ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2023) Volume 34, Issue 2
Effects of anti-osteoarthritic drugs on development of offspring of albino rats during pregnancy and lactation.
Esraa Khaled1*, Abd El Wahab El Ghareeb2, Hamida Hamdi2, Salwa Farouk2
1Department of Biotechnology, Faculty of Post-Graduate Studies for Advanced Sciences, Beni-Suef University, Beni-Suef, Egypt
2Department of Zoology, Faculty of Science, Cairo University, Egypt
- Corresponding Author:
- Esraa Khaled
Department of Biotechnology
Faculty of Post-Graduate Studies for Advanced Sciences
Beni-Suef University
Beni-Suef
Egypt
Accepted date: January 27, 2023
The present study aimed to evaluate the teratogenic effects of the anti-osteoarthritic drug (Genuphil) administered daily orally to the pregnant rats and nursing rats. The pregnant rats were treated during gestation and lactation with doses of 120 and 240 mg/kg. Histopathological studies of pregnant rats and their fetuses during gestation and lactation revealed few inflammatory cells infiltration in the portal area as well as in between the hepatocytes with dilatation in the portal vein, brown pigmented material in the periphery of the dilated central veins, oedema, congestion in the portal vein, granular degenerations in the hepatocytes in the liver. Degenerative change, swelling, coagulative necrosis, degeneration in the lining epithelium of the tubules, swelling of the glomeruli in the lining endothelium of the tufts and focal haemorrhage in between the degenerated tubules were observed in kidney of the treated groups while vacuolization and degeneration in the cerebrum were observed in the brain following Genuphil administration. Histopathological findings showed atritic follicles associated with proliferation of the interstitial stromal cells and severe congestion in the blood vessels of the medullary portion in the ovary while focal haemorrhage and necrosis in the giant cell layer detected in the placenta following Genuphil administration. Genuphil pre-treatment was able to decrease the level of lipid peroxidation while it increased the level of lipid peroxidation during gestation with only 240 mg/kg Genuphil and it decreased the GSH level. Our findings suggest the need for great caution to handle Genuphil especially during pregnancy and lactation.
Keywords
Genuphil, Teratogenicity, Gestation, Lactation.
Introduction
Teratology, the study of abnormal prenatal development and congenital malformations induced by exogenous chemical or physical agents, continues to be a burgeoning area of medical research in the quest for the eradication of preventable birth defects. Identification of agents with teratogenic potential from the plethora of drugs and chemicals that human beings come into contact with in their everyday environment is crucial. Although only some 10% of congenital anomalies are thought to be caused by teratogens [1] representing roughly one in every thousand live births, they compromise the quality of life for millions of individuals worldwide and cost billions of dollars in health care every year.
Osteoarthritis is essentially a debilitating disease characterized by a gradual loss of articular cartilage in synovial joints that causes painful impairment. Functional limitation gradually occurs as a result of joint stiffness and progressive loss of joint motion owing todeformities (loss of the cartilage surface and side growth of osteophytes) accompanied by inflammation of the synovial membrane. The clinical symptoms of OA are pain and functional impairment that includes joint stiffness and dysfunction. In 80% of patients with OA, movement is limited to some degree. This leads to impaired performance in the work place, and 25% of patients cannot perform their main activities of daily life, which often leads to social isolation and depression [2].
Some revolutionary new laboratory and clinical technologies have fastened and broadened our capabilities to discover and gain new insight into this disease. Thus, we are optimistic to see OA drugs able to modulate the key elements driving OA, which is one of the largest burdens on public health and health care systems in the world. Drug therapy should only be considered as one pillar of a three-pronged strategy which also involves a long-term and stage-adjusted individual management using nonpharmacological and surgical treatment [3].
Genuphil is the ultimate joint care formula that contains the essential components responsible for maintaining the normal structure of the articular cartilage and synovial fluid of the joints & reduces the pain and inflammation associated with these conditions. Genuphil contains glucosamine and chondroitin, the two well-known ingredients for their synergistic effect in restoring the normal balance of cartilage metabolism where they stimulate the cartilage anabolic process and inhibit the degradative enzymes thus decreasing the rate of cartilage catabolism. Genuphil contains also, Methyl Sulfonyl Methane (MSM) the natural source of organic sulfur that is essential for proteoglycans synthesis, a process that is mandatory for final assembly of the cartilage. Sulfur also, through forming the disulfide bonds is needed for the collagen synthesis where collagen is the main structural protein of the soft tissue such as cartilage, tendon, ligaments and muscles. This Study is designed to evaluate the teratogenicity of anti-osteoarthritic drug (Genuphil) on development of offspring of albino rats when administered during pregnancy and lactation (Scheme 1).
Materials and Methods
Experimental animals
The present experimental study is carried out on the white albino rat (Rattus norvegicus). The standard guidelines of the Institutional Animal Care and Use Committee (IACUC) were used in handling animals.
Females of 11-13 weeks old were selected for the present study and vaginal smears were prepared every morning and examined under light microscope (according to the method of Snell) for 5 days to select the female with regular estrus [4]. Two females with regular estrus cycle were selected in the pro-estrus stage and caged together with one male overnight under controlled environmental conditions of temperature, humidity and light. The first day of gestation was determined by the presence sperms in the vaginal smear [5].
Experimental design
The Route of administration was oral. The time of administration was scheduled from the 5th day of gestation, daily during both gestation and lactation. Genuphil Drug was manufactured by Eva pharma for pharmaceuticals and medical appliances, Egypt.
Experimental groups
Group A: Control group received distilled water from 5th day of gestation to 19th day of gestation.
Group B: Treated group received 120 mg/kg from 5th day of gestation till the end of lactation.
Group C: Treated group received 240 mg/kg from 5th day of gestation till the end of lactation.
Histological examination
Autopsy samples were taken from ovary, placenta, liver, kidney and brain of mother rats and fetuses in different groups at the 20th day of gestation and from fetuses in seven, fourteen and twenty one days old. They were fixed in 10% formal saline for twenty four hours. Washing was done in tap water then serial dilutions of alcohol (methyl, ethyl and absolute ethyl) were used for dehydration. Specimens were cleared in xylene and embedded in paraffin at 56 degree in hot air oven for twenty four hours. Paraffin bees wax tissue blocks were prepared for sectioning at 4 microns thickness by sledge microtome. The obtained tissue sections were collected on glass slides, deparaffinized, stained by hematoxylin and eosin stain for routine examination then examination was done through the light electric microscope [6].
Sample preparation
Tissue homogenate
➢ Prior to dissection, perfuse tissue with a PBS (Phosphate Buffered Saline) solution, PH 7.4 containing 0.16 mg/ml heparin to remove any red blood cells and clots.
➢ 0.2 and 0.3 gm of the organ tissue (liver and brain) were homogenized in 2ml and 3ml of phosphate buffer per gram tissue.
➢ The homogenate was centrifuged at 4000 r.p.m for 15 minutes.
➢ The supernatant was removed for assay and stored on ice. The sample was freezed at -80°C (If not assaying on the same day) for oxidative stress studies.
Determination of lipid peroxidation
Lipid peroxidation was determined according to the procedure of Satoh and Ohkawa [7,8].
Principle
Thiobarbituric Acid (TBA) reacts with Malondialdehyde (MDA) in acidic medium at temperature of 95°C for 30 min to form thiobarbituric acid reactive product can be measured at 534 nm.
Reagents
10 nmol/mL standard
25 mmol/L chromogen Thiobarbituric acid Detergent Stabilizer
Procedure
| Blank (ml) | Standard (ml) | Sample (ml) | |
|---|---|---|---|
| Sample | - | - | 0.2 |
| Standard | - | 0.2 | - |
| Chromogen | 1.0 | 1.0 | 1.0 |
The samples were mixed well, the test tube was covered with glass bead, heated in boiling water bath for 30 min, cooled, then 0.2 ml of blank was added.
| Blank (ml) | Standard (ml) | Sample (ml) | |
|---|---|---|---|
| Sample | 0.2 | - | - |
The samples were mixed, the absorbance of sample was read against blank and standard against dis. water at 534 nm. Color stable for 6 hrs. Linearity up to 100 nmol.
Determination of Glutathione reduced (GSH) content
Glutathione content was determined according to the procedure of Beutler [9].
