ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 7
Effects of serum paraoxonase and paraoxonase phenotypic distribution on the parameters of first trimester screening
1Department of Family Medicine, Antalya Training and Research Hospital of Ministry of Health, Antalya, Turkey
2Clinic of Obstetrics and Gynaecology, Antalya Training and Research Hospital of Ministry of Health, Antalya, Turkey
3Central Laboratories of Antalya Training and Research Hospital of Ministry of Health, Antalya, Turkey
4Department of Biochemistry, Tepecik Training and Research Hospital of Ministry of Health, Izmir, Turkey
- *Corresponding Author:
- Mehmet Ozen
Antalya Training and Research Hospital
Department of Family Medicine
Antalya Egitimve Arastirma
Hastanesi Varlik Mahallesi Kazim Karabekir Caddesi Soguksu, Turkey
Accepted on January 5, 2017
Background: First trimester screening is a program used to identify any chromosome abnormalities in the first trimester of pregnancy by combining ultrasonography and biochemical markers. The aim of this study is to analyse the relationship between ultrasonography (NT, CRL) and biochemical markers (PAPP-A, β-hCG) with the levels of paraoxonase (PON1), a multifunctional enzyme associated with High-Density Lipoprotein (HDL) and Arylesterase (ARE)-level PON1 phenotypic distribution which are the parameters used in the first trimester screening program.
Materials and Methods: Included in the study were pregnant women (n=114) in weeks 11-13 of pregnancy who had undergone first trimester screening tests. Risks were determined through the use of Benetech PRA software, Serum PON1, ARE and stPON1 values were analysed, and PON1 phenotypic distribution was determined through the double substrate method.
Results: No significant correlation was found between the results of the PON1 and ARE activities and the first trimester screening parameters. The Q allele frequency of the study group was 0.787, while the R allele frequency was 0.213. The PON1 phenotype distributions of the pregnant women were as follows: 66 (58%) QQ, 45 (39.4%) QR and 3 (2.6%) RR phenotypes respectively.
Conclusion: There is a need for more advanced studies to evaluate the relationship between the serum PON1, ARE and PON1 phenotypic distributions of pregnant women in their first trimesters with the complications that may develop in later stages of pregnancy.
Keywords
First trimester screening, Chromosome abnormalities, Pregnancy-associated plasma protein A, Human chorionic gonadotropin, Paraoxonase-1.
Introduction
First trimester screening is carried out in the 11th-14th weeks of pregnancy by evaluating serum markers such as free beta-human chorionic gonadotropin (free β-hCG) and Pregnancy- Associated Plasma Protein A (PAPP-A), and through an ultrasonographic evaluation of fetal nuchal thickness (NT, Nuchal Translucency) [1]. β-hCG is a glycoprotein hormone with α and β subunits, of which the β subunit exerts the biological activity. The most important known function of β- hCG in pregnancy is to provide the necessary hormonal stimulus to the corpus luteum, ensuring it maintains its functions until the onset of the luteo-placental shift [2]. β-hCG levels increase the pregnant women carrying fetuses with trisomy 21, and this increase in β-hCG levels continues as the pregnancy progresses [3]. Intrauterine Growth Retardation (IUGR), preterm delivery, preeclampsia and fetal loss ratios increase in pregnant women known to have high β-hCG levels when compared with those with normal levels of β-hCG [4].
PAPP-A, secreted from the syncytiotrophoblasts, can be found in the maternal serum just after implantation, and its concentration rises throughout the pregnancy. If the fetus being carried has Down syndrome (trisomy 21), the first trimester PAPP-A level falls considerably. In pregnant women with low PAPP-A levels, complications such as IUGR, premature delivery, preeclampsia and stillbirth increase when compared to pregnant women with normal PAPP-A levels [5]. The nuchal thickness (Nuchal Translucency-NT) measurement technique was defined in 1992 by Nicolaides, and relates to the black area visualized by ultrasonography at the back of the baby’s neck in the first trimester of pregnancy [6]. An increased NT value has proven useful in the determination and screening of Down syndrome (trisomy 21) and various other chromosomal abnormalities, including trisomy 13 and trisomy 18.
