ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2015) Volume 26, Issue 3
Effect of Tripterygium wilfordii on LPS-induced acute lung injury and quantitative determination of its active constituents.
1Pharmacy Department of Chaoyang Central Hospital, Chaoyang, Liaoning Province, 122000 China
2College of Pharmacy, Dalian Medical University, Dalian, Liaoning Province, 116044 China
- *Corresponding Author:
- Taowen Pan
College of Pharmacy, Dalian Medical University
Dalian, Liaoning Province, 116044 China
Accepted date: May 01 2015
The objective of the present study is to observe the anti-inflammatory and antioxidant effects of Tripterygium wilfordii extract on LPS-induced acute lung injury (ALI) in rats, and to explore its protective mechanism against ALI. And quantitative determination was performed on the extract. 120 rats were randomly divided into normal control group (saline), model group, dexamethasone group (5 mg/kg, ip), and Tripterygium wilfordii extract low-, mediumand high-dose groups (100, 150, 200 mg/kg, ip), n = 20 in each group. The rats were administered for 3 d, 1 h after the last ip administration, ALI model was induced by ip endotoxin (LPS), 6 h later, the rats were sacrificed, BALF WBC count and protein content, nuclear transcription factor (NF-κB) p65 expression in lung tissue, levels of tumor necrosis factor (TNF-α), interleukin (IL)-6, interleukin (IL)-10, superoxide dismutase (SOD), and malondialdehyde (MDA), as well as neutrophil myeloperoxidase (MPO) activity in each group were observed. Total triterpenoid content in Tripterygium wilfordii extract was determined by UV-V is spectrophotometry with tripterine as the reference. Compared with the control group, in the Tripterygium wilfordii extract medium- and high-dose groups, lung tissue NF-κB p65 expressions were (38.03 ± 4.19)% and (30.69 ± 3.99)%, MDA levels were (1.76 ± 0.21) and (1.36 ± 0.12) nmol/mg, TNF-α levels were (263.66 ± 39.65) and (210.78 ± 30.25) μg/L, IL-6 levels were (289.47 ± 99.99) and (226.58 ± 96.25) ng/L, and MPO activities (2.76 ± 0.54) and (2.43 ± 0.42) U/g, all of which were significantly lower (P<0.05~P<0.01) than those of the model group; IL-10 levels were (17.89 ± 3.89) and (17.29 ± 3.68) μg/L, SOD levels were (69.47 ± 9.23) and (73.65 ± 8.12) U/mg, BALF WBC count and protein content in the model group significantly increased (P<0.05~P<0.01); all of which were significantly higher (P<0.05~P<0.01) than those of the model group. Total triterpenoid content in Tripterygium wilfordii extract was 27.379. Tripterygium wilfordii extract can decrease the pulmonary vascular permeability caused by LPS-induced ALI, reduce inflammation exudation, and lower the degree of oxidative stress injury.
Keywords
Tripterygium wilfordii extract; Acute lung injury; Lipopolysaccharide; Inflammatory factor; Oxidative stress
Introduction
Tripterygium wilfordii Hook. F is a plant in the genus Tripterygium of the family Celastraceae, which has the actions of dispelling wind and eliminating dampness, promoting blood circulation and dredging meridians, dispersing swelling and alleviating pain, removing toxicity and killing parasites, it has been widely used in the treatment of rheumatoid arthritis, glomerulonephritis and various dermatoses, at present, its main medicinal part is the peeled root [1]. The major active constituents of Tripterygium wilfordii crude drug are alkaloids (wilforgine, wilforine), diterpenes (triptolide, triptonide), triterpenes (tripterine, wilforlide), etc., of which triptolide is the main constituent with pharmacodynamic and toxic properties in Tripterygium wilfordii [2,3].