Principle
The method based on the reduction of 5,5'-dithiobis-(2- nitrobenzoic acid) (DTNB) with Glutathione (GSH) to produce a yellow compound. The reduced chromogen is directly proportional to GSH concentration and its absorbance can be measured at 405 nm.
Reagents
500 mmol/L of Trichloroacetic Acid (TCA) 100 mmol/L of Buffer 1.0 mmol/L of DTNB
Procedure
The samples were mixed well then were let to stand for 5 min at R.T. Centrifuged at 3000 rpm for 15 min. Then the following aliquots were taken.
The samples were mixed well. The absorbance was measured after 5-10 min at 405 nm of sample against the blank. Linearity up to 120 mg/dl (4 nmol/l).
Statistical analysis
Statistical analysis was performed using the Analysis of Variance (ANOVA) and duncan’s multiple range test to determine differences between treatments, means at significance level of 0.05. Standard errors of treatment means were also estimated. All statistics were carried out using Statistical Analysis Systems (SAS) program [10].
Results
During gestation period
Histopathological findings on ovary and placenta
Group of rats kept as control
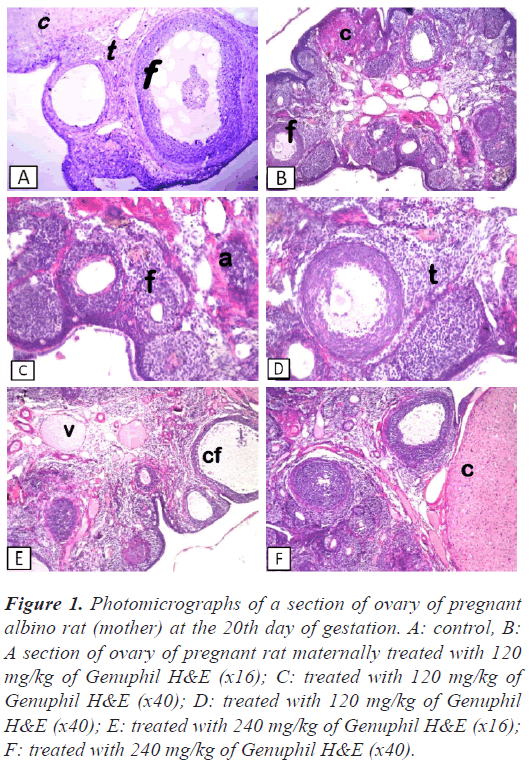
Ovary: There was no histopathological alteration and the normal histological structure of the different stages of follicles, corpus luteum and interstitial stromal cells were recorded in Figure 1.
Figure 1: Photomicrographs of a section of ovary of pregnant albino rat (mother) at the 20th day of gestation. A: control, B: A section of ovary of pregnant rat maternally treated with 120 mg/kg of Genuphil H&E (x16); C: treated with 120 mg/kg of Genuphil H&E (x40); D: treated with 120 mg/kg of Genuphil H&E (x40); E: treated with 240 mg/kg of Genuphil H&E (x16); F: treated with 240 mg/kg of Genuphil H&E (x40).
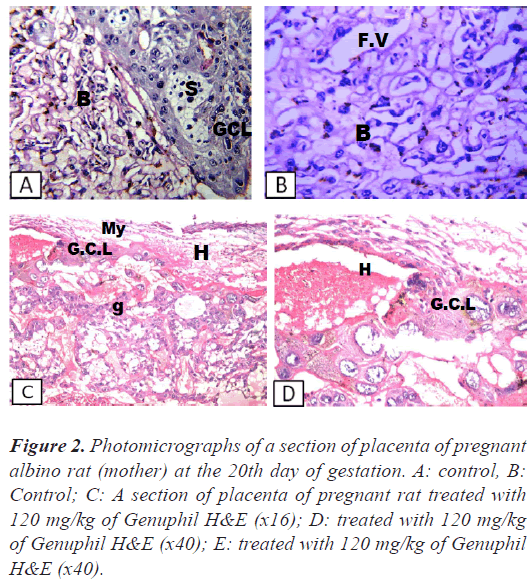
Placenta: There was no histopathological alteration and the normal histological structure of the giant cell layer, trophospongium and labyrinth were recorded in Figure 2.
Figure 2: Photomicrographs of a section of placenta of pregnant albino rat (mother) at the 20th day of gestation. A: control, B: Control; C: A section of placenta of pregnant rat treated with 120 mg/kg of Genuphil H&E (x16); D: treated with 120 mg/kg of Genuphil H&E (x40); E: treated with 120 mg/kg of Genuphil H&E (x40).
Group of rats administrated 120 mg/kg
Ovary: Most of the follicles were atritic associated with proliferation of the interstitial stromal cells (Figure 1).
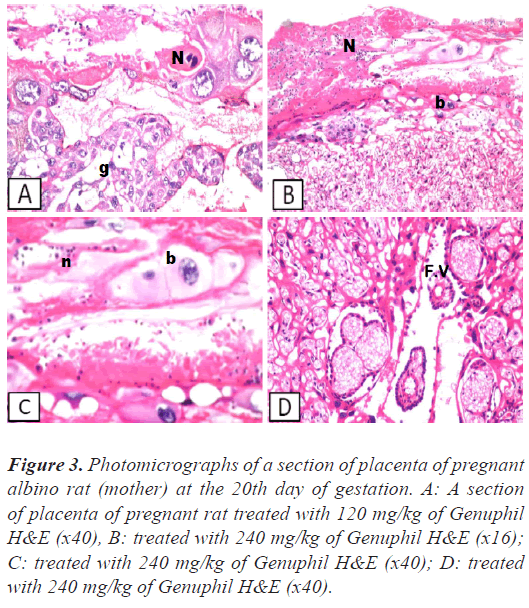
Placenta: Focal haemorrhage and necrosis were detected in the giant cell layer, while the trophospongium , labrynith and fetal villi were intact (Figures 2 and 3).
Group of rats administrated 240 mg/kg
Ovary: Severe congestion was detected in the blood vessels of the medullary portion associated with follicular cystic formation in some follicles while the other showed different stages of maturation and formation of the corpus luteum (Figure 1).
Placenta: Necrosis was noticed in the giant cell and trophospongium (Figure 3), while the underlying labrynith and fetal villi were intact (Figure 3).
Figure 3: Photomicrographs of a section of placenta of pregnant albino rat (mother) at the 20th day of gestation. A: A section of placenta of pregnant rat treated with 120 mg/kg of Genuphil H&E (x40), B: treated with 240 mg/kg of Genuphil H&E (x16); C: treated with 240 mg/kg of Genuphil H&E (x40); D: treated with 240 mg/kg of Genuphil H&E (x40).
Histopathological findings on liver, brain and kidney
Group of rats kept as control
Mother
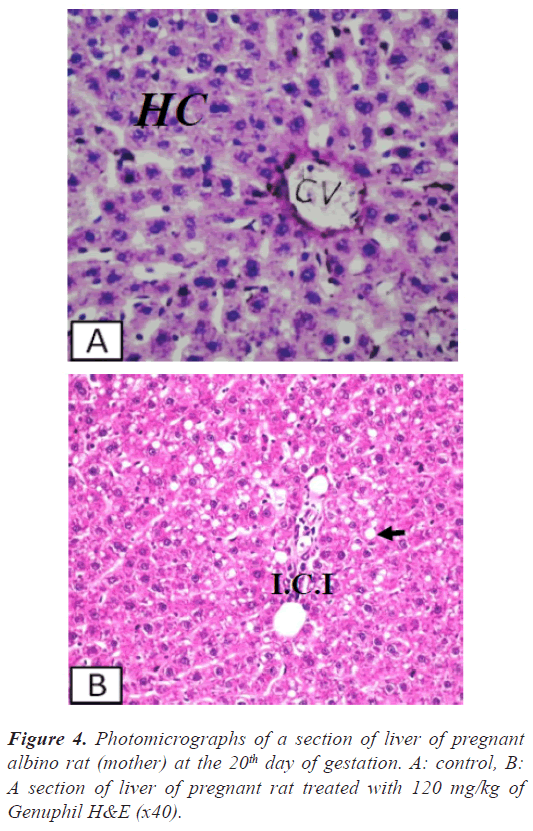
Liver: There was no histopathological alteration and the normal histological structure of the central vein and surrounding hepatocytes were recorded in Figure 4.
