ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 12
Effect of thyroxine combined with donepezil on expression of synaptotagmin-1 and munc-18 in the frontal lobe of adult rats with hypothyroidism
Yanyan Wang1#, Zhangxiang Chen2#, Min Zhu2, Zhangbi Wu2, Hao Yang2, Fen Wang2 and Defa Zhu2*
1Department of Endocrinology, the Fourth Affiliated Hospital of Anhui Medical University, Hefei 230022, PR China
2Department of Geriatric Endocrinology, the First Affiliated Hospital of Anhui Medical University, Hefei 230022, PR China
#Co-first authors: Yanyan Wang and Zhangxiang Chen
- *Corresponding Author:
- Defa Zhu
Department of Geriatric Endocrinology
The First Affiliated Hospital of Anhui Medical University, PR China
Accepted on May 04, 2017
The aim of the current study was to observe the effect of thyroxine combined with donepezil on the expression of synaptotagmin-1 (syt-1) and mammalian uncoordinated-18 (munc-18) in the frontal lobe of adult rats with hypothyroidism and to explore the possible mechanisms of hypothyroidism-induced brain injuries. A total of 45 adult SD rats were randomly divided into a control group (CON), a hypothyroidism group (hypothyroidism), a thyroxine-treatment group (T4), a donepezil-treatment group (DON), and a T4+DON group. The rats were anesthetized with chloral hydrate and blood samples were collected from the abdominal aorta; then, levels of thyroid hormones T3 and T4 and of thyroid stimulating hormone (TSH) were measured in the centrifugal serum. The brain of each rat was sampled on ice and the prefrontal lobe was isolated and stored at -80°C to detect the expression of SYT-1 and munc-18. The expression of syt-1 and munc-18 in the rats’ frontal lobe was analyzed by a western blot analysis and reverse transcriptionpolymerase chain reaction (RT-PCR). Compared with the CON group, the expression of syt-1 and munc-18 in the hypothyroidism group was significantly decreased (P<0.01), even after T4 or DON treatment (P<0.05). However, after T4+DON treatment, expression levels returned to normal values. Hypothyroidism in adults can down-regulate syt-1 and munc-18 in the frontal lobe and a single treatment of either T4 or DON will not be effective. Protein expression can return to normal after a combined treatment with T4 and DON, suggesting that a combined therapy is more conducive to the recovery of damaged proteins in the region of hypothyroidism-induced brain injury.
Keywords
Hypothyroidism, Frontal lobe, Synaptotagmin-1, Munc-18, Thyroxine, Donepezil
Introduction
Thyroxine plays an important role in the development, maturation, and function maintenance of the central nervous system. Previous studies have shown that a change in thyroid hormone levels increases the expression of certain genes in the brain [1]. The basis of neurofunctions is the release of synaptic neurotransmitters, among which synaptotagmin is a key protein involved in the release process, playing important roles in synaptic transmission as well as in short-term and long-term synaptic plasticity [2,3]. Thyroxine (T4) is involved in the regulation of many synaptic proteins [4]. Synaptic proteins are involved in expression regulation and phosphorylation under the control of thyroid hormones, and thyroid hormones may impair the release of neurotransmitters and synaptic plasticity by altering the expression of these proteins. The frontal lobe is the most advanced part in the mammalian brain and an essential central nervous area related to learning and memory [5,6]. Adulthood hypothyroidism can cause frontal lobe damage, and possible underlying mechanisms involve changes in expression of synaptic proteins in the frontal lobe [7,8]. Syt-1 is one brain-specific subtype of synaptotagmin, which regulates the regulation and recycling of synaptic vesicles, promotes the release of neurotransmitters, and is related to learning and memory [9]. Munc-18 is a presynaptic intracellular protein that can promote the anchor of neurotransmitter-containing vesicles on the target membrane and their fusion with the target membrane [10]; it thus plays an important role in the process of neurotransmitter secretion by synaptic vesicles. Studies have shown that hypothyroidism leads to expression damage of syt-1 or munc-18, and while replacement therapy using conventional T4 doses can make serum thyroxine return to normal levels, the expression of synaptic brain proteins fails to fully recover. High-dose thyroxine shock therapy in rats with hypothyroidism can return the expression of a number of intra-hippocampal synaptic proteins to normal levels [8,11,12]. However, the application of a high-dose thyroxine treatment increases the risk of hyperthyroidism; therefore, it is necessary to find new methods to treat hypothyroidism-caused brain damage. The neurotransmitter cholinesterase inhibitor donepezil (DON) has protective effects on cognitive and neurological function [13]. This study investigated the effects of T4+DON on the expression of syt-1 and munc-18 in the frontal lobe of adult rats with hypothyroidism as well as their intervention effects.