Human serum Paraoxonase (PON1) is a protein that contains 354 amino acids and has a molecular weight of 43 kDa that has been associated physically with High-Density Lipoprotein (HDL) [7,8]. Paraoxonase1 (PON1) and Arylesterase (ARE) are two enzymes coded by the same gene that have similar active enzymes. PON1 is an ester hydrolase of the arid alkyl phosphatase class that hydrolyses paraoxon (a potent inhibitor of cholinesterase) and that detoxifies other organophosphates. As an antioxidant enzyme, it protects HDL and low-density lipoprotein (LDL) from oxidation by free radicals, and decreases oxidative stress [9-13]. Arylesterase, like PON1, can detoxify organophosphates, but does not have the genetic polymorphism of PON1.
In this study it is aimed to evaluate the effects of serum PON1 and ARE levels and PON1 phenotypic distribution on the parameters of first trimester screening in pregnant women. As an additional objective, it is aimed to determine the possible roles of these parameters on the development of such chromosomal abnormalities as trisomy 21, trisomy 18 and trisomy 13.
Materials and Methods
Included in the study were pregnant women (n=114) in their first trimesters (11th-13th weeks) who presented at the Antalya Training and Research Hospital Obstetrics and Gynaecology Clinic, Perinatology Division for screening tests. The age, week of pregnancy and ultrasonography findings (NT, CRL) of each of the pregnant women were recorded, as well as smoking habits and any diseases that that may affect PON1 and ARE levels. Diabetic women, twin pregnancies, those who smoke and those who became pregnant following IVF treatment were excluded from the study. Written informed consent was obtained from the whole study group. The study was approved by the local Ethical Committee and all procedures performed in it were in accordance with the 1964 Helsinki declaration and its later amendments.
Serum PAPP-A and β-hCG levels were analysed, and after obtaining the results, a risk assessment was calculated using Benetech PRA software based on information obtained from the forms. The necessary corrections were made, and all assay results were expressed as corrected Multiplies of Median (MoM) values. The threshold for a determination of Down syndrome risk in the first trimester screening tests was accepted to be 1/250. For the determination of combined risks, these ratios were evaluated by converting them to numbers.
Example
Down syndrome risk: 1/4090 → → 1/4090=~0.00024
Down syndrome risk: 0.00024 × 10000=2.44
As the values calculated in this way increase through first trimester screening, the probability of having a child with Down syndrome also increases. The same calculations were made also for risks of age and other trisomies.
Blood samples of the pregnant women were collected in biochemistry test tubes and were centrifuged within 30 minutes in a non-refrigerated centrifuge device for 10 minutes, at 4000 rpm to separate sera. The parameters required for first trimester screening (PAPP-A and β-hCG) were analysed, and the remaining serum samples were stored in eppendorf tubes at -80°C until the assay day. Paraoxonase, arylesterase, total cholesterol, LDL cholesterol, HDL cholesterol and triglyceride measurements were made using the remaining samples. For all biochemical tests, the necessary calibrations and controls were applied.
Analytical methods
Measurement of serum β-hCG levels: Serum intact β-hCG levels were determined by using commercially available assay kits (Beckman Coulter) with an autoanalyzer (Access DxI800, Beckman Coulter Diagnostics). The β-hCG assay is a two-site immunoenzymatic (“sandwich”) assay. A sample is added to a reaction vessel, along with a monoclonal anti-β-hCG antibody conjugated to alkaline phosphatase, TRIS buffered saline with proteins and paramagnetic particles coated with a goat polyclonal anti-β-hCG antibody. After incubation in a reaction vessel, materials bound to the solid phase are held in a magnetic field while unbound materials are washed away. Then, the chemiluminescent substrate Lumi-Phos* 530 is added to the vessel and light generated by the reaction is measured with a luminometer. The limit of detection is 0.5 pg/ml and the coefficient of variation range<0.5% for this assay.
Measurement of serum PAPP-A levels: Serum PAPP-A levels were determined by using commercially available assay kits (Beckman Coulter) with an autoanalyzer (Access DxI800, Beckman Coulter Diagnostics).The PAPP-A assay is a two-site immunoenzymatic (“sandwich”) assay. A sample is added to a reaction vessel, along with a monoclonal anti-PAPP-A antibody conjugated to alkaline phosphatase, TRIS buffered saline with proteins and paramagnetic particles coated with a goat polyclonal anti-PAPP-A antibody. After incubation in a reaction vessel, materials bound to the solid phase are held in a magnetic field while unbound materials are washed away. Then, the chemiluminescent substrate Lumi-Phos* 530 is added to the vessel and light generated by the reaction is measured with a luminometer. The limit of detection is 1 ng/ml and the coefficient of variation range<0.5% for this assay.