Acute lung injury (ALI) is a common clinical syndrome caused by severe infection, trauma, shock, poisoning and other intrapulmonary and extrapulmonary diseases; it has high morbidity and mortality, and is usually manifested as acute progressive dyspnea and refractory hypoxemia. Systemic inflammatory response and oxidative stress are both involved in and mediate the incidence of ALI [4]. Alkaloids are one of the main active constituents of Tripterygium wilfordii, which have broad pharmacological activities; a study [5] has proved that alkaloids from Tripterygium wilfordii have good anti-lipid peroxidation and anti-inflammatory effects. In this experiment, rat model of endotoxin-induced ALI was used to study the anti-inflammatory and antioxidant effects of Tripterygium wilfordii extracts; meanwhile, the mechanism of its anti-inflammatory effect was explored.
Materials
Instruments
Shimadzu UV-2450 UV-Vis spectrophotometer; XS105 analytical balance (Chenguang Electronic Technology Co., Ltd., Shanghai); BUCHI-R200 rotary evaporator, Switzerland. KH-250DB ultrasonic cleaner (Chuangbei Ultrasonic Instrument Co., Ltd., Jiangsu); microplate reader (Tecan, Switzerland); GL-7000 automated biochemical analyzer (Shimadzu, Japan); BECKMAN CS-15R centrifuge (BECKMAN, German); CX31RTSF microscope (Olympus).
Reagents
Methanol, ethanol, ethyl ether and acetic acid (AR, Chengdu Weike Chemical Reagent Factory), dimethyl sulfoxide (DMSO) (Jizhun Chemical Reagent Co., Ltd., Shanghai). Tumor necrosis factor (TNF)-α, interleukin (IL)-6, interleukin (IL)-10, superoxide dismutase (SOD) kits (purchased from Kanghui Biotechnology Co., Ltd., Beijing, batch numbers: 130265, 130106, 130114, 120919); malondialdehyde (MDA), nuclear transcription factor NF-κB, neutrophil myeloperoxidase (MPO) activity assay kits (purchased from Shanghai Jiangong Bioengineering Institute, batch numbers: 130512, 130212, 130614).
Drugs
Tripterygium wilfordii was purchased from the Original Herbal Medicine Division of Yunnan Baiyao (batch number: 201302036), which was identified by the Wuhan Institute of Botany, Chinese Academy of Sciences as Tripterygium wilfordii Hook. F. Crude drug of Tripterygium wilfordii was soaked in ethanol for 6 h, extracted three times under reflux with ethanol, with each time lasting 4 h, the ethyl acetate extracts were combined, and ethanol was removed to give ethanol extract, the ethanol extract was then extracted with ethyl acetate, and ethyl acetate was removed to give an extract, which was prepared to the required concentrations with DMSO during the experiment [6]. Dexamethasone was purchased from Shanghai Luda Pharmaceutical Co., Ltd., (batch number: 110209); lipopolysaccharide (LPS) was purchased from Sigma, USA.
Animals
120 clean SD rats, weighing (240 ± 20) g, provided by the Laboratory Animal Center of Dalian Medical University, animal quality certificate No.: DLYK 2013-00326.
Methods
Preparation of animal model and grouping
The 120 rats were randomly divided into six groups, namely the normal control group, model group, dexamethasone group, and Tripterygium wilfordii extract low-, medium- and high-dose groups, n = 20 in each group. On the first 3 d of the experiment, the rats in the Tripterygium wilfordii extract low-, medium- and high-dose groups were administered ip at daily doses of 100, 150 and 200 mg/kg once daily, control group and model group were administered ip with 2 mL/kg normal saline, and dexamethasone group was administered ip at 5 mg/kg 1 h before model establishment. 1 h after the last administration, rats in each treatment group were administered ip with 5 mg/kg LPS, after model establishment, general state of the rats in each group was observed, 6 h later, rats in the model group presented respiratory distress, with hemorrhagic secretion from mouth and nose.
Specimen collection and observation indices
All animals were anesthetized by giving 50 mg/kg sodium pentobarbital, ip, 6 h after model establishment. Tracheal intubation was performed, and bronchoalveolar lavage was performed with three 3 mL aliquots of normal saline, the bronchoalveolar lavage fluid (BALF) was then collected and centrifuged. Supernatant protein concentration was determined by Coomassie brilliant blue staining. After the precipitated cells were destroyed with red blood cell lysing buffer, white blood cell (WBC) was counted under an optical microscope. Chests of rats were cut open and 200 mg of left lungs were removed, which were made into 10% homogenate in an ice bath, centrifuged at 2500 r/min at 4°C for 15 min, then the supernatant was taken to detect the level of inflammatory factor MDA, as well as SOD and MPO activities.