Materials and Methods
Animals and model preparation
A total of 45 healthy adult male SD rats, weighing 260-300 g, provided by the Nanjing Experimental Animal Center, which were fed a standard rodent diet and had free access to water under normal conditions (21-23°C, humidity 50 ± 5%, normal circadian rhythm) were randomly divided into the following five groups after one week of adaptive feeding: CON, hypothyroidism, T4, DON, T4+DON. All groups except the CON group were given water containing 0.05% propylthiouracil (PTU, w/v) for 6 weeks to establish an adult hypothyroidism rat model. The hypothyroidism rat model was defined as successful if TSH levels increased and T4 levels decreased. Four weeks later and while continuing the model preparation, the T4 group was intraperitoneally injected with L-thyroxine (L-T4) at a dose of 6 μg/100 g body weight per day, while the T4+DON group was administered 0.005% DON-containing water (w/v) as a single treatment. The CON group was given normal water, and was intraperitoneally injected with normal saline 4 weeks later. The rats in each group were weighed once a week and the dosage was adjusted according to their body weight. The treatment lasted 2 weeks. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Anhui Medical University.
Specimen preparation
At the end of the animal modelling, each rat was weighed and injected with 0.3 mL of chloral hydrate per 100 g body weight. The abdominal cavity was opened for sampling blood from the abdominal aorta. The serum was centrifuged at 14000 r/min for 15 min, and then stored at -80°C for measuring levels of T3, T4, and thyroid stimulating hormone (TSH). After blood sampling, each rat was sacrificed. The brain of the rat was then sampled on ice and the prefrontal lobe was isolated and stored in -80°C to measure the expression of Syt-1 and munc-18. The frontal lobe was then dissociated on ice, and stored at -80°C for the determination of Syt-1 and munc-18.
Determination of T3, T4, and TSH
Serum levels of T3, T4, and TSH were measured using radioimmunoassays, while strictly referring to the kit instructions (North Institute of Biological Technology, Beijing, China).
Western blot
The frontal lobe tissue was homogenized on ice together with the protein lysate (10 mmol/L 4-(2-hydroxyethyl)-1- piperazineethanesulfonic acid (HEPES), 1 mmol/L LEDTA, 10% sucrose, pH 7.4, and 2 μL/mL protease inhibitor cocktail) to extract total proteins. The mixture was centrifuged at 2000 r/min for 8 min at 4°C, and the obtained supernatant was then centrifuged at 12000 r/min for 15 min. Proteins were quantified using the bicinchoninic acid (BCA) method. After quantification, all samples were mixed with 4 × protein electrophoresis buffer (3:1) and boiled for 5 min to denature the proteins. After cooling to room temperature, 25 μg of total proteins from each sample were electrophoresed in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDSPAGE), transferred onto one membrane, closed using 5% nonfat dry milk at room temperature for 60 min. Then, the primary syt-1 antibody (1:2500, rabbit polyclonal antibody, Abcam, USA) or the primary munc-18 antibody (1:1000, rabbit polyclonal antibody, Abcam, USA) were added, together with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:4000, rabbit polyclonal antibody, Abcam, USA), for overnight incubation at 4°C. After washing three times for 10 min using 0.05% PBS-T, horseradish peroxidase-labeled rabbit anti-goat IgG (1:100,000, Abcam, USA) was added for a 90- min incubation at room temperature, followed once more by washing three times for 10 min using 0.05% PBS-T; then, ECL coloration was performed and each sample was developed. The optical density ratio of syt-1 and munc-18 to GAPDH was used to indirectly reflect the expression of syt-1 and munc-18 in the sample.