Kidney: There was no histopathological alteration and the normal histological structure of the glomeruli and tubules at the cortex and the tubules at the corticomedullary portion were recorded in Figures 5-6.
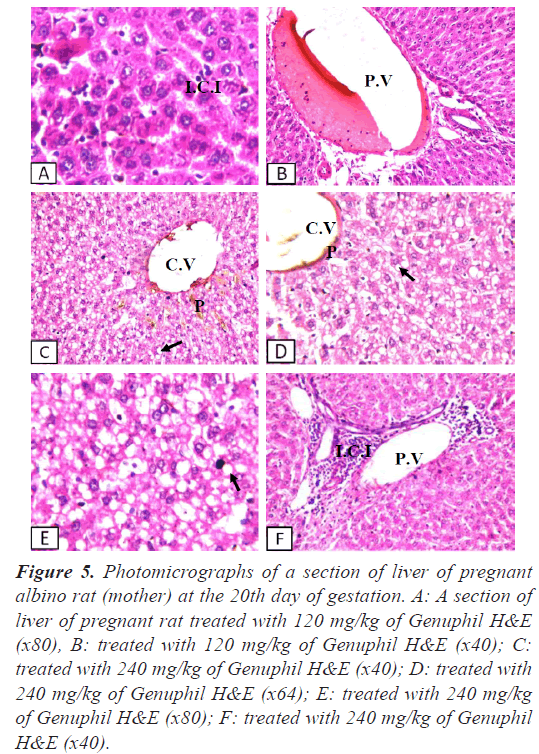
Figure 5: Photomicrographs of a section of liver of pregnant albino rat (mother) at the 20th day of gestation. A: A section of liver of pregnant rat treated with 120 mg/kg of Genuphil H&E (x80), B: treated with 120 mg/kg of Genuphil H&E (x40); C: treated with 240 mg/kg of Genuphil H&E (x40); D: treated with 240 mg/kg of Genuphil H&E (x64); E: treated with 240 mg/kg of Genuphil H&E (x80); F: treated with 240 mg/kg of Genuphil H&E (x40).
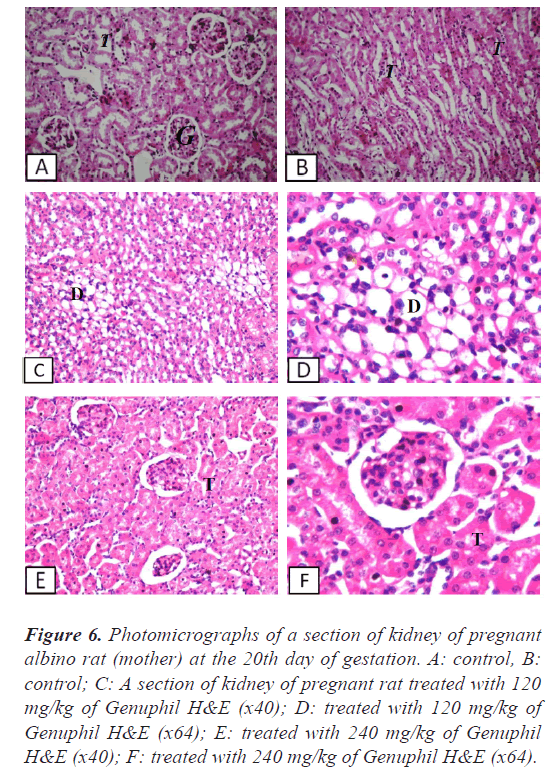
Figure 6: Photomicrographs of a section of kidney of pregnant albino rat (mother) at the 20th day of gestation. A: control, B: control; C: A section of kidney of pregnant rat treated with 120 mg/kg of Genuphil H&E (x40); D: treated with 120 mg/kg of Genuphil H&E (x64); E: treated with 240 mg/kg of Genuphil H&E (x40); F: treated with 240 mg/kg of Genuphil H&E (x64).
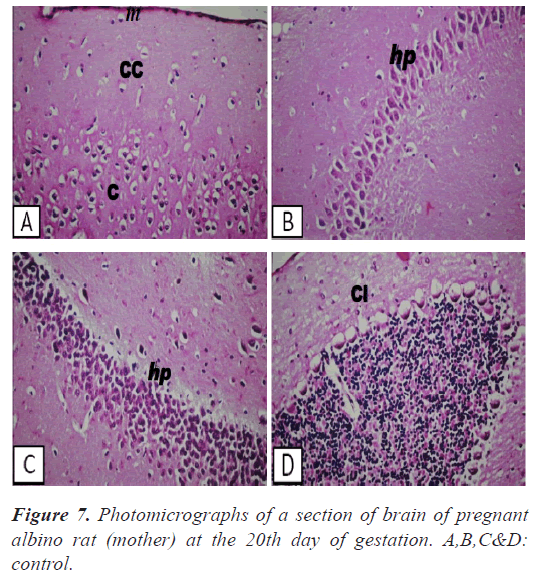
Brain: There was no histopathological alteration and the normal histological structure of the meninges, cerebral cortex, cerebrum, hippocampus, cerebellum and medulla oblongata were recorded in Figures 7-8.
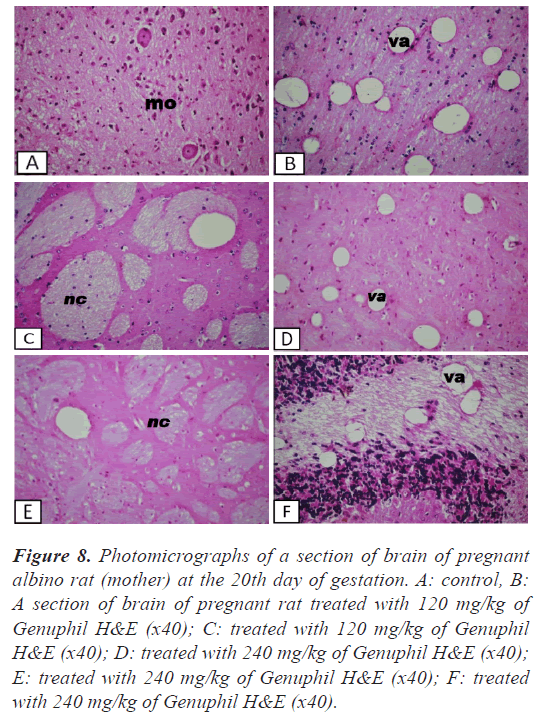
Figure 8: Photomicrographs of a section of brain of pregnant albino rat (mother) at the 20th day of gestation. A: control, B: A section of brain of pregnant rat treated with 120 mg/kg of Genuphil H&E (x40); C: treated with 120 mg/kg of Genuphil H&E (x40); D: treated with 240 mg/kg of Genuphil H&E (x40); E: treated with 240 mg/kg of Genuphil H&E (x40); F: treated with 240 mg/kg of Genuphil H&E (x40).
Fetus
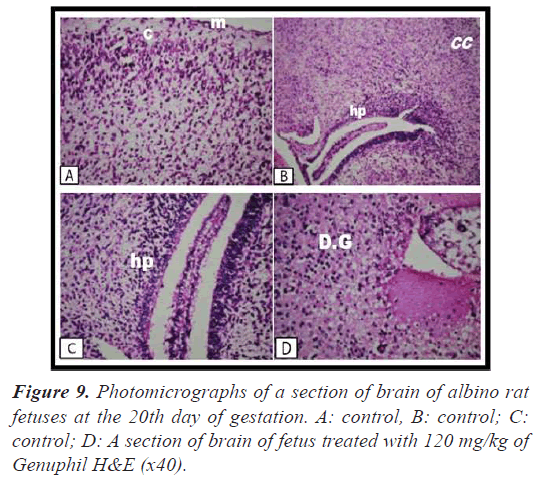
Brain: There was no histopathological alteration as recorded in Figure 9.
Group of rats administrated 120 mg/kg Genuphil
Mother
Liver: Few inflammatory cells infiltration was detected in the portal area as well as in between the fatty changed hepatocytes with dilatation in the portal vein (Figures 4-5).