NF-κB p65 expression in lung tissue
Right lower lungs were removed and fixed in 10% formalin, paraffin-embedded, and sliced, followed by determination of NF-κB p65 expression by immunohistochemistry.
Statistical processing
Statistical analysis was performed using SPSS 16.0 statistical software, all the data were expressed as x ± s, statistical processing was performed by t test and analysis of variance, P<0.05 was considered statistically significant.
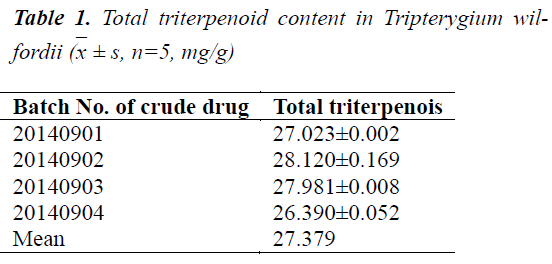
Determination of total triterpenoid lactone (vanillinperchloric acid colorimetric method) content in Tripterygium wilfordii by UV-Vis spectrophotometry
Preparation of reference solution
10.0 mg of tripterine reference substance was accurately weighed, dissolved in methanol, and diluted to the mark to prepare a 2.0 mg/mL reference stock solution.
Preparation of test solution
About 0.2 g of Tripterygium wilfordii extract (sifted through a 40-mesh sieve after crushing) was accurately weighed, placed in a stoppered conical flask, added precisely with 50 mL of methanol, then stopped, weighed, and ultrasonically extracted for 30 min. After the lost weight was replenished with methanol, the solution was shaken well, and filtered to give the test solution.
Selection of detection wavelength
Each 0.1 mL of reference and test solutions was taken, and evaporated to dryness in a water bath at 100°C. Then, each was added with 0.8 mL of 5% vanillin-glacial acetic acid solution and 2 mL of perchloric acid, shaken well, and heated in a 70°C water bath for 45 min. After removing and cooling in tap water for 5 min, the solutions were added with 5 mL of glacial acetic acid, shaken well, and reacted. 10 min later, the solutions were placed in a cuvette, and scanned between 200-800 nm with glacial acetic acid as the blank solution. The results showed that the absorptions of reference substance and test sample were both larger at 538 nm, so 538 nm was selected as the detection wavelength.
Investigation of linearity
Appropriate amount of tripterine reference substance was accurately drawn, dissolved in methanol, and diluted quantitatively to prepare standard solutions with concentrations of 100.0, 115.0, 130.0, 145.0, 160.0 and 175.0 mg/L, followed by measurement of absorbance at 538 nm. Regression equation of tripterine was obtained with absorbance as ordinate and concentration as abscissa as y = 3.4329x-0.0121, r = 0.9997.
Accuracy test
Tripterine reference solution was taken, and determined repeatedly for six consecutive times. RSD was calculated to be 0.28%, which indicated the good accuracy of instrument.
Stability test
The prepared Tripterygium wilfordii extract test solution was taken, and measured for absorbance at 0, 10, 20, 30, 40, 50 and 60 min, respectively, to investigate the colori metric stability within 60 min. Results revealed that RSD was 2.12% (n = 7) at 60 min, indicating the good colorimetric stability of sample within 0-60 min.
Reproducibility test
Tripterygium wilfordii crude drug was taken, prepared into six parallel test solutions, and measured for absorbance at 538 nm. RSD was calculated to be 2.06% (n = 6).
Recovery test
Six aliquots of Tripterygium wilfordii extracts were prepared into test solutions by addition of tripterine reference substance the same in amount as the test sample, and measured for absorbance at 538 nm. Sample recovery was calculated to be (97.314 ± 2.623)% (RSD = 2.78%).