Total RNA extraction and real-time reverse transcriptionpolymerase chain reaction (RT-PCR)
Total RNA was extracted strictly referring to the kit instructions (Molecular Research Center, Cincinnati, USA) and purity was measured (expressed by the ratio of the absorbance values at 260 nm and 280 nm). Of the total RNA, 1 μg was then reverse transcribed to the corresponding cDNA (AMV; Promega Corporation, USA). PCR amplification was performed as follows: predenaturation at 95°C for 5 min, denaturation at 95°C for 30 s, annealing at 60°C for 30 s, extension at 72°C for 30 s, for 30 cycles. All reactions were performed in a Light Cycler® 480 FQ-PCR instrument (Roche Diagnostics GmbH, Mannheim, Germany). Gene expression levels were represented by the relative computed tomography (CT) value and normalized by comparing to the internal reference GAPDH. The RT-PCR primers are shown in Table 1.
| Primer | Sequence | Fragment size (bp) |
|---|---|---|
| Munc18 | Forward: 5’-AGACATCATGACCGAGGGGA-3’ | 128 |
| Reverse: 5’-AGACGGGGTGATGAGGTACA-3’ | ||
| SYN | Forward: 5’-CTTGTCCCACACAATGCCACT-3’ | 150 |
| Reverse: 5’-AAGGACCGCAACTATGGCT-3’ | ||
| GAPDH | Forward: 5’-AGTGCCAGCCTCGTCTCATA-3’ | 91 |
| Reverse: 5’-GAGAAGGCAGCCCTGGTAAC-3’ |
Table 1. RT-PCR primers.
Statistical analysis
All data were analyzed using SPSS 16.0. The experimental data were expressed as mean ± standard deviation (x̅ ± s). For intergroup comparison, we used one-way ANOVA, and the LSD method was implemented for multiple comparisons among groups. P<0.05 was considered statistically significant.
Results
Analysis of T3, T4, and TSH
Compared with the CON group, serum levels of T3 and T4 in the hypothyroidism and the DON group were decreased, while TSH was significantly increased (P<0.01). Levels of T3, T4, and TSH in the T4 and the T4+DON group showed no significant differences compared to the CON group (Table 2).
| Group | T3 (nmol/L) | T4 (nmol/L) | TSH (umol/L) |
|---|---|---|---|
| CON | 0.82±0.06 | 49.98±3.96 | 1.13±0.21 |
| Hypo | 0.59±0.16** | 19.23±4.02** | 20.65±10.38** |
| T4 | 0.80±0.21 | 50.10±4.98 | 1.09±0.49 |
| DON | 0.61±0.19** | 18.36±5.13** | 21.36±11.04** |
| T4+DON | 0.82±0.08 | 51.29±6.17 | 1.21±0.39 |
Note: compared with Group CON: **P<0.01.
Table 2. T3, T4 and TSH levels in each group (n=9, x ± s).