Kidney: There was degenerative change in the lining epithelium of the tubules at the corticomedullary (Figure 6).
Brain: Vacuolozation (Figure 8), as well as focal circumscribed encephalomalacia (Figure 8) were detected in the cerebrum.
Fetus
Brain: There was diffuse gliosis in the cerebrum (Figures 9-10).
fetuses at the 20th day of gestation. A: control, B: control; C: control; D: A section of brain of fetus treated with 120 mg/kg of Genuphil H&E (x40).
Group of rats administrated 240 mg/kg Genuphil
Mother
Liver: Dilatation was detected in the central vein associated with brown to yellow pigmentation in between the fatty changed hepatocytes (Figure 5). The portal area showed dilatation in the portal vein with inflammatory cells infiltration (Figure 5).
Kidney: There was swelling in the lining epithelium of the tubules (Figure 6).
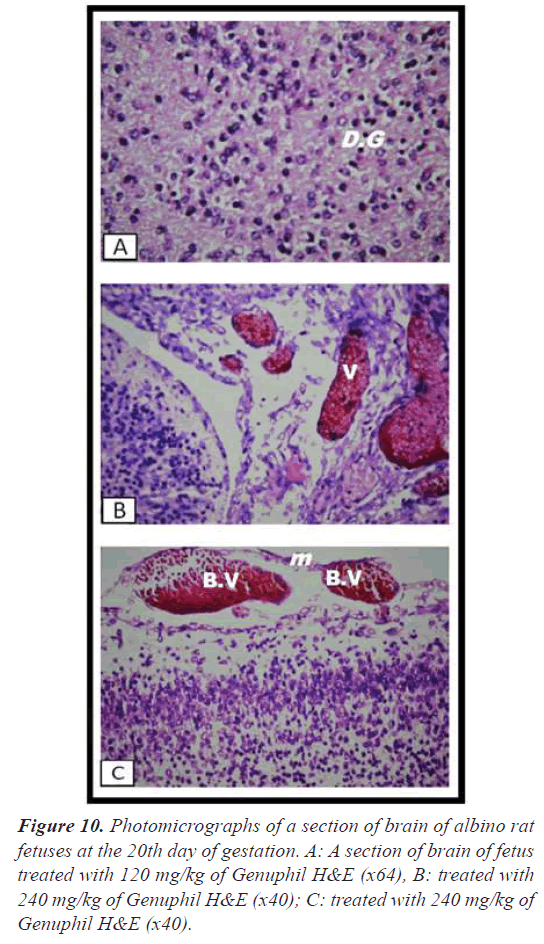
Brain: The cerebrum showed vacuolization and focal encephalomalacia (Figure 8), while the cerebellum showed only vacuolization (Figure 8).
Fetus
Brain: There was severe congestion in the plexus as well as in the meningeal blood vessels (Figure 10).
Oxidative stress investigations during gestation
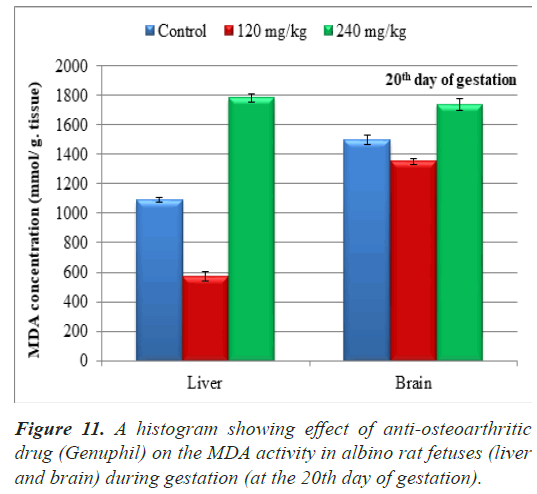
Lipid peroxide (Malondialdehyde): The results of MDA in sample of fetuses from pregnant rats at the 20th day of gestation were represented in Table 1.
Along the duration of the experiment with the age progress from 20th day of gestation to 21st of lactation, in both 120 and 240 mg/kg treated groups, a high significant decrease (P<0.01) in the MDA concentration was found in all tested samples (liver and brain) in comparison with the control one except in liver and brain of 240 mg/kg treated group at the 20th day of gestation, a high significant increase (P<0.01) in the MDA concentration in sample (liver and brain) in comparison with the control group (Table 1), (Figure 11).
The average of malondialdehyde in samples (liver) of fetuses maternally treated with 120 mg/kg of Genuphil from 5th day of gestation to 19th day of gestation showed high significant decrease (P<0.01) as compared to the control group (Table 1), (Figure 11). A high Significant increase (P<0.01) in MDA was found in samples (liver) of fetuses maternally treated with 240 mg/kg of Genuphil from 5th day of gestation to 19th day of gestation as compared to the control group (Table 1), (Figure 11).
| Control | 120 mg/kg | 240 mg/kg | |
|---|---|---|---|
| Malondialdehyde in sample (liver) (nmol/g.tissue) | 1089.29 ± 14.8961936b | 573.5930733 ± 30.7405040c | 1781.39 ± 26.6769941a |
| Malondialdehyde in sample (brain) (nmol/g.tissue) | 1089.29 ± 14.8961936b | 1352.81 ± 19.5645140c | 1738.10 ± 41.8202873a |
Note: The tissues (Liver and brain) taken at the 20th day of
gestation from fetuses of pregnant rats.
Data are represented as mean ± standard error
Means with the same letter are not significantly different
(c,a) highly significantly different from b at (P<0.01)
Table 1. Effect of Genuphil on lipid peroxide (Malondialdehyde, nmol/g.tissue) in liver and brain of fetuses at the 20th day of gestation (during gestation).
The average of malondialdehyde in samples (brain) of fetuses maternally treated with 120 mg/kg of Genuphil from 5th day of gestation to 19th day of gestation showed high significant decrease (P<0.01) as compared to the control group (Table 1), (Figure 11).
High Significant increase (P<0.01) in MDA was found in samples (brain) of fetuses maternally treated with 240 mg/kg of Genuphil from 5th day of gestation to 19th day of gestation as compared to the control group (Table 1), (Figure 11).
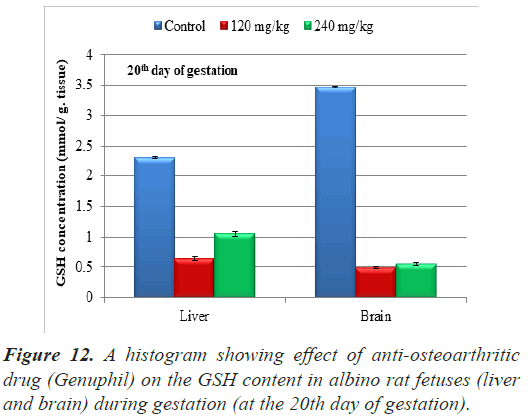
Glutathione reduced (GSH): The results of Glutathione reduced (GSH) concentration in samples (liver and brain) of fetuses from pregnant rats at the 20th day of gestation were represented in Table 2.
In both 120 and 240 mg/kg treated groups, there was highly significant decrease (P<0.01) in Glutathione reduced (GSH) concentration in Liver of fetuses from pregnant rats at the 20th day of gestation as compared to the control group (Table 2), (Figure 12).
| Control | 120 mg/kg | 240 mg/kg | |
|---|---|---|---|
| Glutathione (GSH) Concentration (liver) (mmol/ g. tissue ) | 2.3088000 ± 0.0162126a | 0.6364000 ± 0.0337492c | 1.0508000 ± 0.0408008b |
| Glutathione (GSH) Concentration (brain) (mmol/g.tissue) | 3.4632000 ± 0.0162126a | 0.4884000 ± 0.0162126b | 0.5476000 ± 0.0272899b |
Note: The tissues (Liver and brain) taken at the 20th day of
gestation from fetuses of pregnant rats.
Data are represented as mean ± standard error
Means with the same letter are not significantly different
(c,b) highly significantly different from a at (P<0.01)
Table 2. Effect of Genuphil on Glutathione reduced (GSH, nmol/g.tissue) in liver and brain of fetuses at the 20th day of gestation (during gestation).