Quantitative determination of test solutions
Different batches of Tripterygium wilfordii crude drugs were prepared separately into test solutions, and measured for absorbance at 538 nm. Total contents of triterpenoids in Tripterygium wilfordii of various origins were calculated based on the regression equation. The results are shown in Table 1.
Results
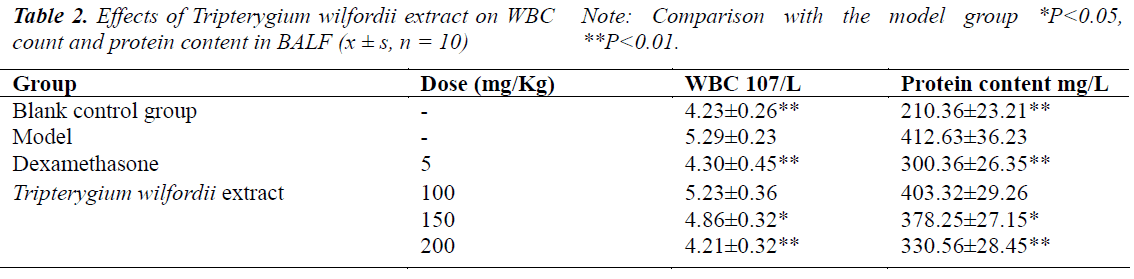
Effects on WBC count and protein content in BALF in rats with ALI
Protein level and WBC count in BALF of rats in the model group were significantly higher (P<0.01) than the control group. After treatment with Tripterygium wilfordii extract, protein level and WBC count in BALF were both lower than the rats in the model group, and the effects were especially significant (P<0.01) in the high-dose group, see Table 2.
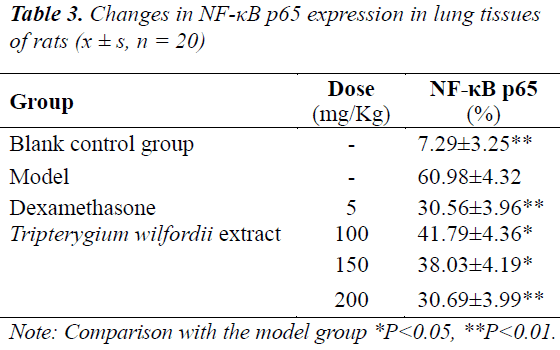
Effects on NF-κB p65 expression in lung tissues of rats with ALI
NF-κB p65 expressions in lung tissue cells of rats in the model group were all significantly higher (P<0.01) than the control group. After treatment with Tripterygium wilfordii extract, NF-κB p65 expression in each treatment group was significantly lowered, of which the effect was significant (P<0.01) in the high-dose group, see Table 3.
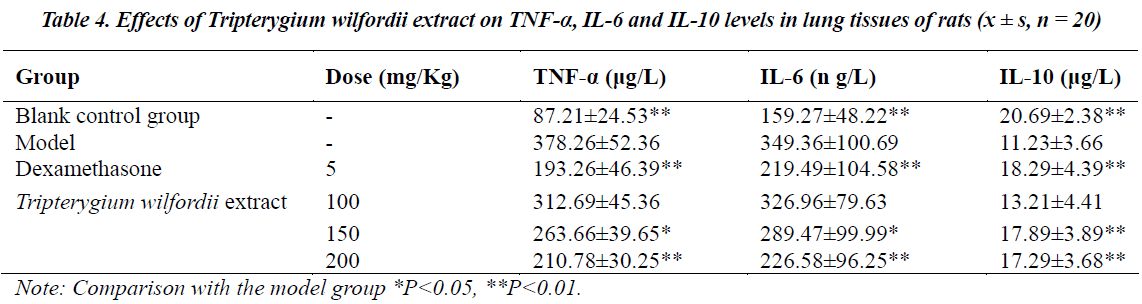
Effects on TNF-α, IL-6 and IL-10 in lung tissues of rats with ALI
TNF-α and IL-6 in the model group were significantly higher (P<0.01) than the control group, while IL-10 was significantly lower (P<0.01) than the control group. After treatment with Tripterygium wilfordii extract, TNF-α and IL-6 concentrations in each treatment group were significantly lower than the model group, while IL-10 was significantly higher than the control group, the effects were especially significant (P<0.01) in the medium- and highdose groups, see Table 4.