Western blot
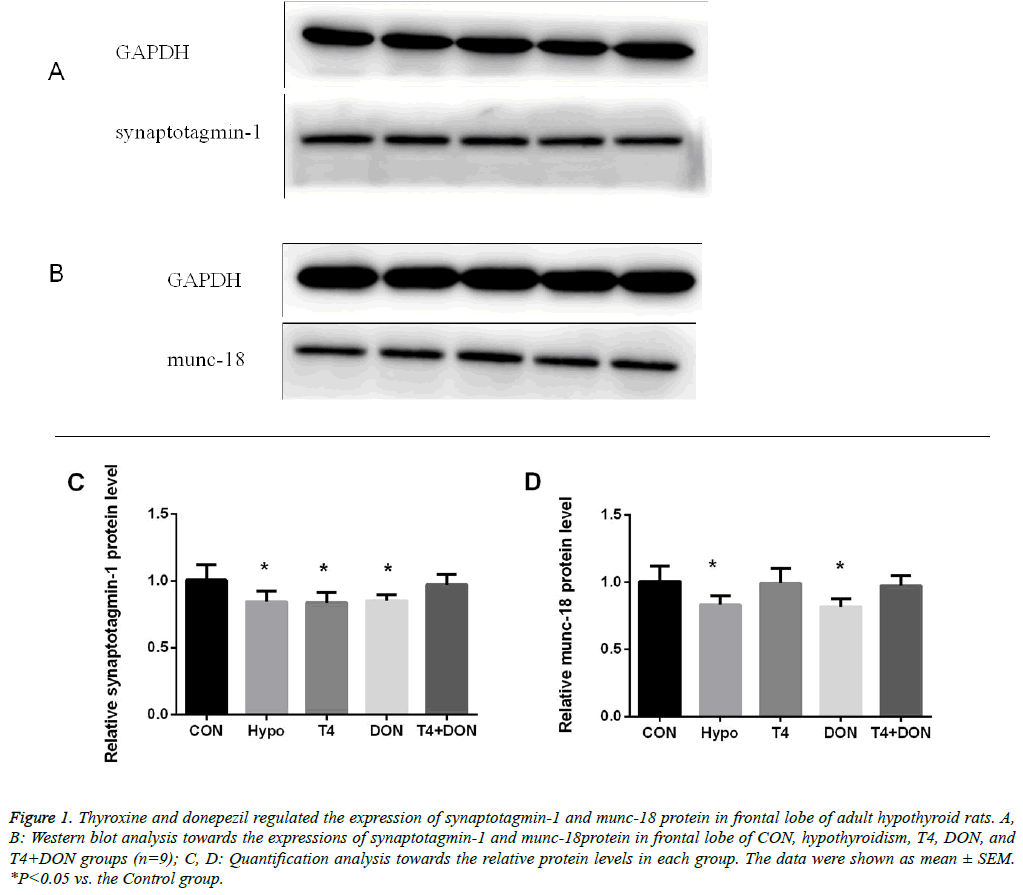
The western blot results showed that compared with the CON group, syt-1 was down-regulated in the frontal lobe of the hypothyroidism group, accounting for only 83.9% of syt-1 levels in the CON group (P<0.05). The recovery of the downregulated syt-1 protein in the T4 and the DON group was not significant, accounting for 84.1% and 84.8% (P<0.05) of levels in the CON group, respectively. The expression of syt-1 protein in the frontal lobe of the T4+DON group accounted for 96.5% of protein levels in the CON group, but the difference was not statistically significant (Figures 1A-1C). Levels of munc-18 were down-regulated in the frontal lobe of the hypothyroidism group, accounting for 82.4% of levels in the CON group (P<0.05). In the DON group, the down-regulated levels of munc-18 protein were recovered compared to the hypothyroidism group (P<0.05), but there was still a marginal difference in the levels of munc-18 protein between the CON and DON groups, accounting for 80.8% of CON group levels (P<0.05). The levels of munc-18 in the T4 and the T4+DON group was 98.3% and 96.5% of the levels of those proteins in the CON group, respectively, and there was no significant intergroup difference (Figures 1B and 1D).
Figure 1: Thyroxine and donepezil regulated the expression of synaptotagmin-1 and munc-18 protein in frontal lobe of adult hypothyroid rats. A, B: Western blot analysis towards the expressions of synaptotagmin-1 and munc-18protein in frontal lobe of CON, hypothyroidism, T4, DON, and T4+DON groups (n=9); C, D: Quantification analysis towards the relative protein levels in each group. The data were shown as mean ± SEM. *P<0.05 vs. the Control group.
