ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 13
Effect of rosiglitazone on the expression of tumor necrosis factor-α in the liver tissue of mice with non-alcoholic steatohepatitis
1Department of Infectious Diseases, Beijing Shijitan Hospital, Capital Medical University, Beijing, PR China
2Department of TCM-WW Integration for Liver Diseases, the Third Hospital of Hebei Medical University, Shijiazhuang, PR China
- *Corresponding Author:
- Lei Wang
Department of Infectious Diseases, Beijing Shijitan Hospital
Capital Medical University, PR China
Accepted date: May 12, 2017
The aim of this study was to investigate the effect of rosiglitazone on the expression of Tumor Necrosis Factor-α (TNF-α) in the liver tissue of mice with Non-Alcoholic Steatohepatitis (NASH) and its role in NASH progression. Fifteen healthy male C57BL/6J mice were randomly divided into three groups: the control group (MCD+), the model group (MCD-), and the rosiglitazone group (MCD ± R). The serum alanine aminotransferase (ALT) and triglyceride (TG) levels were detected using the enzyme method. Hematoxylin-Eosin (HE) staining was performed to observe steatosis, inflammation, and fibrosis in the liver tissue. Reverse Transcription Polymerase Chain Reaction (RT-PCR) and western blotting were performed to detect the expression of TNF-α mRNA and protein. Liver pathology: the MCD+ group showed no significant liver steatosis, inflammation, and fibrosis; the MCD- group showed obvious steatosis and inflammation. However, the MCD ± R group exhibited reduced lesions. The expression of TNF-α mRNA and protein in the liver tissue among the groups was as follows: group MCD ≥ group MCD ± R>group MCD+. In the mouse models of NASH, the TNF-α expression in the liver tissue was related to the progression of NASH; Rosiglitazone can inhibit the expression of TNF-α and prevent the progression of NASH, thus providing new ideas for selecting therapeutic targets for NASH.
Keywords
Non-alcoholic steatohepatitis, Tumor necrosis factor-α, Peroxisome proliferator-activated receptor γ agonist, Rosiglitazone.
Introduction
Non-Alcoholic Steatohepatitis (NASH) is the key link between the progression of simple hepatic steatosis to cirrhosis or even liver cancer [1,2]. Its incidence increases annually and is about 20% throughout the world currently [1]. NASH is the most common cause of enzyme-associated liver function abnormalities and chronic liver diseases in Europe, America, and other Western countries. Recent data suggest that NASH also shows a similar prevalence in the Middle East, Far East, Africa, Caribbean, and Latin America as in the West. NASH has gained increasing attention in recent years as an important risk factor for diabetes, atherosclerosis, and hyperlipidemia [3-5]. With the increasing incidence of fatty liver and its associated complications such as cirrhosis and liver cancer, the pathogenesis of NASH and the research of therapeutic drug targets have become the research focus in recent years [6,7]. Many cytokines are involved in the occurrence and formation of fatty liver and inflammation, among which Peroxisome Proliferator Activated Receptor γ (PPARγ) is a key transcription factor that regulates adipocyte differentiation and induction and controls the gene expression of a variety of fat cells. Thiazolidinediones (TZDs) are synthetic PPARγ agonists that regulate fat metabolism and immune responses and exert an anti-inflammatory effect by upregulating the level of PPARγ [8]. Tumor Necrosis Factor-α (TNF-α) is another important factor that regulates the body metabolism, immune function, and inflammatory tissue damage [9]; however, the role and relationship of the PPARγ agonist rosiglitazone with TNF-α in NASH and their impact on the progression of NASH are still unclear. This study used a high-fat diet deficient in choline and Methionine (MCD) to establish the NASH mouse model [10] combined with rosiglitazone intervention in order to assess the role of TNF-α in the pathogenesis of NASH. Rosiglitazone affects the expression of TNF-α and its relationship with NASH, providing the basis for the selection of treatment protocols for NASH.
Materials and Methods
Animals
Healthy male C57BL/6J mice, weighing 20~25 g, were provided by the Central Institute for Experimental Animals, Chinese Academy of Medical Sciences. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Capital Medical University.
