ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 16
Effects of siRNA interference of CDKL1 expression on proliferation and apoptosis of gastric cancer cells
Xiaohong Pan1, Yang Yan2*, Qiken Li3, Jiefeng Xu4, Yunfeng Zou5, Jianbo Zhou1, Xiaofeng Huang1, Guofeng Chen6 and Zilong Li6
1Department of Gastroenterology, Yuyao People’s Hospital, Medical School of Ningbo University, Yuyao, PR China
2Department of Radiology, Yuyao People’s Hospital, Medical School of Ningbo University, Yuyao, PR China
3Department of Gastrointestinal Tumor Surgery, Zhejiang Cancer Hospital, Hangzhou, China
4Department of Medical Biological Experimental Center, Yuyao People’s Hospital, Medical School of Ningbo University, Yuyao, PR China
5Department of Pathology, Yuyao People’s Hospital, Medical School of Ningbo University, Yuyao, PR China
6Department of Emergency Medicine, Yuyao People’s Hospital, Medical School of Ningbo University, Yuyao, PR China
- *Corresponding Author:
- Yang Yan
Department of Radiology
Yuyao People’s Hospital
Medical School of Ningbo University
Yuyao, PR China
Accepted on August 03, 2017
Objective: Cyclin Dependent Kinases (CDKs) play an important role in mediating cell cycle progression, mitosis and proliferation. Cyclin Dependent Kinase Like 1 (CDKL1) has high levels of homology with CDKs and has been shown to be involved in the regulation of cell cycle mediation, although little is known about the exact mechanism. This study used RNA interference (RNAi) approach to downregulate CDKL1 expression in gastric cancer cells, to investigate its role in mediating gastric cancer cell cycle, proliferation, apoptosis as well as its correlation with pathogenesis.
Patients and Methods: Tumor tissues were collected from gastric cancer patients to compare CDKL1 expression across different pathological grades. Its expression was also compared among high differentiated gastric cancer cell line MKN-28 cell, moderate differentiated cell line SGC-7901 cell, low differentiated cell line MGC-803 cell and normal gastric mucosal epithelial cell line GES-1 cell. Cultured MGC-803 cells were transfected with si-CDKL1 to measure cell apoptosis and cycle by flow cytometry and cell proliferation by EdU staining.
Results: Gastric cancer tissues had higher CDKL1 expression than normal mucosal tissues, with higher expression in patients with advanced grades. Gastric cancer cell lines had higher CDKL1 expression than normal cells, with higher expression in those low differentiated cells. siRNA interference downregulated CDKL1 expression in MGC-803 cells, elevated cell apoptosis, induced G0/G1 phase arrest and reduced proliferation potency.
Conclusions: CDKL1 up-regulation is correlated with gastric cancer pathogenesis. CDKL1 downregulation induced gastric cell cycle arresting, inhibited cell proliferation and facilitated cell apoptosis.
Keywords
Cyclin dependent kinase like 1 (CDKL1), Gastric cancer, Cell cycle, Cell proliferation, Cell apoptosis.
Introduction
Gastric Cancer (GC) is a common malignant tumor in digestive tract with high incidence plus mortality rate among all cancers all over the world [1,2]. GC has insidious disease onset with asymptotic features at early stage [3,4]. However, rapid progression afterwards to terminal stage has several features including high malignancy, frequent invasion or distal metastasis, and lower sensitivity for chemo/radio-therapy, making treatment inefficacy and patient’s survival or prognosis unfavourable [5,6]. GC occurrence and progression is a complicated process involving multiple steps and factors for regulation [7]. Cell proliferation and apoptosis are two aspects during tumor cell biological process. When cell proliferation was abnormally elevated whilst apoptosis was decreased, cells will present dysregulated growth which is induced by gene abnormality, leading to development of tumor pathogenesis [8-10]. Normal cell proliferation and growth velocity are dependent on the precise regulation of cell cycle. During tumor pathogenesis, most oncogenes and tumor suppressor genes do not solely regulate cell proliferation or growth, but affect tumor pathogenesis via regulating cell cycle progression [11-13].