Highly significant decrease (P<0.01) in Glutathione reduced (GSH) concentration was found in samples (brain) of fetuses maternally treated with 120 mg/kg of Genuphil from 5th day of gestation to 19th day of gestation as compared to the control group (Table 2), (Figure 12).
Glutathione reduced (GSH) concentration in samples (brain) of fetuses maternally treated with 240 mg/kg of Genuphil from 5th day of gestation to 19th day of gestation showed highly significant decrease (P<0.01) as compared to the control group (Table 2), (Figure 12).
During lactation
Histopathological findings
Group of fetuses in seven days old
Group kept as control
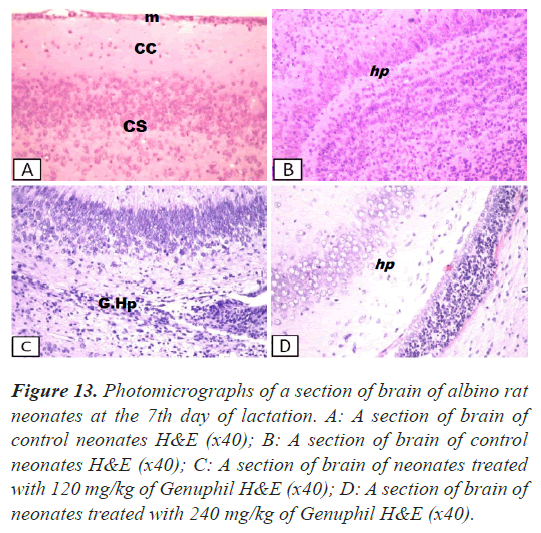
Brain: There was no histopathological alteration and the normal histological structure of the meninges, cerebral cortex, cerebrum and hippocampus were recorded in Figure 13.
Figure 13: Photomicrographs of a section of brain of albino rat neonates at the 7th day of lactation. A: A section of brain of control neonates H&E (x40); B: A section of brain of control neonates H&E (x40); C: A section of brain of neonates treated with 120 mg/kg of Genuphil H&E (x40); D: A section of brain of neonates treated with 240 mg/kg of Genuphil H&E (x40).
Group administrated 120 mg/kg
Brain: There was diffuse gliosis in the hippocampus (Figure 13).
Group administrated 240 mg/kg
Brain: Oedema was observed in the hippocampus (Figure 13).
Group of fetuses in fourteen days old
Group kept as control
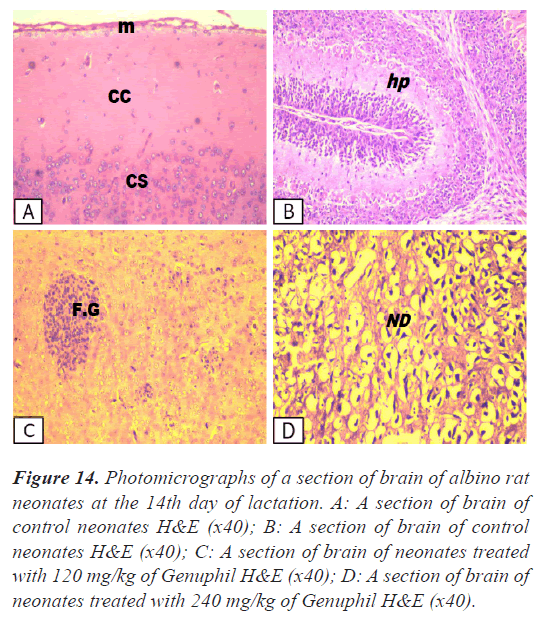
Brain: There was no histopathological alteration as recorded in Figure 14.
Figure 14: Photomicrographs of a section of brain of albino rat neonates at the 14th day of lactation. A: A section of brain of control neonates H&E (x40); B: A section of brain of control neonates H&E (x40); C: A section of brain of neonates treated with 120 mg/kg of Genuphil H&E (x40); D: A section of brain of neonates treated with 240 mg/kg of Genuphil H&E (x40).
Group administrated 120 mg/kg
Brain: The cerebrum showed focal gliosis (Figure 14).
Group administrated 240 mg/kg
Brain: The cerebrum showed degeneration and oedema in the neuronal cells (Figure 14).
Group of fetuses in twenty one days old
Group Kept as control
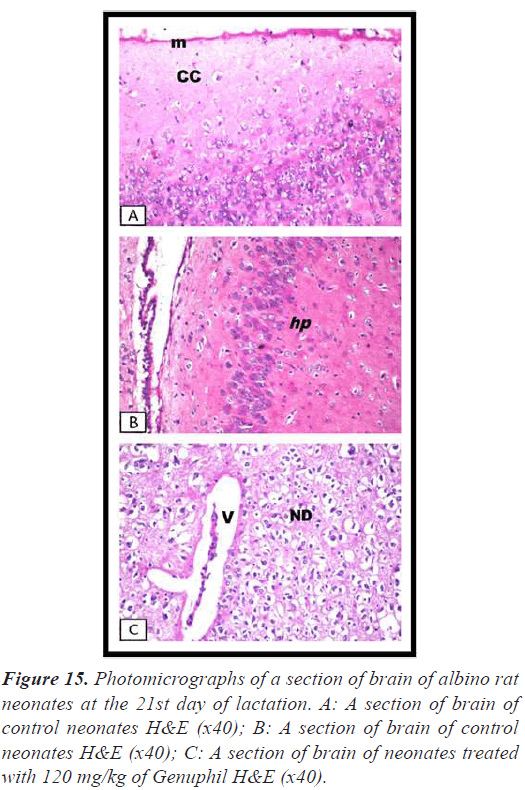
Brain: There was no histopathological alteration as recorded in Figure 15.
Group administrated 120 mg/kg
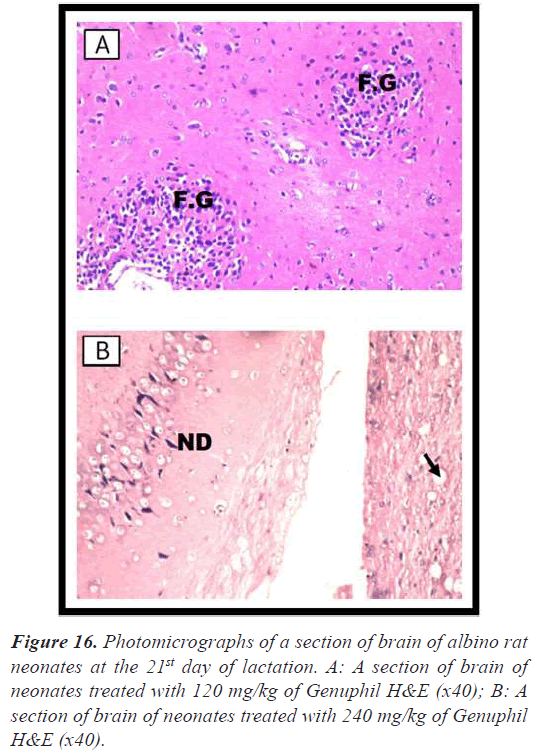
Brain: The cerebrum showed neuronal edema and degeneration with sever dilatation in the blood vessels (Figure 15), and focal gliosis (Figure 16).
Group administrated 240 mg/kg
Brain: Vacuolization and neuronal degeneration were detected in the hippocampus (Figure 16).
Oxidative stress investigations during lactation
Lipid peroxide (Malondialdehyde)
Group of fetuses in seven days old
The results of MDA in samples of fetuses in seven days old were represented in Table 3.
| Control | 120 mg/kg | 240 mg/kg | |
|---|---|---|---|
| Malondialdehyde in sample (liver) (nmol/g.tissue) | 1945.54 ± 21.7567670a | 1418.51 ± 6.9542770b | 1242.83 ± 14.5795949c |
| Malondialdehyde in sample (brain) (nmol/g.tissue) | 1338.25 ± 35.6627612a | 566.3390670 ±15.1526217b | 413.5954133 ± 8.0287143c |
Note: The tissues (Liver and brain) taken from fetuses in seven
days old.