RT-PCR
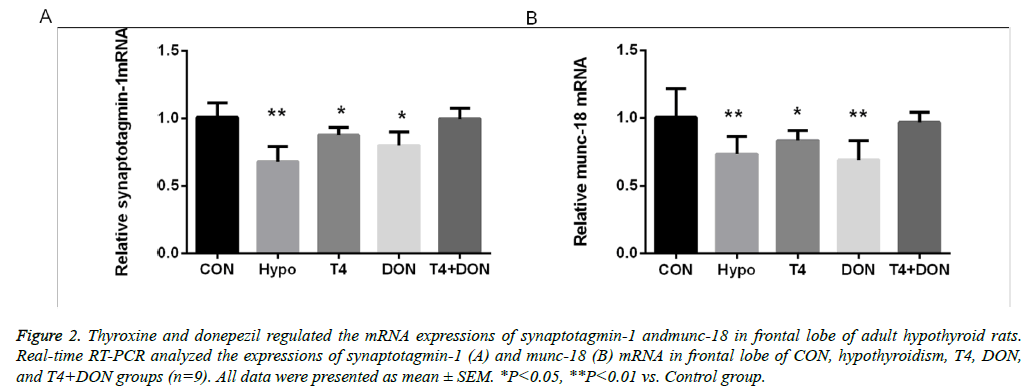
The RT-PCR results showed that, compared with the CON group, syt-1 was down-regulated in the frontal lobe of the hypothyroidism group, accounting for 67.2% of levels in the CON group (P<0.01). However, the down-regulated syt-1 protein levels in the T4 and the DON group were recovered compared to the hypothyroidism group, accounting for 86.9% and 79.3% of those in the CON group, respectively (P<0.05). The expression of syt-1 protein in the frontal lobe of the T4+DON group represented 99.0% of that in the CON group, and the difference was not statistically significant (Figure 2A). Levels of munc-18 were significantly down-regulated in the frontal lobe of the hypothyroidism group, accounting for 72.8% of the CON group levels (P<0.01). In the T4 group, the down-regulated munc-18 protein was recovered compared to the hypothyroidism group (P<0.05), accounting for 83.0% of munc-18 levels in the CON group (P<0.05). In the DON group, the down-regulated munc-18 protein was not obviously recovered, accounting for 68.6% of the levels in the CON group (P<0.01). Expression of munc-18 in the T4+DON group represented 96.2% of that in the CON group, and there was no significant intergroup difference (Figure 2B).
Figure 2: Thyroxine and donepezil regulated the mRNA expressions of synaptotagmin-1 andmunc-18 in frontal lobe of adult hypothyroid rats. Real-time RT-PCR analyzed the expressions of synaptotagmin-1 (A) and munc-18 (B) mRNA in frontal lobe of CON, hypothyroidism, T4, DON, and T4+DON groups (n=9). All data were presented as mean ± SEM. *P<0.05, **P<0.01 vs. Control group.
Discussion
The brain is an important target organ of thyroid hormones, and thyroid hormone deficiency in fetuses and infants has obvious effects on the brain. Many studies have confirmed that adult hypothyroidism also leads to structural and functional damage of the brain [14-16]. The frontal lobe is the advanced part of the brain, is closely related to cognitive functions, and is the important anatomical basis and nerve center related to learning and memory [5]. Hypothyroidism can cause cognitive impairment in the frontal lobe, especially learning and memory disorders. The mechanisms of hypothyroidism, among which synaptic plasticity may be an important aspect, are complex. Thyroid hormones may alter the expression of some neurotransmitters, receptors, proteins, and genes in neurons and synapses, thereby impairing the release of neurotransmitters and synaptic plasticity. The regulation of synaptic transmission and synaptic plasticity requires the participation of a series of synaptic proteins, and syt-1 and munc-18 are two important synaptic proteins involved in the whole process of anchoring, initiation, and fusion of synaptic vesicles.
