informed consents were obtained from all the subjects recruited into our study.
References
- Wang KQ, Hou YQ, Li QH, Zhao DP, Duan YC, Ran ZS, Li XQ. Inhibitory effect of LY294002 on CD3mAb-activated T cells and Mtb-Ag-activated γδΤ cells via TCR signal transduction pathway. Int J Clin Exp Pathol 2017;10: 5538-5544.
- Wang KQ, Hou YQ, Gu CX, Zhao DP, Duan YC, Ran ZS, Li QH, Li XQ. Inhibitory effect of the mitogen activated protein kinase specific inhibitor PD98059 on Mtb-Ag-activated γδΤ cells. Int J Clin Exp Pathol 2017;10: 9644-9648.
- Wang KQ, Hou YQ, Duan YC, Wang YR, Li XQ. A method for detecting intracellular IL-2 in γδΤ cells. Biomed Res 2018;29: 3144-3148.
- Wei L, Wang KQ, Ran ZS, Liu QH, Chen YY, Ji B, Meng L, Cao WW, An X. Auxiliary diagnostic value of γδΤ cell, IL-17, and IFN-γ levels in peripheral blood and bronchoalveolar lavage fluid for lung cancer complicated with chronic obstructive pulmonary disease. Int J Clin Exp Med 2018;11: 7183-7191.
- Wang YS, Zhou Y, Wang KQ. γδT cells in bacterium research progress in the mechanism of disease infection. Chin J Microbiol Immunol 2016;36: 555-560.
- Ramutton T, Buccheri S, Dieli F, Todaro M, Stassi G, Meraviglia S. γδΤ cells as a potential tool in colon cancer immunotherapy. Immunotherapy 2014;6: 989-999.
- Wang KQ, Hou YQ, Li Y. A simple and rapid amplification method for obtaining γδΤ cells. Clin J Hematol 2004;25:58.
- Wang KQ, Hou YQ, Gu CX, Zhao DP, Duan YC, Wang YR, Ran ZS, Li XQ. Western blotting was used to detect ZAP-70 molecule from γδΤ cells in peripheral blood. Int J Clin Exp Med 2019; 11:1785-1790.
- Han LY, Fei SJ, Chen FX, Liu JQ, Chen GL. Effect of zoledronate on human γδΤ cells killing gastric cancer cell lines SGC-7901. World Clin J Digestol 2009;17: 181-185.
- Gui WY, Ma Y, Chen FX, He X. Stimulation of gamma delta T cell by isopentenyl pyrophosphate and its effect of anti-HBV in vitro. Clin J Clini Hepatol 2008; 24: 334-337.
- Xi Y, Miao T, Wan L, Fenq T, Gong T, Li M. Amplification efficency and optimization of culture conditions of γδΤ cells in peripheral blood by different phosphate compounds. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2014;30: 868-871.
- Xia L, Liu YX, Chi YF, Wang J, Gao F, Li L, Yu J, Liu BL. Comparative study γδΤ cells on human peripheral blood using different inductive methods. Clin J Lab Diagn 2011;15: 1998-2000.
- Ding Y, Sheng LL, Qiu XJ, Xu D, Li BQ. Comparison of Mycobacterium tuberculosis heat-resistant antigens and phosphoantigens on activation and proliferation of human peripheral γδΤ cells. J Bengbu Med Co 2012;37: 745-747.
- Chen Y, Lv HZ, Li BQ, Wang W, Zhang HF. Stimulatory effect of purified mycobacterium tuberculosis peptide antigen on human γδΤ lymphocyte proliferation. Chin J Immul 2004;20: 661-664.