Measurement of paraoxonase and arylesterase enzyme activities in serum: PON1 and ARE enzyme activities were measured using commercially available kits (Relassay®, Turkey). The fully automated PON1 activity measurement method consists of two different sequential reagents; the first reagent is an appropriate Tris buffer containing calcium ions, which is a cofactor of PON1 enzyme. A linear increase in the absorbance of p-nitrophenol, produced from paraoxon, is followed in a kinetic measurement mode. The non-enzymatic hydrolysis of paraoxon was subtracted from the total rate of hydrolysis. The molar absorptivity of p-nitrophenol is 18,290 M-1 cm-1 and one unit of paraoxonase activity is equal to 1 mol of paraoxon hydrolyzed per liter per minute at 37°C [14]. Phenylacetate was used as a substrate to measure IS activity. PON1, present in the sample, hydrolyses phenylacetate to its products, phenol and acetic acid. The produced phenol is colorimetrically measured via oxidative coupling with 4- aminoantipyrine and potassium ferricyanide. Nonenzymatic hydrolysis of phenyl acetate was then subtracted from the total rate of hydrolysis. The molar absorptivity of the colored complex is 4000 M-1 cm-1 and one unit of arylesterase activity is equal to 1 mmol of phenylacetate hydrolyzed per liter per minute at 37°C [15].
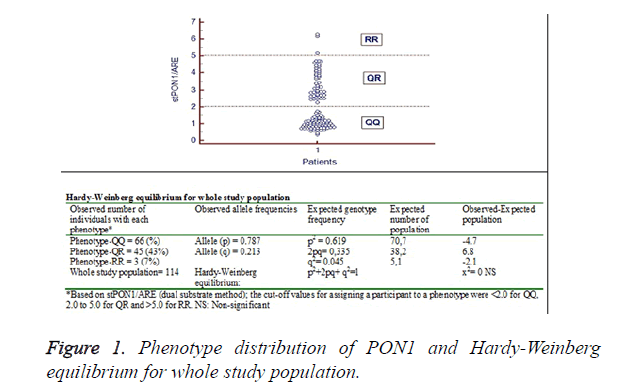
Paraoxonase phenotype distribution: The genetic polymorphism Q/R in codon 192 is responsible for three isotypes: QQ (low activity), QR (intermediate activity), and RR (high activity). The phenotype distribution of PON1 was determined using the dual substrate method. The ratio of paraoxon hydrolysis in the presence of 1 moL/L NaCl (salt stimulated paraoxonase: stPON) to phenylacetate hydrolysis was used to assign individuals to one of the phenotypes [14]. The ratio provided by dividing salt-stimulated paraoxonase by arylesterase enzyme activity demonstrated a trimodal PON1 frequency distribution in whole study population (Figure 1). Accordingly, the cut-off values for assigning a participant to a phenotype were<2.0 for QQ, 2.0 to 5.0 for QR and>5.0 for RR. Allele-Q and allele-R were in excellent agreement with the Hardy-Weinberg equilibrium (Figure 1).
Routine parameters
The levels of Triglycerides (TG), Total Cholesterol (TC), HDL cholesterol and Low density Lipoprotein cholesterol (LDL) were determined by using commercially available assay kits (Beckman Coulter) and an autoanalyzer (Beckman AU5800; Beckman Coulter Diagnostics, USA).
Statistical analysis
The demographic and biochemical data of the pregnant women was expressed as mean ± SD and in terms of the reference interval (minimum and maximum). In parameters indicating normal distribution, a student’s t-test and Pearson correlation analysis were used, while a Mann-Whitney U-test and Spearman correlation analysis were applied for parameters showing a non-normal distribution. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA) software, version 18.0, and a p value<0.05 was accepted as statistically significant.