The SNARE core complex is the smallest functional unit of membrane fusion [17], and a large influx of Ca2+ can promote the complete polymerization of the spiral beam of the SNARE core complex. This induces the fusion of synaptic vesicle membranes and presynaptic membranes to release intravesicular neurotransmitters [18]; during this process, syt-1 is a critical factor in mediating the anchoring of synaptic vesicles onto the presynaptic membrane [19]. Syt-1 is one of the most important Ca2+ receptors in the process of membrane fusion and exocytosis, accounting for 7% of the total amount of vesicular proteins [20]. It may be the most important Ca2+ receptor in regulating the rapid synaptic transmission [21,22]. Mice with syt-1 gene knockout showed severe Ca2+-induced synaptic vesicle efflux dysfunction, which further confirms the roles of syt-1 in the process of membrane fusion and exocytosis [23].
Munc-18 is a synaptic protein located in the presynaptic cytosol and mainly expressed in the brain. As munc-18 is a hydrophilic protein related to secretion, it can combine with SNARE in a variety of ways. It is a main component of the synaptic vesicle fusion protein complex, can promote the anchoring of neurotransmitter-containing vesicles onto the target membrane and the fusion of the vesicles to the target membrane [9], and plays an important role in the process of neurotransmitter secretion from synaptic vesicles via "excretion" [24], thereby promoting the release of neurotransmitters. Mutations of munc-18 will result in the complete blockade of neurotransmitter secretion [25], and apoptosis of embryonic neural cells can be observed in mice with munc-18 mutation-caused neurotransmitter secretion deficiency [26]. In this study, the expression of syt-1 and munc-18 in the frontal lobe of the hypothyroidism group was reduced compared to the CON group. Studies [11,27,28] found that syt-1 and munc-18 levels in the hippocampus or cerebellum of rats with hypothyroidism were decreased, which are consistent with our findings, and the quantitative PCR analysis in the current study also showed similar changes for mRNA levels. These results suggest that thyroid hormones can regulate protein synthesis in the central nervous system.
L-thyroxine replacement therapy is a standard clinical treatment against hypothyroidism. The reduction in expression of syt-1 and munc-18 in the frontal lobe of the hypothyroidism rats treated with physiological doses of thyroxine or donepezil was partially restored, which was consistent with the results of earlier studies [11,29]. The incomplete expression recovery of syt-1 and munc-18 in the prefrontal cortex may be associated with short treatment times and insufficient doses. Alzoubi et al. [30] found that a 6-week physiological-dose thyroxine treatment can reverse synaptic protein expression abnormalities in the hippocampus. Van-Doorn et al. [31] found that the concentration of thyroid hormones in the central nervous system was much lower than their serum concentrations; when the physiological-dose hormone replacement recovers serum thyroid hormone expression, the thyroid hormone level in the central nervous system may therefore not return to normal value.
T4+DON treatment can return hypothyroidism-caused changes to normal values, which was found to be more effective than the application of T4 or DON alone, and was consistent with the findings of previous studies [27,32]. As a neurotransmitter cholinesterase inhibitor, DON combines with cholinesterase, thus preventing the hydrolysis of neurotransmitter acetylcholine in the brain and increasing the acetylcholine concentration in the synaptic cleft; in clinical settings, because of its independent neuroprotective effects, DON is mainly used for the treatment of mild to moderate cognitive dysfunction. Previous studies [33,34] have suggested that DON may play a neuroprotective role by improving the structures of neuronal organelles and synapses in the hippocampus. Certain studies [13] also showed that DON can improve the expression of synaptic proteins in the hippocampus of rats with Tau by inducing the anti-inflammatory effects of acetylcholine. Kotani et al. [35] found that DON increased the connections between neurons and synapses. In the present study, syt-1 and munc-18 levels returned to normal in the frontal lobe of the T4+DON group, and the recovery of such abnormally expressed synaptic proteins may reflect the neuroprotective effects of DON.
Collectively, our findings show that hypothyroidism can cause the aberrant expression of syt-1 and munc-18 in the frontal lobe, and that T4 or DON alone cannot fully restore such damages. The combination of T4+DON brought down the protein expression levels to normal values, suggesting that a combined therapy is more conducive to the recovery of proteins in brain injury caused by hypothyroidism. These findings can therefore provide a theoretical basis as well as research directions for the clinical treatment of hypothyroidinduced brain injury.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81272152).