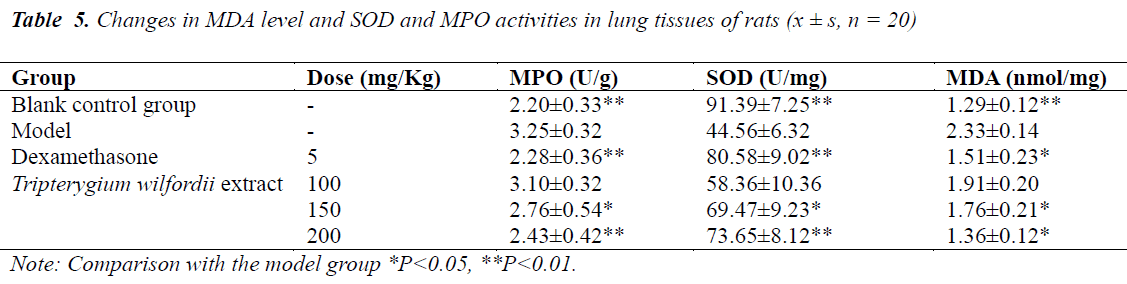
Effects on MPO and SOD activities and MDA level in lung tissues of rats with ALI
MPO activity and MDA level in the model group were significantly higher (P<0.01) than the control group, while SOD activity was significantly lower (P<0.01) than the control group. After treatment with Tripterygium wilfordii extract, MPO activity and MDA level in each treatment group significantly decreased, while SOD activity was significantly higher compared with the model group, the effects were especially significant (P<0.05~P<0.01) in the medium- and high-dose groups, see Table 5.
Discussion
LPS-induced acute lung injury is often accompanied by the release of inflammatory cytokines and inflammatory response [7], which cause infiltration of neutrophils, and release of oxygen free radicals, proteases and other inflammatory mediators, protein concentration in BALF can reflect the permeability of epithelial cells and degree of pulmonary edema [8], lung tissue MPO reflects the number of neutrophils, and indirectly reflects the degree of local neutrophil infiltration in lung tissues, BALF WBC count and lung tissue MPO index can indirectly reflect the amounts of neutrophil infiltration and accumulation in the lung. In this experiment, 6 h after the induction of rat model of LPS-induced lung injury, the protein content and WBC count in BALF, and lung tissue MPO activity were all significantly higher than the blank control group, which can fully demonstrate the successful establishment of animal model in the present experiment.
Currently, it is believed that in the development and progression of ALI, a large number of different proinflammatory cytokines are produced in local lung tissues, which form a complex cytokine network, thus causing complex inflammatory responses [9]. Among them, NF-κB p65 is a protein factor with pleiotropic transcriptional regulation; up-regulation of its activity can activate factors such as TNF-α and IL-6, and produce a variety of inflammatory substances [10]. Increased amount of TNF-α can promote neutrophil exudation, phagocytosis and release of oxygen free radicals, and induce alveolar epithelial cells to produce other pro-inflammatory cytokines [7]. While endogenous and exogenous IL-10 have a protective effect on ALI caused by several different causes, and improve the survival of animals. Therefore, IL-10 level is directly associated with the severity of the inflammatory response [11].
ALI is often accompanied by inflammatory response, as well as the release of oxygen free radicals. SOD is a specific enzyme which directly scavenges oxygen free radicals; MDA is the product of fatty acid and oxygen free radical chain reactions by oxidants, which can reflect the severity of free radical attack on cells [12,13]. As a marker enzyme of neutrophil activation, changes in MPO activity reflects the degree of activation of neutrophils [14].