Results
The mean (± SD) age and week of pregnancy values of the pregnant women were 28.7 ± 5.2 and 12.3 ± 0.7, respectively. CRL and NT values were 59.7 ± 9.3 and 1.32 ± 0.35 mm, respectively. The PAPP-A and β-hCG concentrations in the study group were 916.6 ± 688 ng/ml and 69028 ± 31706 mIU/ml, respectively. The values of first trimester screening parameters (mean ± SD; min-max), as well as risks of genetic abnormalities are presented in Table 1.
| Parameters | Mean ± SD | Reference interval (min-max) |
|---|---|---|
| Age, year | 28.7 ± 5.2 | 17-35 |
| Gestational age, weeks | 12.3 ± 0.7 | 11-13 |
| CRL, mm | 59.7 ± 9.3 | 42-83 |
| PAPP-A, ng/ml | 916.6 ± 688 | 133-3935 |
| PAPP-A, MoM | 0.91 ± 0.63 | 0.17-3.48 |
| β-hCG, mIU/ml | 69028 ± 31706 | 13401-190351 |
| β-hCG, MoM | 0.88 ± 0.48 | 0.17-2.84 |
| NT, mm | 1.32 ± 0.35 | 0.7-2.3 |
| NT, MoM | 1.09 ± 0.29 | 0.55-1.84 |
| *The risk of Down synd. | 3.8 ± 5.4 | 0.2-29.4 |
| *The risk of Age | 14.6 ± 13.8 | 6.4-87 |
| *The risk of Trisomy 21 | 0.78 ± 1.7 | 0.1-12.2 |
| *Risks converted to numbers. | ||
Table 1. Data from the first trimester screening of the pregnant women.
The mean ± SD values of PON1 and ARE were found to be 174.6 ± 111 U/L and 218.1 ± 69.7 kU/L, respectively, while those of stPON1 were 441 ± 315 U/L. Total cholesterol, triglyceride and HDL-C levels were 167 ± 33.5, 130 ± 58 and 46.4 ± 11 mg/dl, respectively. These values, together with those of other serum lipids, are presented in Table 2 (mean ± SD; reference interval).
| Parameters | Mean ± SD | Reference range |
|---|---|---|
| PON 1, U/L | 174.6 ± 111 | - |
| ARE, kU/L | 218.1 ± 69.7 | - |
| stPON1, U/L | 441 ± 315 | - |
| T.CHOL., mg/dl | 167 ± 33.5 | 0-200 |
| TRIG, mg/dl | 130 ± 58 | 0-150 |
| HDL-C, mg/dl | 46.4 ± 11 | 40-60 |
| LDL-C, mg/dl | 95.4 ± 24 | 0-130 |
| VLDL-C, mg/dl | 26.5 ± 12 | 10-40 |
| TC: Total Cholesterol; TRIG: Triglyceride; HDL-C: LDL-C: LDL VLDL-C: Cholesterol was calculated by using Friedewald Formula: Total cholesterol-(VLDL+HDL), where VLDL=Triglyceride/5. | ||
Table 2. PON1 and ARE activities and lipid parameters in the pregnant women.
No significant correlations were found between first trimester screening test parameters listed in Table 1 (CRL, NT, PAPP-A, β-hCG, genetic abnormality risks, etc.) and HDL-associated antioxidant enzymes PON1 and ARE activities, or stPON1 activity ratio values. The results of the Pearson and Spearman analyses are given in Table 3.
| Parameters | PON1, rho, p | ARE, rho, p | stPON1, rho, p |
|---|---|---|---|
| Age, year | r=0.122, p=0.19 | r=0.01, p=0.90 | r=0.375, p=0.14 |
| Gestational age, weeks | r=0.144, p=0.12 | r=0.07, p=0.94 | r=0.141, p=0.11 |
| CRL, mm | r=0.176, p=0.06 | r=0.03, p=0.71 | r=0.186, p=0.06 |
| PAPP-A, ng/ml | r=0.127, p=0.17 | r=-0.129, p=0.16 | r=0.135, p=0.16 |
| PAPP-A, MoM | r=0.07, p=0.41 | r=-0.116, p=0.21 | r=0.07, p=0.45 |
| β-hCG, mIU/ml | r=0.07, p=0.43 | r=0.02, p=0.75 | r=0.05, p=0.56 |
| β-hCG, MoM | r=0.09, p=0.32 | r=0.07, p=0.41 | r=0.07, p=0.45 |
| NT, mm | r=-0.02, p=0.81 | r=0.08, p=0.34 | r=-0.03, p=0.79 |
| NT, MoM | r=-0.09, p=0.30 | r=0.04, p=0.64 | r=-0.09, p=0.29 |
| The risk of down synd. | r=-0.02, p=0.75 | r=0.15, p=0.10 | r=-0.03, p=0.70 |
| The risk of age | r=0.11, p=0.21 | r=-0.01, p=0.99 | r=0.14, p=0.13 |
| The risk of trisomy 21 | r=-0.19, p=0.07 | r=0.09, p=0.29 | r=-0.19, p=0.07 |
Table 3. Correlations between first trimester screening test parameters and PON1 and ARE activities.