Data are represented as mean ± standard error
Means with the same letter are not significantly different
(b,c) highly significantly different from a at (P<0.01)
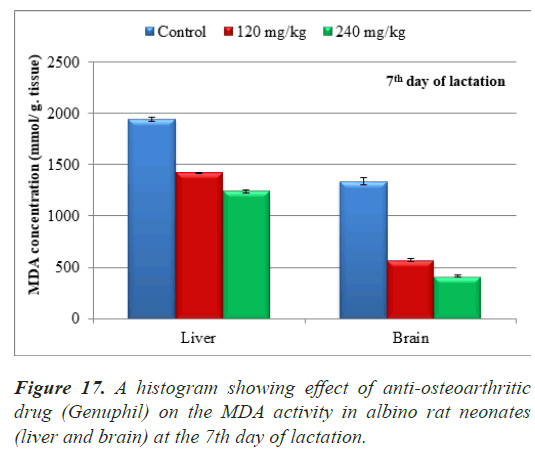
Table 3. Effect of Genuphil on lipid peroxide (Malondialdehyde, nmol/g.tissue) in liver and brain of fetuses in seven days old.
The average of malondialdehyde in samples (liver) of neonates maternally treated with 120 and 240 mg/kg of Genuphil from 5th day of gestation to 6th day of lactation showed highly significant decrease (P<0.01) as compared to the control group (Table 3), (Figure 17).
The average of malondialdehyde in samples (brain) of neonates maternally treated with 120 and 240 mg/kg of Genuphil from 5th day of gestation to 6th day of lactation showed highly significant decrease (P<0.01) as compared to the control group (Table 3), (Figure 17).
Group of fetuses in fourteen days old
The results of MDA in samples of fetuses in fourteen days old were represented in Table 4.
| Control | 120 mg/kg | 240 mg/kg | |
|---|---|---|---|
| Malondialdehyde in sample (liver) (nmol/g.tissue) | 763.1782947 ±7.8558922a | 499.2248083 ± 13.1165885b | 532.1705427 ± 14.6489067b |
| Malondialdehyde in sample (brain) (nmol/g.tissue) | 1349.61 ± 40.5486376a | 165.8914733 ± 3.7096076c | 513.9534883 ± 8.9164137b |
Note: The tissues (Liver and brain) taken from fetuses in
fourteen days old
Data are represented as mean ± standard error
Means with the same letter are not significantly different
(b,c) highly significantly different from a at (P<0.01)
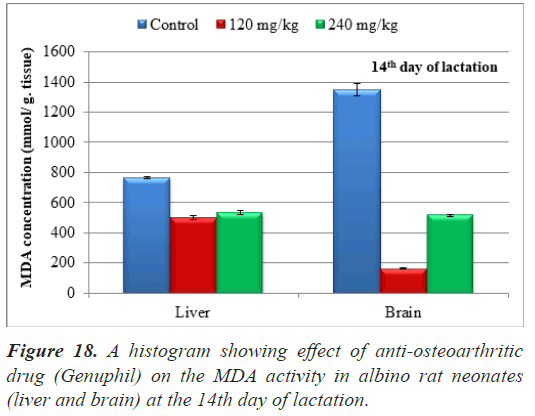
Table 4. Effect of Genuphil on lipid peroxide (Malondialdehyde, nmol/g.tissue) in liver and brain of fetuses in fourteen days old.
The average of malondialdehyde in samples (liver) of neonates maternally treated with 120 mg/kg of Genuphil from 5th day of gestation to 13th day of lactation showed highly significant decrease (P<0.01) as compared to the control group (Table 4), (Figure 18).
The average of malondialdehyde in samples (liver) of neonates maternally treated with 240 mg/kg of Genuphil from 5th day of gestation to 13th day of lactation showed highly significant decrease (P<0.01) as compared to the control group (Table 4), (Figure 18).
Highly Significant decrease (P<0.01) in MDA was found in samples (brain) of neonates maternally treated with 120 & 240 mg/kg of Genuphil from 5th day of gestation to 13th day of lactation as compared to the control group (Table 4), (Figure 18).
Group of fetuses in twenty one days old
The results of MDA in samples of fetuses in twenty one days old were represented in Table 5.
| Control | 120 mg/kg | 240 mg/kg | |
|---|---|---|---|
| Malondialdehyde in sample (liver) (nmol/g.tissue) | 1169.52 ± 2.3836480a |
345.7977208 ± 23.3966499c | 566.2393157 ± 26.5622728b |
| Malondialdehyde in sample (brain) (nmol/g.tissue) | 894.5868967 ±1.9635397a | 371.7948723 ± 0.7802317b | 341.8803417 ± 14.2378906c |
Note: The tissues (Liver and brain) taken from fetuses in twenty
one days old
Data are represented as mean ± standard error
Means with the same letter are not significantly different
(b,c) highly significantly different from a at (P<0.01)
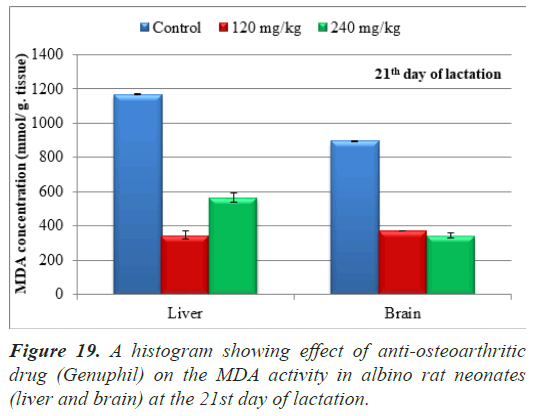
Table 5. Effect of Genuphil on lipid peroxide (Malondialdehyde, nmol/g.tissue) in liver and brain of fetuses in twenty one days old (during lactation).
The average of malondialdehyde in samples (liver) of neonates maternally treated with 120 and 240 mg/kg of from 5th day of gestation to 20th day of lactation showed highly significant decrease (P<0.01) as compared to the control group (Table 5), (Figure 19).
The average of malondialdehyde in samples (brain) of neonates maternally treated with 120 and 240 mg/kg of genuphil from 5th day of gestation to 20th day of lactation showed highly significant decrease (P<0.01) as compared to the control group (Table 5), (Figure 19).
Glutathione reduced (GSH)
Group of fetuses in seven days old
The results of Glutathione reduced (GSH) concentration in samples (liver and brain) of neonates at the 7th day of lactation were represented in Table 6.
| Control | 120 mg/kg | 240 mg/kg | |
|---|---|---|---|
| Glutathione (GSH) Concentration (Liver) (mmol/ g. tissue ) | 3.6852000 ± 0.0486378a | 0.4440000 ± 0.0162126c | 0.7548000 ± 0.0162126b |
| Glutathione (GSH) Concentration (brain) (mmol/g.tissue) | 7.5776000 ± 0.0731066a | 0.5624000 ± 0.0408008b | 0.2960000 ± 0.0408008c |
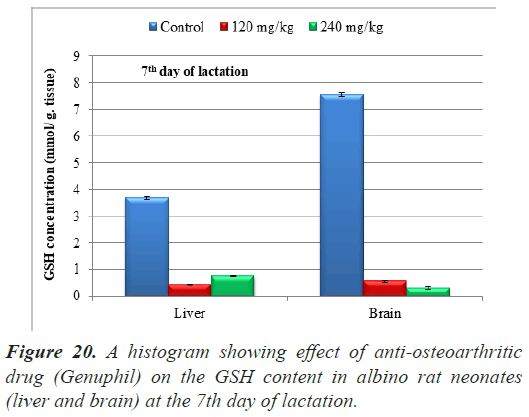
Note: The tissues (Liver and brain) taken from fetuses in seven days old. Data are represented as mean ± standard error. Means with the same letter are not significantly different. (b,c) highly significantly different from a at (P<0.01).
Table 6. Effect of Genuphil on Glutathione reduced (GSH, nmol/g.tissue) in liver and brain of fetuses in seven days old.
In both 120 and 240 mg/kg treated groups, there was highly significant decrease (P<0.01) in Glutathione reduced (GSH) concentration in Liver of neonates at the 7th day of lactation as compared to the control group (Table 6), (Figure 20).
Highly significant decrease (P<0.01) in Glutathione reduced (GSH) concentration was found in samples (brain) of neonates maternally treated with 120 and 240 mg/kg of Genuphil from 5th day of gestation to 6th day of lactation as compared to the control group (Table 6), (Figure 20).