Reagents
The reagents used for the experiment were total RNA extraction reagent kit, RNA inhibitor, AMV reverse transcriptase, Taq DNA polymerase, TNF-α amplification primer, and β-actin (SBS Genentech Co. Ltd., Beijing, China). The DNA ladder (Marker) was provided by Shanghai TWbiotech Co. Ltd., China. The goat anti-mouse TNF-α polyclonal antibody and horseradish peroxidase-labeled goat anti-rabbit IgG serum were purchased from Santa Cruz, USA. Rosiglitazone (Avandia) was purchased from GlaxoSmithKline Pharmaceuticals Ltd.
Establishment of NASH mouse model
Fifteen healthy male C57BL/6J mice were fed with adequate diet containing DL-methionine (3 g/kg) and choline chloride (2 g/kg) for one week, and then randomly divided into three groups (n=5): the control group, which were fed with the above diet (expressed as MCD+); the model group, fed with a highfat diet deficient in methionine and choline (expressed as MCD-); the rosiglitazone intervention group, fed with MCDplus rosiglitazone (50 mg/kg/d) feeding (expressed as MCD ± R). Three weeks later, all the animals were killed under deep anesthesia, and the serum specimens were sampled for the determination of ALT and TG using an enzymatic method; partial liver tissue was fixed in 10% formalin for Hematoxylin- Eosin (HE) staining and observation; the rest of the liver tissue was quickly frozen with liquid nitrogen, and stored at -80°C for the detection of TNF-α mRNA and protein expression.
Hepatic histopathological analysis
Paraffin embedding, conventional slicing, HE staining, and light microscopy were performed on the liver tissue to observe the histological changes. Referring to "The diagnosis and treatment guidelines of NASH" [11] and "prevention and treatment programs of viral hepatitis" [12], steatosis is divided into four degrees (F0~4), inflammatory activities are divided into 3 degrees (G0~3), and liver fibrosis is divided into four phases (S0~4).
Detection of TNF-α mRNA expression
The Reverse Transcription Polymerase Chain Reaction (RTPCR) method was used for the detection of TNF-α mRNA expression. The total RNA from the liver tissue was extracted using the Trizol one-step extraction kit, reversely transcribed into cDNA, and the TNF-α gene fragment was amplified using this cDNA (the template), Taq DNA polymerase, and β-actin (the internal reference). The primers of mouse TNF-α used for amplification: upstream: 5'-GGC AGG TCT ACT TTG GAG TCA TTG C-3', downstream: 5'-ACA TTC GAG GCT CCA GTG AAT TCG G-3'; the amplified fragment was 300 bp; β-actin: upstream: 5'-GAC AGG ATG CAG AAG GAG ATT ACT G-3', downstream: 5'-GCT GAT CCA CAT CTG CTG GAA-3'; the amplified fragment was 144 bp. PCR reaction parameters: pre-denaturation at 94°C for 5 min and then denaturation at 94°C for 45 s, annealing at 57°C for 45 s, extension at 72°C for 60 s, with a total of 30 cycles, followed by extension at 72°C for 5 min. Electrophoresis was then performed on the PCR products using 8% polyacrylamide gel at 100 V for 2 h; after staining in 0.5 μg/ml ethidium bromide for 10 min. The BIO-PROFIF image analysis system was used to scan the absorbance; the absorbance ratio of TNF-α/β-actin was used to calculate the relative content of TNF-α mRNA.
Detection of TNF-α protein expression
The Western blotting method was used to detect the expression of TNF-α protein. The tissue lysate containing protease inhibitors was used to extract the protein homogenates of the liver tissue; the concentration was then determined at UV 750 nm. The tissue sample containing 150 μg of proteins was denatured, and loaded onto 10% SDS-PAGE for electrophoresis. After the proteins were transferred onto the gel at 4°C for 2 h, the PVDF membrane was blocked with 5% skim milk in 1× TBST-diluted for 1 h and then incubated with the goat anti-mouse TNF-α polyclonal antibody (dilution: 1:150) overnight at 4°C; after rinsing with 1X TBST (5 min × 3), the membrane was incubated with horseradish peroxidaselabeled rabbit anti-goat polyclonal secondary antibody (dilution 1:2000) at room temperature for 1 h, followed by DAB coloring. At least three animals were selected from each group and each experiment was repeated at least three times. The ID digital imaging analysis system software (Kodak, USA) was used for the analysis of images, with the gray value expressed as the Integral Optical Density (IOD), and the level of β-actin was used as the loading control for the same amount of protein.