The phenotypic distribution of PON1 was evaluated using the double substrate method and Q allele and R allele frequencies were found to be 0.787 and 0.213, respectively. The PON1 phenotypic distributions of the pregnant women were as follows: QQ, 66 (58%); QR, 45 (39.4%) and RR, 3 (2.6%), respectively. The pregnant women were divided into two groups: Q allele (QQ) and R allele (QR+RR). Except PON1 activity, no significant difference was identified between the first trimester screening test parameters, lipid panel or ARE activity levels of the two groups (Table 4).
| Parameters | QQ (n=66) | QR+RR (n=48) | p |
|---|---|---|---|
| CRL, mm | 60 (51-64) | 61.2 (54-68) | 0.08 |
| PAPP-A, ng/ml | 621 (361-1207) | 725 (473-1266) | 0.4 |
| PAPP-A, MoM | 0.8 (0.42-1.19) | 0.72 (0.44-1.23) | 0.97 |
| β-hCG, mIU/ml | 64.874 (50.422-83.434) | 61.876 (44.608-85.599) | 0.69 |
| β-hCG, MoM | 0.76 (0.61-1.05) | 0.72 (0.56-1.06) | 0.76 |
| NT, mm | 1.3 (1-1.6) | 1.2 (1-1.55) | 0.59 |
| NT, MoM | 1.10 (0.91-1.27) | 1.02 (0.83-1.23) | 0.19 |
| The risk of down synd | 1.7 (0.38-5.32) | 1.09 (0.24-4.12) | 0.51 |
| The risk of Age | 8.33 (7.5-13.6) | 10.1 (7.9-17.4) | 0.07 |
| The risk of Trisomy 21 | 0.10 (0.1-0.7) | 0.10 (0.1-0.17) | 0.09 |
| PON1 U/L | 97.1 ± 31 | 280 ± 94 | <0.01 |
| ARE kU/L | 203 (167-267) | 211 (173-244) | 0.92 |
| T C mg/dl | 162 ± 33 | 174 ± 34 | 0.08 |
| TRIG, mg/dl | 122 (89-155) | 120 (91-165) | 0.39 |
| HDL-C, mg/dl | 44 (37-53) | 44.5 (39-55) | 0.27 |
| LDL-C, mg/dl | 92 ± 24 | 100 ± 24 | 0.1 |
Table 4. Relationships between first trimester screening parameters and PON1 phenotypic distribution. These parameters did not differ significantly between the Q allele carriers and R allele carriers.
The study group was followed up beyond the third trimester and none of the subjects developed any pregnancy complications.
Discussion
In the present study, in which the relationships between first trimester screening tests and HDL-associated antioxidant enzymes PON1 and ARE were evaluated, and no significant correlations were detected between the serum PON1 and ARE levels and the first trimester screening tests. PON1 phenotypic distribution was determined using the dual substrate method (stPON1/ARE), and the Q allele and R allele frequencies were found to be 0.787 and 0.213, respectively (Figure 1). QQ, QR and RR phenotypes were determined to be 58 percent, 39.4 percent and 2.6 percent, respectively. The PON1 R allele frequency identified in the present study was found to be similar to that determined in the Turkish population in previous studies. In these genotype studies, PON1 R allele frequency was found to be 0.28 by Ellidag et al. [16], 0.38 by Karakaya et al. [17] and 0.31 by Aynacioglu et al. [18]. These results indicate that the gene polymorphism distribution in the Turkish population resembles that of other Caucasian populations, but different to Far Eastern countries, where RR phenotypic distribution is high [19].