Cell cycle is under precise regulation of three factors, including Cyclin Dependent Kinases (CDKs), cyclins and Cyclin Dependent Kinase Inhibitors (CDKIs), among which CDKs play critical regulatory functions [14,15]. CDKs were firstly discovered in yeast species in 1970’s, and were then identified in humans [16]. Human CDKs family consists of seven members (CDK1 to CDK7), all of which belong to serine/ threonine protein kinase with highly conservative structures. CDKs can regulate G1/S phase transition of cell cycle, facilitate S phase entry, and modulate G2 to M phase transition [17]. Cyclin dependent kinase like 1 (CDKL1) is a member of cell division control protein 2 (CDC2) related serine/threonine protein kinase family, and shares high structural similarity with CDKs protein, and may play important roles in cell cycle regulation [18]. CDKL1 was firstly discovered in 1995, but with few studied on its functional role in tumor pathogenesis [19]. This study used RNA interference (RNAi) approach to reduce CDKL1 expression in GC cells, in order to investigate its role in GC cell proliferation and apoptosis regulation.
Materials and Methods
Major reagent and materials
Human highly differentiated GC cell line MKN-28, moderately differentiated GC cell line SGC-7901, lower differentiated GC cell line MGC-803, and normal human gastric mucosal epithelial cell line GES-1 were purchased from Xinyu Biotech (China). RPMI 1640, Opti-MEM culture medium, and FBS were purchased from Gibco (US). Transfection reagent Lipofectamine 2000 was purchased from Invitrogen (US). RNA extraction kit Rneasy MiNi Kit, fluorescent quantification reagent QuantiTect SYBR Green RT-PCR Kit was purchased from Qiagen (Germany). PCR primer was provided by GE Pharmacon (US). Si-CDKL1 and si-NC were synthesized by Gimma Pharmacology (China). Rabbit antihuman CDKL1 was purchased from Abcam (US). HRP conjugated secondary antibody was purchased from Jackson Immuno Research (US). Annexin V-FITC/PI apoptosis test kit, BCA protein quantification kit, Caspase-3 activity assay kit, and PI dye were purchased from Beyotime (China). EdU Flow Cytometry Kit and RNAs were purchased from Sigma (US). Triton X-100 was purchased from Suobao Biotech (China).
Clinical information
A total of 58 GC patients who received treatment in Yuyao People’s Hospital, Medical School of Ningbo University from July 2016 to December 2016 were recruited for collecting GC tissue samples during surgery and for confirmed diagnosis by histopathology examination. There were 19, 21 and 18 cases at stage I, stage II and stage III, respectively. Another 20 normal gastric mucosal tissue samples were recruited as the control group.
This study was approved by ethics committee of Yuyao People’s Hospital, Medical School of Ningbo University and all the enrolled objects had signed informed consents.
Cell culture
MKN-28, SGC-7901, MGC-803 and GES-1 cells were all cultured in RPMI 1640 medium containing 10% FBS and 1% penicillin-streptomycin, and kept in a 37°C incubator with 5% CO2. Cells at log-growth phase with satisfactory status were used for further experiments.
Cell transfection
MGC-803 cells were inoculated into 6-well plate. Original culture medium was removed when reaching 60%-70% confluence for changing Opti-MEM medium. 5 μL Lipofectamine 2000, 5 μL si-NC, 5 μL si-CDKL1 were separately dissolved into 50 μL Opti-MEM medium for 5 min room temperature incubation. Lipofectamine 2000 was gently mixed with si-NC or si-CDKL1 for 20 min room temperature incubation, and was then added into 6-well plate. After continuous incubation for 6 h, Opti-MEM medium was removed for changing fresh RPMI1640 medium containing 10% FBS. After 72 h incubation, cells were collected for further assays.