Group of fetuses in fourteen days old
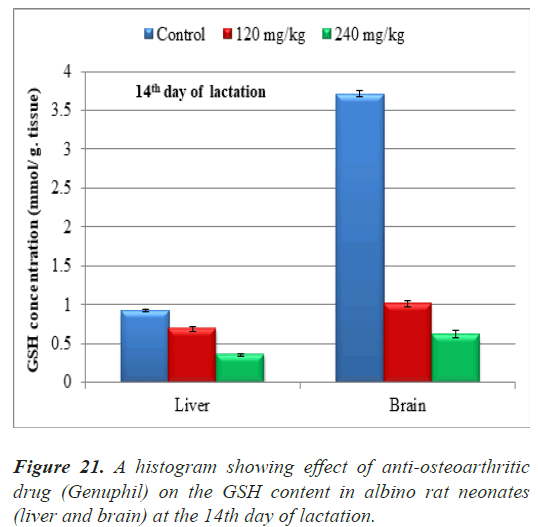
The results of Glutathione reduced (GSH) concentration in samples (liver and brain) of neonates at the 14th day of lactation were represented in Table 7.
| Control | 120 mg/kg | 240 mg/kg | |
|---|---|---|---|
| Glutathione (GSH) Concentration (Liver) (mmol/ g. tissue ) | 0.9324000 ± 0.0162126a | 0.6882000 ± 0.0297844b | 0.3552000 ± 0.0162126c |
| Glutathione (GSH) Concentration (brain) (mmol/g.tissue) | 3.7148000 ± 0.0408008a | 1.0138000 ± 0.0332589b | 0.6216000 ± 0.0472674c |
Note: The tissues (Liver and brain) taken from fetuses in
fourteen days old.
Data are represented as mean ± standard error.
Means with the same letter are not significantly different.
(b,c) highly significantly different from a at (P<0.01).
Table 7. Effect of Genuphil on Glutathione reduced (GSH, nmol/g.tissue) in liver and brain of fetuses in fourteen days old.
both 120 and 240 mg/kg treated groups, there was highly significant decrease (P<0.01) in Glutathione reduced (GSH) concentration in Liver of neonates at the 14th day of lactation as compared to the control group (Table 7), (Figure 21).
Highly significant decrease (P<0.01) in Glutathione reduced (GSH) concentration was found in samples (brain) of fetuses maternally treated with 120 and 240 mg/kg of Genuphil from 5th day of gestation to 13th day of lactation as compared to the control group (Table 7), (Figure 21).
Group of fetuses in twenty one days old
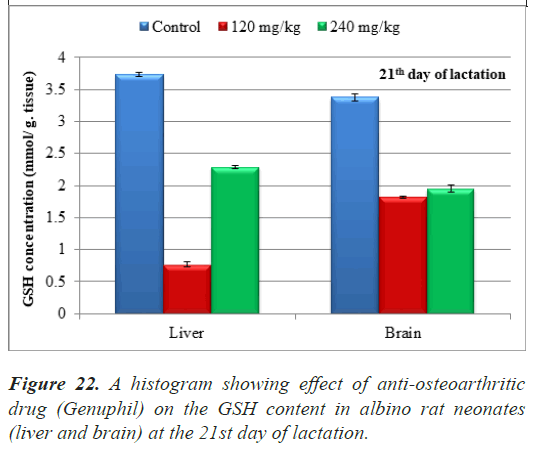
The results of Glutathione reduced (GSH) concentration in samples (liver and brain) of neonates at the 21st day of lactation were represented in Table 8.
| Control | 120 mg/kg | 240 mg/kg | |
|---|---|---|---|
| Glutathione (GSH) Concentration (Liver) (mmol/ g. tissue ) | 3.7296000 ± 0.0324252a | 0.7696000 ± 0.0337492c | 2.2940000 ± 0.0247651b |
| Glutathione (GSH) Concentration (brain) (mmol/g.tissue) | 3.3744000 ± 0.0486378a | 1.8204000 ± 0.0162126c | 1.9536000 ± 0.0486378b |
Table 8. Effect of Genuphil on Glutathione reduced (GSH, nmol/g.tissue) in liver and brain of fetuses in twenty one days old.
Note: The tissues (Liver and brain) taken from fetuses in twenty
one days old
Data are represented as mean ± standard error
Means with the same letter are not significantly different
(b,c) highly significantly different from a at (P<0.01)
In both 120 and 240 mg/kg treated groups, there was highly significant decrease (P<0.01) in Glutathione reduced (GSH) concentration in Liver of neonates at the 21st day of lactation as compared to the control group (Table 8), (Figure 22).
Highly significant decrease (P<0.01) in Glutathione reduced (GSH) concentration was found in samples (brain) of neonates maternally treated with 120 and 240 mg/kg of Genuphil from 5th day of gestation to 20th day of lactation as compared to the control group (Table 8), (Figure 22).
Discussion
A pregnancy-related increase in gastric pH would affect the absorption of weak acids and bases [11]. Concurrent use of other common medications used in pregnancy such as antacids, and nutritional supplements such as vitamins and iron, could also bind and inactivate some drugs [12]. Intramuscular absorption of drugs is generally more rapid due to increased blood flow, which enhances systemic drug absorption and the rate of onset of action. An exception to this would be late in pregnancy when blood flow to the lower extremities is decreased, potentially decreasing absorption of drugs administered to this site [12]. Lastly, estrogen and progesterone alter hepatic enzyme activity, which can increase drug accumulation or decrease elimination of some drugs [13].
Most drugs are transferred from the maternal circulation to the fetal circulation by diffusion. The rate of transfer is dependent on the chemical properties of the drug such as the degree of protein binding, ionic dissociation, lipid solubility, and molecular weight [14]. Fetal proteins appear to bind to various drugs less readily than maternal proteins, and maternal plasma albumin decreases during pregnancy, while fetal albumin progressively increases. This results in differences of concentrations depending on gestational age. Only unbound drugs are capable of crossing the placenta; because of this, protein bound drugs (such as digoxin or ampicillin) can reach higher concentrations in the fetus [11].
Because fetal pH is usually slightly more acidic than the maternal pH, weak bases are more likely to cross the placenta. However, once crossing the placenta and making contact with relatively acidic fetal blood, these molecules become more ionized; this phenomenon is known as “ion trapping” [11]. Lipid soluble drugs will also pass more readily through cell membranes and, therefore, can pass easily to the placenta. For example, antibiotics and opiates are highly lipid soluble and pass readily to the placenta [14].
The molecular weight of a drug also has an impact on its ability to cross the placenta. As a general rule, the larger a drug molecule, the higher the molecular weight. As such, drugs with a lower molecular weight (500 g/mol) cross the placenta readily, while drugs with a molecular weight between 600 and 1000 cross at a slower rate. A few drugs with a high molecular weight (1000 g/mol), such as heparin and insulin, fail to cross the placenta in significant amounts [14].
Transplacental transfer of drugs increases in the third trimester due to increased maternal and placental blood flow, and decreased thickness and increased surface area of the placenta. Ion trapping may produce drug concentrations in the fetus that exceed that of the mother. However, for most drugs the fetal blood concentrations tend to be 50 to 100% of the maternal blood concentrations [12].
The ratio of drug concentration in milk to the drug concentration in maternal plasma is known as the M/P ratio. The ratio is a useful estimate for the amount of drug that is likely to transfer into breast milk. The major mechanism for the transfer of drugs into milk appears to be diffusion between the maternal plasma and the maternal milk compartment, as the M/P ratios are independent of plasma levels or volume of milk. The breast milk concentration usually does not exceed the maternal plasma concentration, and even when the M/P ratio approaches 1.0, the dosage of drug ingested by the infant rarely reaches a therapeutic concentration [11].
There is no information and previous studies available about the safety of Genuphil during pregnancy. Therefore, we made a further study about the effect of this medicine during pregnancy and lactation.
In our study, histopathological findings showed atritic follicles associated with proliferation of the interstitial stromal cells in ovary of the pregnant rats treated with 120 mg/kg of Genuphil from 5th day to 19th day of gestation while Severe congestion in the blood vessels of the medullary portion associated with follicular cystic formation in some follicles was observed in ovary with dose 240 mg/kg of Genuphil.