PAPP-A was determined to be associated not only with chromosome abnormalities, but also with complicated pregnancy outcomes [20,21]. In a study by Smith et al. carried out on women in the 8th-14th weeks of pregnancy, it was determined that risks of intrauterine growth retardation, moderate and very preterm deliveries, preeclampsia and stillbirth increase in pregnant women with low PAPP-A levels. Besides, the Insulin-like Growth Factor (IGF) found in trophoblasts in the first and early second trimesters plays an important role in the prognosis of pregnancy. PAPP-A is the protease of Insulin-like Growth Factor Binding Protein-4 (IGFBP-4) [22], and so when PAPP-A levels are low, IGFBP-4 levels are increased, meaning that free IGF levels decrease in the maternal serum [23,24]. Dugoff et al. identified that the risk of preeclampsia and gestational hypertension increases when maternal serum PAPP-A levels are low in the first trimester [25]. There are a number of studies that have determined that low PAPP-A levels are related with increased ratios of Intrauterine Growth Retardation (IUGR) and Smallfor Gestational Age (SGA) babies [22-26]. Low β-hCG levels in the first trimester were indicated to be mostly related to pregnancy-induced hypertension. Charas et al. determined that first trimester maternal serum free β-hCG levels are low in 15 percent of pregnancy-induced hypertension cases, and in 20 percent of gestational diabetes cases [27]. Silvana et al. found that first trimester free β-hCG levels are found to be low in pregnant women with gestational hypertension and preeclampsia when compared to normal pregnancies [28]. Although there are studies proclaiming that first trimester PAPP-A and β-hCG values are good predictors of late pregnancy complications, no relationship could be identified between these parameters and PON1, stPON1, ARE and PON1 phenotypic distribution.
Pregnancies complicated by gestational diabetes must be carefully followed up, given the maternal and fetal risks involved [29]. There have been a number of studies evaluating serum PON1 and ARE levels in cases of gestational diabetes, which found them to be low [30,31]. In a genotype study by Al-Hakeem et al. of pregnant women with gestational diabetes, higher frequencies of gestational diabetes were determined in the pregnant women carrying R allele (QR+RR) [32]. In our study, the number of pregnant women carrying R allele was 48 (42%), however these pregnant women could not be followed up for an evaluation of the gestational risk of diabetes. As said, this study may lead to the application of further studies related to this subject.
As mentioned previously, pregnant women with low PAPP-A levels are at higher risk of preeclampsia and stillbirth. In studies carried out with preeclamptic pregnancies, the results related to PON1 and ARE enzymes were found to be contradictory. Yaghmaei et al. [33] and Baker et al. [34] found increased PON1 activity in cases of preeclampsia, while Kumru et al. determined lower PON1 activity [35]. In a study by Sarandol et al. carried out for 21 cases of mild preeclampsia, 21 severe preeclampsia cases and 20 normally pregnant women with no complications, serum PON1 and ARE activity did not differ significantly among the three groups [36]. Kim et al. made a study of 32 preeclampsia cases and 57 normally pregnant women, but found no significant difference in terms of frequency of the PON1 genotype [37].
We conclude that chromosomal abnormalities represent significant problems to parents and affected children. First-trimester screening tests and programs help physicians determine these abnormalities. In this study we have investigated the relationship between HDL-associated antioxidant enzymes PON1 and ARE and first trimester tests that have high predictive values in determining the chromosomal abnormalities and complications in pregnancies. No correlations were determined between the first trimester screening tests and PON1 and ARE levels. Furthermore, in a study in which PON1 phenotypic distribution was analysed, no correlation was determined between the first trimester screening tests and the Q allele or R allele carriers either. After we have completed our study, we followed up these pregnant women in their later periods and none of them developed any pregnancy complications. While fortunate for the women involved, this is the main limitation of our study, as identifying any potential relationship between pregnancy complications and the parameters we have investigated became impossible. In this regard, there is a need for more advanced and larger studies to evaluate the relationship between PON1 and ARE activity levels, and PON1 phenotypic distributions of women in their first trimesters and any complications that may develop in later stages of pregnancy.
References
- Snijders RJ, Noble P, Sebire N, Souka A, Nicolaides KH. UK multicentre project on assessment of risk of trisomy 21 by first- and second-trimester DS screening maternal age and fetal nuchal-translucency thickness at 10-14 weeks of gestation. Fetal Medicine Foundation First Trimester Screening Group. Lancet 1998; 352: 343-346.