Statistical analysis
SPSS10.0 statistical software was used to process the data, and the experimental results were expressed as x̄ ± s. The test for homogeneity of variance was performed before ANOVA, and the Student-Newman-Keuls method was used for the intergroup comparison, with P<0.05 considered as statistically significant.
Results
Changes in serum biochemical parameters
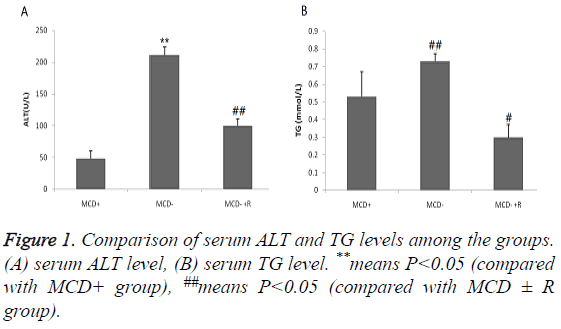
The serum ALT and TG levels in the MCD-group were significantly higher than those in the other two groups, and rosiglitazone can significantly reduce the serum levels of these two indexes. The serum ALT levels were in the order of group MCD ≥ group MCD ± R>group MCD+ (P<0.01), and the serum TG levels were in the order of group MCD≥ group MCD+>group MCD R (P<0.01, Figure 1).
Liver histopathological observation
The liver histopathology of the animals in the MCD+ group showed no obvious abnormalities (Figure 2A). The liver tissue in the group MCD- showed obvious water-like degeneration, bullous steatosis (most obvious in acinar 3 band), hepatosinusoidal gap narrowing, and lobular punctate or focal liver cell necrosis, accompanied by the infiltration of a large number of inflammatory cells, among which monocytes and lymphocytes accounted the most, together with a small amount of neutrophils (F2~3/G1~2/S1) (Figure 2B). The MCD ±R group showed significantly reduced damages than the MCDgroup, the liver cells were mildly swollen, and showed slight steatosis (F0~1/G0/S0); no significant liver cell necrosis and inflammatory cell infiltration can be seen (Figure 2C).
Figure 2: The effect of rosiglitazone on the liver histology in MCDdiet- induced steatohepatitis. Haematoxylin and eosin-stained liver sections from mice fed: (A) the control diet, (B) the MCD diet, (C) the MCD diet supplemented with rosiglitazone (50 mg kg-1d-1) (black arrows indicate hepatic steatosis and inflammation). Experimental duration is 3 weeks. Slides are representative of 5 animals per group (original magnification, 200X).