References
- Dickson PW, Aldred AR, Menting JG, Marley PD, Sawyer WH, Schreiber G. Thyroxine transport in choroid plexus. J Biol Chem 1987; 262: 13907-13915.
- Schreiber G, Aldred AR, Jaworowski A, Nilsson C, Achen MG, Segal MB. Thyroxine transport from blood to brain via transthyretin synthesis in choroid plexus. Am J Physiol 1990; 258: R338-345.
- Alzoubi KH, Gerges NZ, Alkadhi KA. Levothyroxin restores hypothyroidism-induced impairment of LTP of hippocampal CA1: electrophysiological and molecular studies. Exp Neurol 2005; 195: 330-341.
- Hélie S, Ell SW, Ashby FG. Learning robust cortico-cortical associations with the basal ganglia: an integrative review. Cortex 2014; 64: 123-135.
- Puig MV, Antzoulatos EG, Miller EK. Prefrontal dopamine in associative learning and memory. Neuroscience 2014; 282: 217-229.
- Sui L, Wang F, Li BM. Adult-onset hypothyroidism impairs paired-pulse facilitation and long-term potentiation of the rat dorsal hippocampo-medial prefrontal cortex pathway in vivo. BrainRes 2006; 1096: 53-60.
- Yang HY, Sun CP, Jia XM, Gui L, Zhu DF, Ma WQ. Effect of thyroxine on SNARE complex and synaptotagmin-1 expression in the prefrontal cortex of rats with adult-onset hypothyroidism. J Endocrinol Invest 2011; 35: 312-316.
- Chen GH, Wang YJ, Qin S, Yang QG, Zhou JN, Liu RY. Age-related spatial cognitive impairment is correlated with increase of synaptotagmin 1 in dorsal hippocampus in SAMP8 mice. Neurobiol Aging 2007; 28: 611-618.
- Weimer RM, Richmond JE, Davis WS, Hadwiger G, Nonet ML, Jorgensen EM. Defects in synaptic vesicle docking in unc-18 mutants. Nat Neurosci 2003; 6: 1023-1030.
- Zhu Y, Ning D, Wang F, Liu C, Xu Y, Jia X, Zhu D. Effect of thyroxine on munc-18 and syntaxin-1 expression in dorsal hippocampus of adult-onset hypothyroid rats. Eur J Histochem 2012; 56: e22.
- Liu CL, Xu YX, Zhan Y, Hu HL, Jia XM, Chen GH, Zhu DF. Effect of thyroxine on synaptotagmin 1 and SNAP-25 expression in dorsal hippocampus of adult-onset hypothyroid rats. J Endocrinol Invest 2011; 34: 280-286.
- Yoshiyama Y, Kojima A, Ishikawa C, Arai K. Anti-inflammatory action of donepezil ameliorates tau pathology, synaptic loss, and neurodegeneration in a tauopathy mouse model. J Alzheimers Dis 2010; 22: 295-306.
- Khedr EM, El Toony LF, Tarkhan MN, Abdella G. Peripheral and central nervous nervous system alterations in hypothyroidism: electrophysiological findings. Neuropsychobiology 2000; 41: 88-94.
- Gerges NZ, Stringer JL, Alkadhi KA. Combination of hypothyroidism and stress abolishes early LTP in the CA1 but not dentate gyrus of hippocampus of adult rats. Brain Res 2001; 922: 250-260.
- Palha JA, Nissanov J, Fernandes R, Sousa JC, Bertrand L, Dratman MB, Morreale de Escobar G, Gottesman M, Saraiva MJ. Thyroid hormone distribution in the mouse brain: the role of transthyretin. Neuroscience 2002; 113: 837-847.
- Domanska MK, Kiessling V, Stein A, Fasshauer D, Tamm LK. Single vesicle millisecond fusion kinetics reveals number of SNARE complexes optimal for fast SNARE-mediated membrane fusion. J Biol Chem 2009; 285: 32158-32166.
- Jahn R, Südhof TC. Membrane fusion and exocytosis. Annu Rev Biochem 1999; 68: 863-911.