- Stenman UH, Tiitinen A, Alfthan H, Valmu L. The classification, functions and clinical use of different isoforms of HCG. Hum Reprod Update 2006; 12: 769-784.
- Chandra S, Scott H, Dodds L, Watts C, Blight C. Unexplained elevated maternal serum alpha-fetoprotein and/or human chorionic gonadotropin and the risk of adverse outcomes. Am J Obstet Gynecol 2003; 189: 775-781.
- Davidson EJ, Riley SC, Roberts SA, Shearing CH, Groome NP, Martin CW. Maternal serum activin, inhibin, human chorionic gonadotrophin and alpha-fetoprotein as second trimester predictors of pre-eclampsia. BJOG 2003; 110: 46-52.
- Smith GC, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, Connor JM. Early pregnancy levels of pregnancy associated plasma protein A and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab2002; 87: 1762-1767
- Nicolaides KH, Azar G, Byrne D, Mansur C, Marks K. Fetal nuchal translucency: Ultrasound screening for chromosomal defects in first trimester of pregnancy. BMJ 1992; 304: 967-969
- Eren E, Yilmaz N, Aydin O. High density lipoprotein and its dysfunction. Open Biochem J 2012; 6: 78-93.
- Ellidag HY, Eren E, Aydin O, Yaldaram M, Sezer C. Multiple myeloma: relationship to antioxidant esterases. Med Princ Pract 2014; 23: 18-23.
- Yilmaz N. Relationship between paraoxonase and homocysteine: crossroads of oxidative diseases. Arch Med Sci 2012; 8: 138-153.
- Ellidag HY, Aydin O, Eren E, Yilmaz N, Ergin M. Decreased HDL-dependent paraoxonase and arylesterase enzyme activity may indicate a worse prognosis in multiple myeloma. Asian Pac J Cancer Prev 2014; 15: 9847-9851.
- Gluba A, Pietrucha T, Banach M, Piotrowski G, Rysz J. The role of polymorphisms within paraoxonases (192 Gln/Arg in PON1 and 311Ser/Cys in PON2) in the modulation of cardiovascular risk: a pilot study. Angiology 2010; 61: 157-165.
- Li P, Bu SH, Lu XT, Li LX, Xu AJ. Relationships between PON1 Q192R polymorphism and clinical outcome of antiplatelet treatment after percutaneous coronary intervention: a meta-analysis. Mol Biol Rep 2014; 41: 6263-6273.
- Humbert R, Adler DA, Disteche CM, Hassett C, Omiecinski CJ, Furlong CE. The molecular basis of thehuman serum paraoxonase activity polymorphism. Nat Genet 1993; 3: 73-76.
- Eckerson HW, Wyte CM, La Du BN. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet 1983; 35: 1126-1138.
- Haagen L, Brock A. A new automated method for phenotyping arylesterase (E.C.3.1.1.2.) based upon inhibition of enzymatic hydrolysis of 4-nitrophenyl acetate. Eur J Clin Chem Clin Biochem 1992; 30: 391-395.
- Ellidag HY, Eren E, Aydin O, Neselioglu S, Yilmaz N. The phenotype distribution of paraoxonase-1 in patients with multiple myeloma, bladder, and colorectal cancer. J Med Biochem 2014; 33: 252-258.
- Karakaya A, Ibis S, Kural T, Kose SK, Karakaya AE. Serum paraoxonase activity and phenotype distribution in Turkish subjects with coronary heartdisease and its relationship to serum lipids and lipoproteins. Chem Biol Interact 1999; 118: 193-200.
- Aynacioglu AS, Cascorbi I, Mrozikiewicz PM, Nacak M, Tapanyigit EE. Paraoxonase 1 mutations in a Turkish population. Toxicol Appl Pharmacol 1999; 157: 174-177.
- Wang M, Lang X, Zou L, Huang S, Xu Z. Four genetic polymorphisms of paraoxonase gene and risk of coronary heart disease: a meta-analysis based on 88 case-control studies. Atherosclerosis 2011; 214: 377-385.
- Smith GC, Crossley JA, Aitken DA, Pell JP, Cameron AD. First-trimester placentation and the risk of antepartum stillbirth. JAMA 2004; 292: 2249-2254.