This experiment shows that Tripterygium wilfordii extract can lower BALF levels of albumin and WBC, reduce alveolar-capillary membrane permeability, and improve the degree of pulmonary edema. Tripterygium wilfordii extract can promote the expression of SOD, improve the free radical scavenging capacity in rats, and has a protective effect on oxidative stress in LPSinduced lung injury model rats. This study shows that Tripterygium wilfordii extract can effectively inhibit NF-κB p65 expression, decrease the production of proinflammatory factors such as TNF-α and IL-6, and increase the level of IL-10, effectively inhibit the release of mediators such as oxygen free radicals, lipid metabolite MDA and MPO, suppress inflammatory responses in many ways, and reduce the degree of lung injury.
In summary, Tripterygium wilfordii extract can decrease the pulmonary vascular permeability caused by LPS-induced ALI, reduce inflammation exudation, and lower the degree of oxidative stress injury. The mechanisms of its actions may be associated with downregulation of NF-κB p65 expression, reduction of antiinflammatory cytokines such as TNF-α and IL-6, reduction of metabolites such as MPO and MDA, and increase of SOD expression.
References
- "Chinese MateriaMedica" Editorial Board, State Administrationof Traditional Chinese Medicine. Chinese MateriaMedica. Shanghai: Shanghai Scientific & Technical Publishers 1999: 4134-4135.
- Si JP, Ruan XC, Guo BL, Huang WH, Xu YK. Current situation and sustainable utilization of Tripterygium resources.Journal of Chinese Medicinal Materials 2005; 28: 10-11.
- Bi XQ, Tao JY, Bi XL.Research progress in root, bark and aerial parts of Tripterygiumwilfordii. Hubei Journal of Traditional Chinese Medicine 2004; 26: 55-56.
- Jiang XH, Huang XM, He YZ. Effect of Tanreqing Injection on Anti-Oxidant Activities of Acute Lung Injury in Rats. hinese Archives of Traditional Chinese Medicine 2012; 30: 1043-1044.
- Jiang T, Qin LP, Zheng HC, Luan L, Han T. Studies on the Flavonoids and AntilipidPeroxidantion of Bidensbipinnata. Natural Product Research and Development 2006; 18: 765-765.
- Cheng ZZ, Xiao YS, Fang YJ, Wang WD. Exploration of extraction technology of Tripterygiumwilfordii.Chinese Journal of Pharmaceuticals 1990; 21: 435-436.
- Ge DJ, Liu GJ. Inhibition of the PTEN protects rats against LPS-induced acute lung injury. Chinese Pharmacological Bulletin 2010; 26: 1199-1199.
- Xu J, Li TP, Wu HQ. Effects of ebselen on inflammatory factors in rats with acute lung injury. Chongqing Medicine 2012; 41: 836-839.
- Moreno JJ. Antiflammin-2 prevents HL-60 adhesion to endothelial cells and prostanoid production induced by lipopolysaccharides. J PharmacolExpTher 2001; 296: 884-889.
- Zouki C, Ouellet S, Filep JG. The anti-inflammatory peptides, antiflammins, regulate the expression of adhesionmoleculesonhuman leukocytes and prevent neutrophil adhesion to endothelial cells. FASEB J 2000; 14: 572-580.
- Kamal AM, Hayhoe RP, Paramasivam A, Cooper D,Flower RJ,SolitoE,Perretti M. Antiflammin-2 activatesthe human formy-l peptide receptor like 1. ScientificWorld Journal, 2006, 6:1375-1384
- Lin B, Zhang YY, Xu H, Chen DF. Antioxidant effect of total polysaccharides from Radix Bupleuri in rats with acute lung injury. Chinese Journal of Clinical Pharmacy 2010; 19: 6-10.
- Huang XL, Wang SM, Fan YM, Ding CH, Ling YL. Role of heme oxygenase-1 in dachengqitang ameliorating lipopolysaccharide-induced acute lung injury in mice. China Journal of Chinese MateriaMedica 2012; 37: 250- 254.
- Ge JX, Shao DH, Wang H. Protection of mildronate on acute lung injury in mice. Journal of Jiangsu University (Medicine Edition) 2012; 22: 58-60.
 ± s, n=5, mg/g)
± s, n=5, mg/g)