- Hui E, Johnson CP, Yao J, Dunning FM, Chapman ER. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca2+-regulated fusion. Cell 2009; 138: 709-721.
- Lindau M. Synaptotagmin function illuminated. J Gen Physiol 2003; 122: 251-253.
- Stevens CF, Sullivan JM. The synaptotagmin C2A domain is part of the calcium sensor controlling fast synaptic transmission. Neuron 2003; 39: 299-308.
- Adolfsen B, Littleton JT. Genetic and molecular analysis of the synaptotagmin family. Cell Mol Life Sci 2001; 58: 393-402.
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Südhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell 1994; 79: 717-727.
- Okamoto M, Südhof TC. Mints, Munc18-interacting proteins in synaptic vesicle exocytosis. J Biol Chem 1997; 272: 31459-31464.
- Harrison SD, Broadie K, van de Goor J, Rubin GM. Mutations in the Drosophila Rop gene suggest a funetion in general secretion and synaptic transmission. Neuron 1994; 13: 555-566.
- Honarpour N, Tabuchi K, Stark JM, Hammer RE, Südhof TC, Parada LF, Wang X, Richardson JA, Herz J. Embryonic neuronal death due to neurotrophin and neurotransmitter deprivation occurs independent of Apaf-1. Neuroscience 2001; 106: 263-274.
- Yang H, Zha X, Cai Y, Wang F, Wu Z, Wu B, Jia X, Zhu D. Impacts of thyroxine combined with donepezil on hippocampal ultrastructures and expressions of synaptotagmin-1 and SNAP-25 in adult rats with hypothyroidism. Int J Clin Exp Med 2015; 8: 17922-17931.
- Wang Y, Zhong J, Wei W, Gong J, Dong J, Yu F, Wang Y, Chen J. Developmental iodine deficiency and hypothyroidism impair neural development, upregulate caveolin-1, and downregulate synaptotagmin-1 in the rat cerebellum. Biol Trace Elem Res 2011; 144: 1039-1049.
- Wang N, Cai Y, Wang F, Zeng X, Jia X, Tao F, Zhu D. Effects of thyroxin and donepezil on hippocampal acetylcholine content and syntaxin-1 and munc-18 expression in adult rats with hypothyroidism. Exp Ther Med 2014; 7: 529-536.
- Alzoubi KH, Gerges NZ, Aleisa AM, Alkadhi KA. Levothyroxin restores hypothyroidism-induced impairment of hippocampus-dependent learning and memory: Behavioral, electrophysiological, and molecular studies. Hippocampus 2009; 19: 66-78.
- van Doorn J, Roelfsema F, van der Heide D. Concentrations of thyroxine and 3,5,3'-triiodothyronine at 34 different sites in euthyroid rats as determined by an isotopic equilibrium technique. Endocrinology 1985; 117: 1201-1208.
- Wang F, Zeng X, Zhu Y, Ning D, Liu J, Liu C, Jia X, Zhu D. Effects of thyroxine and donepezil on hippocampal acetylcholine content, acetylcholinesterase activity, synaptotagmin-1 and SNAP-25 expression in hypothyroid adult rats. Mol Med Rep 2014; 11: 775-782.
- Saxena G, Patro IK, Nath C. ICV STZ induced impairment in memory and neuronal mitochondrial function: A protective role of nicotinic receptor. Behav Brain Res 2011; 224: 50-57.
- Alcántara-González F, Mendoza-Perez CR, Zaragoza N, Juarez I, Arroyo-García LE, Gamboa C, De La Cruz F, Zamudio S, Garcia-Dolores F, Flores G. Combined administration of cerebrolysin and donepezil induces plastic changes in prefrontal cortex in aged mice. Synapse 2012; 66: 938-949.
- Kotani S, Yamauchi T, Teramoto T, Ogura H. Donepezil, an acetylcholinesterase inhibitor, enhances adult hippocampal neurogenesis. Chem Boil Interact 2008; 175: 227-230.