- Smith GC, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, Connor JM. Early pregnancy levels of pregnancy associated plasma protein A and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab 2002; 87: 1762-1767.
- Lawrence JB, Oxvig C, Overgaard MT, Sottrup JL, Gleich GJ. The insulin-like growth factor (IGF) dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy associated plasma protein-A. Proc Natl Acad Sci USA 1999; 96: 3149-3153.
- Giudice LC, Conover CA, Bale L, Faessen GH, Ilg K, Sun I. Identification and regulation of the IGFBP-4 protease and its physiological inhibitor in human trophoblasts and endometrial stroma: evidence for paracrine regulation of IGF II bioavailability in the placental bed during human implantation. J Clin Endocrinol Metab 2002; 87: 2359-2366.
- Irwin JC, Suen LF, Martina NA, Mark SP, Giudice LC. Role of the IGF system in trophoblast invasion and pre-eclampsia. Hum Reprod 1999; 14: 90-96.
- Dugoff L, Hobbins JC, Malone FD, Porter TF, Luthy D. First trimester maternal serum PAPP-A and free beta-HCG concentrations and nuchal tramslucency are associated with obstetric complications: a population based screening study (the FASTER trial). Am J Obstet Gynecol 2004; 191: 1446-1451.
- Krantz D, Goetz L, Ssmpson JL, Thom E, Zachary J. Association of extreme first trimester free human chorionic gonadotropin beta, PAPP-A, and nuchal translucency with intrauterin growth restriction and other adverse pregnancy outcomes. Am J Obstet Gynecol 2004; 191: 1446-1451.
- Ong CY, Liao AW, Spencer K, Munim S, Nicolaides KH. First trimester maternal serum free beta human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. BJOG 2000; 107: 1265-1270.
- Canini S, Prefumo F, Pastorino D, Crocetti L, Afflito CG. Association between birth weight and first trimester free beta-human chorionic gonadotropin and pregnancy associated plasma protein-A. Fertil Steril 2008; 89: 174-178.
- Bischof P, Bruce D, Cunningham P, Klopper A. Measurement of pregnancy associated plasma protein A (PAPP-A). Clin Chim Acta 1979; 95: 243-247.
- Gelisgen R, Genc H, Kayali R, Oncul M, Benian A. Protein oxidation markers in women with and without gestational diabetes mellitus: a possible relation with paraoxonase activity. Diabetes Res Clin Pract 2011; 94: 404-409.
- Camuzcuoglu H, Toy H, Cakir H, Celik H, Erel O. Decreased paraoxonase and arylesterase activities in the pathogenesis of future atherosclerotic heart disease in women with gestational diabetes mellitus. J Womens Health (Larchmt) 2009; 18: 1435-1439.
- Al-Hakeem MM, Abotalib Z, Alharbi KK, Khan IA. Relationship between the paraoxonase 1 gene glutamine 192 to arginine polymorphism and gestational diabetes mellitus in Saudi women. Clin Biochem 2014; 47: 122-125.
- Yaghmaei M, Hashemi M, Azarian A, Moazeni-Roodi A, Mokhtari M. Association of L55M and Q192R polymorphisms of paraoxonase-1 gene with preeclampsia. Arch Med Res 2011; 42: 324-328.
- Baker AM, Klein RL, Haeri S, Moss KL, Boggess KA. Association of midgestational paraoxonase 1 activity with pregnancies complicated by preeclampsia. Am J Perinatol 2010; 27: 205-210.
- Kumru S, Aydin S, Gursu MF, Ozcan Z. Changes of serum paraoxonase (an HDL-cholesterol-associated lipophilic antioxidant) and arylesterase activities in severe preeclamptic women. Eur J Obstet Gynecol Reprod Biol 2004; 114: 177-181.
- Sarandol E, Dirican M, Uncu G. Oxidizability of apolipoprotein B-containing lipoproteins and serum paraoxonase/arylesterase activities in preeclampsia. Clin Biochem 2004; 37: 990-996.
- Kim YJ, Park H, Lee HY, Ahn YM, Ha EH. Paraoxonase gene polymorphism, serum lipid, and oxidized low-density lipoprotein in preeclampsia. Eur J Obstet Gynecol Reprod Biol 2007; 133: 47-52.