In the present study, pre-treatment with 120 mg/kg of Genuphil from the 5th day to the 19th day of gestation caused Focal haemorrhage and necrosis in the giant cell layer in the placenta while Necrosis was noticed in the giant cell and trophospongium in the placenta with dose 240 mg/kg.
In our study, histopathological examinations revealed Few inflammatory cells infiltration in the portal area as well as in between the fatty changed hepatocytes with dilatation in the portal vein in the liver of the pregnant rats treated with 120 mg/kg of Genuphil from the 5th day to the 19th day of gestation while Dilatation in the central vein associated with brown to yellow pigmentation in between the fatty changed hepatocytes and dilatation in the portal vein with inflammatory cells infiltration were detected in the liver of the pregnant rats treated with 240 mg/kg of Genuphil from the 5th day to the 19th day of gestation.
During gestation, we found that the kidney of the pregnant rats treated with 120 mg/kg of Genuphil from 5th day to 19th day of gestation showed degenerative change in the lining epithelium of the tubules at the corticomedullary while swelling in the lining epithelium of the tubules was noticed in the kidney of pregnant rats with dose 240 mg/ kg Genuphil.
In our study, Vacuolization as well as focal circumscribed encephalomalacia were detected in the cerebrum of the brain of the pregnant rats treated with 120 mg/kg of Genuphil from 5th day to 19th day of gestation while vacuolization, focal encephalomalacia in the cerebrum and only vacuolization in the cerebellum were detected in the brain of pregnant rat with the dose 240 mg/kg Genuphil.
Histopathological findings were observed in the brain of fetuses maternally treated with 120 and 240 mg/kg of Genuphil from 5th day to 19th day of gestation. Diffuse gliosis in the cerebrum was observed in the brain of fetuses maternally treated with 120 mg/kg of Genuphil from 5th day to 19th day of gestation while severe congestion in the plexus as well as in the meningeal blood vessels was detected in the brain of the fetuses with the dose 240 mg/ kg of Genuphil.
Diffuse gliosis in the hippocampus was observed in the brain of fetuses in seven days old after oral administration of 120 mg/kg of Genuphil from 5th day of gestation to 6th day of lactation while edema was detected in the hippocampus of the brain of fetuses in seven days old with the dose 240 mg/kg of Genuphil.
The cerebrum of the brain of fetuses in fourteen days old showed focal gliosis after oral administration of 120 mg/ kg of Genuphil from 5th day of gestation to 13th day of lactation while the cerebrum showed degeneration and oedema in the neuronal cells with the dose 240 mg/kg of Genuphil.
The cerebrum of the brain of neonates at the 21st day of lactation showed neuronal edema and degeneration with severe dilatation in the blood vessels, and focal gliosis after oral administration of 120 mg/kg of Genuphil from 5th day of gestation to 20th day of lactation while Vacuolization and neuronal degeneration were detected in the hippocampus of the brain of neonates at the 21st day of lactation with the dose 240 mg/kg of Genuphil.
Oxidative stress is involved in the aetiology of defective embryo development. Reactive Oxygen Species (ROS) may originate from embryo metabolism and/or embryo surroundings. Embryo metabolism generates ROS via several enzymatic mechanisms. The relative contribution of each source seems different depending on the species, the stage of development, and the culture conditions. Several exogenous factors and culture conditions can enhance the production of ROS by embryos. ROS can alter most types of cellular molecules, and also induce development block and retardation. Multiple mechanisms of embryo protection against ROS exist, and these have complementary actions. External protection, present in follicular and tubal fluids, mainly comprises nonenzymatic antioxidants such as hypotaurine, taurine and ascorbic acid. Internal protection mainly comprises antioxidant enzymes: Superoxide dismutase, glutathione peroxidase and gamma-glutamylcysteine synthetase. Transcripts encoding for these enzymes are present in the oocyte, embryo and oviduct. It may be important that these transcripts are stored during oocyte maturation in order to allow the embryo to acquire the aptitude to develop. It is now common to add antioxidant compounds to culture media. Nevertheless, maintaining the prooxidant- antioxidant equilibrium in embryos through such supplementation is a complex problem [15].
Oxygen radicals, or Reactive Oxygen Species (ROS) act as primary or secondary messengers to promote cell growth or death. Many instances demonstrate an important direct role of ROS in development because redox status regulates key transcription factors that influence cell signaling pathways involved in proliferation, differentiation, and apoptosis. Therefore, oxidative stress can alter many important reactions that affect embryonic development both positively and negatively. During particular periods in development, the embryo is more or less susceptible to oxidative stress, and teratogens, which can modify redox status, such as thalidomide, phenytoin, and ethanol, will disrupt fetal development. Various events in pregnancy such as diabetes also alter the redox state. Fortunately, antioxidants can obviate these effects through modification of gene expression, transcription factor signaling, and cell cycle alterations [16].
Ubiquitous free radical production occurs continuously in all living cells mainly as a byproduct of aerobic metabolism and under physiological circumstances being counterbalanced by cellular antioxidant system. Therefore, under normal conditions free radical level does not exceed defense cells capacity. Excessive free radical formation, originating either from endogenous or exogenous sources, leads to oxidative stress damaging tissues, lipids, carbohydrates, proteins and DNA. The damage is minimized or stopped by antioxidant defense activation in one of the three ways: preventing free radicals production, scavenging excessive radicals and/or accelerating radicals decomposition [17].
According to our best knowledge we have not found published data on the effect of Genuphil on lipid peroxidation and Glutathione reduced (GSH) level, which we did.
Our results showed that prenatal exposure to 120 mg/ kg of Genuphil Caused significant decrease in the lipid peroxidation and Glutathione reduced (GSH) level in liver and brain compared to control group.
MSM is used orally and topically. There is limited formal safety data and no long-term assessment. However, MSM is rapidly excreted from the body [18,19] and animal toxicity studies of MSM showed only minor adverse events using doses of 1.5 g/kg and 2.0 g/kg of MSM for 90 days. This dose represents a human dose of 30-42 g/d, which is equivalent to 5-7 times the proposed maximum recommended human dose of 6 g/d [20]. MSM alone and in combination with glucosamine in the treatment of patients with osteoarthritis modestly reduced pain and swelling but did not reduce joint stiffness [21].
A further study confirmed MSM had no toxic effects on either pregnant rats or their foetus [18]. Only minor adverse effects are associated with MSM administration in humans and include allergy, GI upsets and skin rashes.
MSM as one of the three constituents of Genuphil (Glucosamine sulfate, chondroitin sulfate and MSM) might contain components which could be playing a contributory antioxidant role either by augmenting or complementing the endogenous antioxidant defense system, thereby reducing the oxidative stress status and that might explain the decrease in lipid peroxidation in our study.
In our study, administration of the higher dose 240 mg/ kg of Genuphil caused significant increase in the lipid peroxidation in the liver and the brain compared to control group while significant decrease in Glutathione reduced (GSH) level in liver and brain compared to control group. This increase in lipid peroxidation might be due to slight decay in efficacy of antioxidant defense system and nutritional status of the rats.
In our study, with the continuation of treatment with Genuphil instead of higher lipid peroxidation, we was getting exactly the opposite but with a clear pattern of decrease. We found significant decrease in the lipid peroxidation and Glutathione reduced (GSH) level in liver and brain compared to control group after administration of Genuphil (120 and 240 mg/kg) from 5th day of Gestation to 6th, 13th and 20th day of lactation.
Interestingly, two patients showed very low detectable lipid peroxidation products in their serum [22].
After reading several studies we think that non enzymatic antioxidants may be the reason for these results. Stress adaptation/disadaptation might be a possible explanation.
Birth defects are reported to occur in 3-5% of all newborn. Of these, 1-3% are thought to be attributed to adverse effects from a pharmacologic agent [23].
Conclusion
This previous study might also explain the decrease in the lipid peroxidation and Glutathione reduced (GSH) level in liver and brain of these neonates. Often the decision to use a particular medication has to be based on the potential risks to the fetus and benefits to the pregnant woman. This is of particular concern and requires that providers have an understanding of the risks and benefits of the use of pharmacologic agents. It is evident that Genuphil is teratogenic and much care must be given to prevent the increased risk if genuphil is administrated during pregnancy and lactation.