Detection of TNF-α mRNA and protein expressions in mouse liver tissue
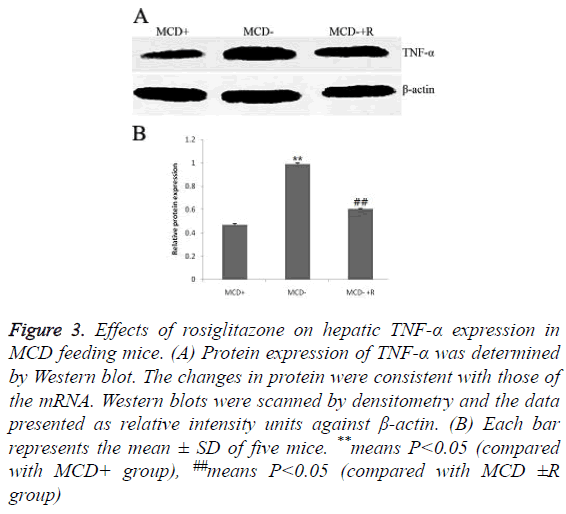
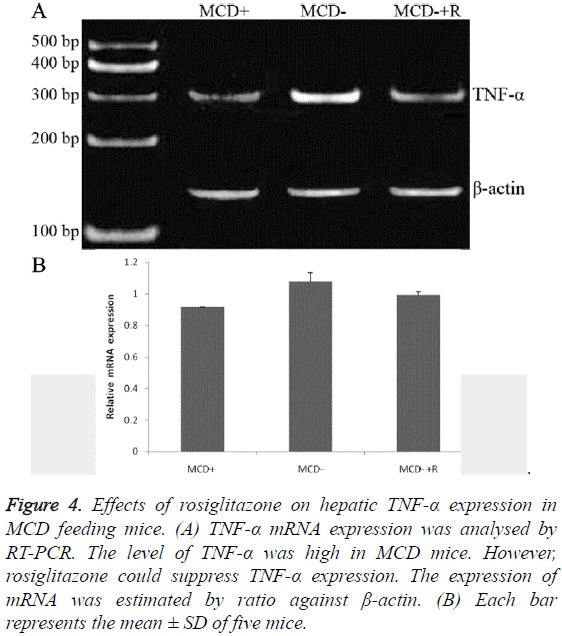
The expressions of TNF-α protein were in the order of group MCD ≥ group MCD ± R>group MCD+ (0.99 ± 0.01, 0.60 ± 0.01, 0.47 ± 0.01, P<0.01) (Figures 3A and 3B). The expressions of TNF-α mRNA were in the order of group MCD ≥ group MCD ± R>group MCD+ (1.11 ± 0.01, 0.99 ± 0.02, 0.91 ± 0.00, P<0.01, Figures 4A and 4B).
Figure 3: Effects of rosiglitazone on hepatic TNF-α expression in MCD feeding mice. (A) Protein expression of TNF-α was determined by Western blot. The changes in protein were consistent with those of the mRNA. Western blots were scanned by densitometry and the data presented as relative intensity units against β-actin. (B) Each bar represents the mean ± SD of five mice. **means P<0.05 (compared with MCD+ group), ##means P<0.05 (compared with MCD ±R group)
Figure 4: Effects of rosiglitazone on hepatic TNF-α expression in MCD feeding mice. (A) TNF-α mRNA expression was analysed by RT-PCR. The level of TNF-α was high in MCD mice. However, rosiglitazone could suppress TNF-α expression. The expression of mRNA was estimated by ratio against β-actin. (B) Each bar represents the mean ± SD of five mice.
Discussion
Hepatic steatosis and inflammation are regulated by a variety of cytokines. TNF-α is an important factor regulating the body metabolism, immune functions, and inflammatory tissue damages [13-16]. PPARγ and its agonists play important roles in adipocyte differentiation and induction as well as in controlling the gene expressions of various cytokines produced by the fat cells. The latter can upregulate PPARγ to play an important role in regulating fat metabolism and immune functions and exerting anti-inflammatory effects [17,18]. However, the roles of TNF-α and PPAR-γ agonists in NASH and the factors that may impact their expressions are still unclear. This study used a high-fat MCD diet to establish the mouse model of NASH in order to investigate the interactions and relationship between these two during NASH progression.
Our results showed that in the MCD-induced NASH mouse model, the expression of TNF-α was significantly upregulated and exhibited a changing trend consistent with the degrees of steatosis and inflammation as well as the serum ALT and TG levels. This indicates that TNF-α is one of the key factors promoting the formation of liver steatosis and inflammation, and it is positively correlated with the development of NASH, which is consistent with the expected results of this study. According to our results, the possible regulatory mechanisms of TNF-α in the pathogenesis of NASH may be: 1). stimulation of the decomposition of glycogen in the fat tissue resulting in an increase of Free Fatty Acids (FFA) [19]. The excessive FFA then acts on the liver cells, which can lead to an increase in the swelling of liver mitochondria and permeability, liver cell degeneration, necrosis, and inflammatory cell infiltration [20]; 2). FFA can also lead to the increase of lipid peroxidation, which generates reactive oxygen species, free radicals, and malondialdehyde. The fluidity and permeability of cell membranes causes disorders, resulting in liver cell dysfunction or necrosis. In addition, when arachidonic acid is stimulated by reactive oxygen species, it can produce leukotrienes that induce neutrophil chemotaxis [21]; 3). FFA can also be upregulated by CYP2E1, thus increasing the oxygen consumption in liver tissue. Therefore, the reduced coenzyme II-dependent microsomal lipid peroxidation will be further strengthened, thus further aggravating the liver damages; 4) Induction of the release of IL-1, IL-6, or other cytokines, thereby increasing the inflammation of the liver. The specific regulatory mechanisms of TNF-α in the pathogenesis of NASH still need further studies.
The treatment of NASH should be based on lifestyle interventions such as reasonable diet and increased physical activities, together with drug therapies [22]; however, currently, there is lack of effective drugs for the treatment of NASH owing to its unclear pathogenesis. Targeted gene regulation is the focus of the current research. PPAR-γ, a ligand-activated nuclear transcription factor that belongs to the nuclear receptor superfamily, can modify the expression of chemokines in monocyte/macrophage, inhibit the aggregation and activation of neutrophils and polymorphonuclear leukocytes, inhibit the adhesion of monocytes to endothelial cells, and inhibit the production of pro-inflammatory cytokines; thus, PPAR-γ plays a role in mediating antiinflammatory effects [23]. Rosiglitazone belongs to TZDs and is a PPAR-γ agonist with high affinity. It plays important roles in regulating lipid and glucose metabolism as well as in the physiological and pathological processes of inflammation, hypertension, or coronary heart diseases. It has minor liver toxic side effects. Therefore, its effects in the treatment of NASH have received widespread attention. In the model group, the presence of steatosis is tightly associated with chronic hepatic inflammation, an effect in part mediated by activation of the Ikk-b/NF-kB signaling pathway. In the models of MCD MCDinduced steatosis, increased NF-kB activity is associated with elevated hepatic expression of inflammatory cytokines such as TNF-a, interleukin- 6 (IL-6) and interleukin 1-beta (IL-1b), and activation of Kupffer cells. The results of this study showed that the mice in the group MCD-+R showed significant reduction in steatosis and inflammation of the liver tissue compared with the group MCD-; the expression of TNF-α was also decreased significantly (P<0.01), indicating that rosiglitazone can decrease the expression of TNF-α, improve liver steatosis and inflammatory injury, and reduce the serum ALT and TG levels. This is considered to be related to the ability of rosiglitazone to activate PPAR-γ and inhibit the TNF- α expression in adipose tissue and inflammatory cells; Based on our results, rosiglitazone can inhibit the expression of TNF- α in the liver tissue of mice with non-alcoholic steatohepatitis, and improve the inflammation symptom in NASH. We think that the PPARγ agonist rosiglitazone impressed the release of TNF-α associated with IL-1, IL-6, or other cytokines, thereby attenuating the inflammation of the liver. Since the study subjects selected for this study were mice, the related research findings still need to be deepened in order to investigate the clinical significance in humans.
In summary, in the MCD-induced NASH mouse model, the changes in serum ALT and TG levels showed similar trends as observed in the expression levels of hepatic TNF-α mRNA and protein; Rosiglitazone can modify the activities of the liver TNF-α; therefore, it can mitigate the MCD-induced hepatic steatosis and inflammation, indicating that rosiglitazone may be one of the effective drugs for the treatment of NASH.
Conflict of Interest
All authors have no conflict of interest regarding this paper.
References
- Nalbantoglu IL, Brunt EM. Role of liver biopsy in nonalcoholic fatty liver disease. World J Gastroenterol 2014; 20: 9026-9037.
- Chang PE, Goh GB, Ngu JH, Tan HK, Tan CK. Clinical applications, limitations and future role of transient elastography in the management of liver disease. World J Gastrointest Pharmacol Ther 2016; 7: 91-106.
- Milic S, Lulic D, Stimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol 2014; 20: 9330-9337.
- Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J Gastroenterol 2014; 20: 1724-1745.
- Leite NC, Villela-Nogueira CA, Cardoso CR, Salles GF. Non-alcoholic fatty liver disease and diabetes: From physiopathological interplay to diagnosis and treatment. World J Gastroenterol 2014; 20: 8377-8392.
- Gupta V, Mah XJ, Garcia MC, Antonypillai C, van der Poorten D. Oily fish, coffee and walnuts: dietary treatment for nonalcoholic fatty liver disease. World J Gastroenterol 2015; 21: 10621-10635.
- Schwenger KJ, Allard JP. Clinical approaches to non-alcoholic fatty liver disease. World J Gastroenterol 2014; 20: 1712-1723.
- Schmilovitz-Weiss H, Hochhauser E, Cohen M, Chepurko Y, Yitzhaki S, Grossman E, Leibowitz A, Ackerman Z, Ben-Ari Z. Rosiglitazone and bezafibrate modulate gene expression in a rat model of non-alcoholic fatty liver disease-a historical prospective. Lipids Health Dis 2013; 12: 41.
- Lim JW, Dillon J, Miller M. Proteomic and genomic studies of non-alcoholic fatty liver disease-clues in the pathogenesis. World J Gastroenterol 2014; 20: 8325-8340.
- Willebrords J, Pereira IV, Maes M, Crespo Yanguas S, Colle I, Van Den Bossche B, Da Silva TC, de Oliveira CP, Andraus W, Alves VA, Cogliati B, Vinken M. Strategies, models and biomarkers in experimental non-alcoholic fatty liver disease research. Prog Lipid Res 2015; 59: 106-125.
- Labrecque DR, Abbas Z, Anania F, Ferenci P, Khan AG, Goh KL, Hamid SS, Isakov V, Lizarzabal M, Peñaranda MM, Ramos JF, Sarin S, Stimac D, Thomson AB, Umar M, Krabshuis J, LeMair A; World Gastroenterology Organisation. World Gastroenterology Organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Clin Gastroenterol 2014; 48: 467-473.
- Brook G, Bhagani S, Kulasegaram R, Torkington A, Mutimer D, Hodges E, Hesketh L, Farnworth S, Sullivan V, Gore C, Devitt E, Sullivan AK, Clinical Effectiveness Group British Association for Sexual Health and HIV. United Kingdom National Guideline on the Management of the viral hepatitides A, B and C 2015. Int J STD AIDS 2016; 27: 501-525.
- Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal overgrowth, intestinal permeability, endotoxaemia, and tumor necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut 2001; 48: 206-211.
- Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett 2008; 582: 117-131.
- Chen X, Xun K, Chen L, Wang Y. TNF-alpha, a potent lipid metabolism regulator. Cell Biochem Funct 2009; 27: 407-416.
- Zhang J, Tan Y, Yao F, Zhang Q. Polydatin alleviates non-alcoholic fatty liver disease in rats by inhibiting the expression of TNF-α and SREBP-1c. Mol Med Rep 2012; 6: 815-820.
- Kallwitz ER, McLachlan A, Cotler SJ. Role of peroxisome proliferators-activated receptors in the pathogenesis and treatment of nonalcoholic fatty liver disease. World J Gastroenterol 2008; 14: 22-28.
- Lemoine M, Serfaty L, Cervera P, Capeau J, Ratziu V. Hepatic molecular effects of rosiglitazone in human non-alcoholic steatohepatitis suggests long-term pro-inflammatory damage. Hepatol Res 2014; 44: 1241-1247.
- Castro Cabezas M, Erkelens DW, van Dijk H. Free fatty acids: mediators of insulin resistance and atherosclerosis. Ned Tijdschr Geneeskd 2002; 146: 103-109.
- Boden G, She P, Mozzoli M, Cheung P, Gumireddy K, Reddy P, Xiang X, Luo Z, Ruderman N. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappa B pathway in rat liver. Diabetes 2005; 54: 3458-3465.
- Day CP. Pathogenesis of steatohepatitis. Best Pract Res Clin Gastroenterol 2002; 16: 663-678.
- Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology 2003; 38: 413-419.
- Panzer U, Schneider A, Guan Y, Reinking R, Zahner G, Harendza S, Wolf G, Thaiss F, Stahl RA. Effects of different PPAR gamma agonists on MCP 1 expression and monocytere cruitment in experiment glomerulonephritis. Kindney Int 2002; 62: 455-464.