qRT-PCR for measuring gene expression
Rneasy MiNi Kit was used to extract RNA following manual instructions. QuantiTect SYBR Green RT-PCR kit was used to test gene expression. Primer sequences were: CDKL1PF: 5’- CGAAT GCTCA AGCAA CTCAA GC-3’; CDKL1PR: 5’- GCCAA GTTAT GCTCT TCACG AG-3’; β-actin PF: 5’- GAACC CTAAG GCCAA C-3’; β-actin PR: 5’-TGTCA CGCAC GATTT CC-3’. In a 20 μL qRT-PCR reaction system, the followings were added: 10.0 μL 2 X QuantiTect SYBR Green RT-PCR Master Mix, 1.0 μL forward and reverse primer (at 0.5 μm/L), 1.0 μg RNA template, 0.5 μL QuantiTect RT Mix, and ddH2O. Reverse transcription conditions were: 50°C for 30 min. PCR conditions were: 95°C pre-denature for 5 min, followed by 40 cycles each containing 94°C for 15 s denature, 60°C for 30 s annealing, and 72°C for 30 s elongation. Gene expression was measured on ABI ViiATM7 real time fluorescent quantitative PCR cycler. The qPCR results were calculated using the 2-ΔΔCT method and normalized relative to internal control.
Western blot
500 μL RIPA lysis buffer was mixed with 20 mg tissues or 5 × 106 cells. After mixture, cells or tissue were lysed on ice for 15 min. Protein concentration and quality were detected in the supernatant, and then were separated in 10% SDS-PAGE. After transferring into PVDF membrane, CDKL1 (1:200 dilution) or β-actin (1:500) primary antibody was added and incubated at 4°C overnight. The membrane was washed in PBST for 3 times (5 min each). HRP conjugated secondary antibody (1:5000) was added for 60 min room temperature incubation. After PBST washing for 3 times (5 min each), ECL chromogenic substrate was added for detecting protein expression.
Flow cytometry analysis of cell apoptosis
Following manual instruction of Annexin V-FITC/PI apoptosis test kit, cells from all treatment groups were collected and digested. After digestion, cells were rinsed in PBS, and were re-suspended in 100 μL binding buffer. 5 μL Annexin V-FITC was firstly added and incubated at 4°C under dark for 15 min, followed by 5 min dark incubation in 5 μL PI. At the end time point, Beckman-Coulter EPICS XL-MCL flow cytometry was used to test cell apoptosis.
Spectrometry for testing Caspase-3 activity
Following manual instruction of Casapse-3 activity assay kit, pNA standard samples were prepared to plot the standard curve. Cells were then digested in trypsin and subsequently lysed on ice followed by collection of supernatant by centrifugation. The supernatant was then transferred to newly pre-cold tubes. Test buffer was added into 96-well plate along with the samples Ac-DEVD-pNA. The mixture was incubated at 37°C for 2 h. A 405 absorbance value was measured to evaluate the Caspase-3 activity in test samples.
Flow cytometry analysis of cell proliferation
EdU Flow Cytometry kit was used to test cell proliferation. In brief, cells were re-suspended in complete medium and mixed with EdU solution (final concentration 10 μM). With 37°C incubation for 2 h, cells were inoculated into 6-well plate for 48 h culture to collect cells by trypsin. Cells after digestion was washed in PBS containing 1% BSA, and fixed in 100 μL 4% paraformaldehyde for 15 min at room temperature. The membrane was then washed in PBS containing BSA. 100 μL permeabilization buffer containing saponin was added, followed by addition of test buffer containing PBS, catalyst solution, cyanine 5 dye azide and buffer and incubation under dark for 30 min at room temperature. 3 mL quenching buffer containing saponin was used to wash cells, which were resuspended in 500 μL wash reagent II. Cell proliferation was measured on a Beckman-Coulter EPICS XL-MCL flow cytometry.
Flow cytometry measurement of cell cycle
Cells were digested in trypsin and fixed in 70% ethanol. PBS containing 25 μg/mL PI, 25 μg/mL RNase A, and 0.1% Triton X-100 were added for 30 min reaction under dark at 4°C. Cell cycle was measured on a Beckman-Coulter EPICS XL-MCL flow cytometry.
MTT analysis of cell proliferation
After transfection with si-NC, si-CDKL1, MGC-083 cell was seeded into 96-well plate and culture for 24 h, 48 h and 72 h followed by addition of 10 μL MTT reagents. After reaction for 4 h, supernatant was discard and 150 μL DMSO was added followed by measuring the absorption value at the wavelength of 450 nm.
Statistical analysis
SPSS 18.0 software was used for data statistics and analysis. Measurement data were presented as mean ± standard deviation (SD). Comparison of measurement data between groups was performed by student t-test. A statistical significance was defined when p<0.05.
Results
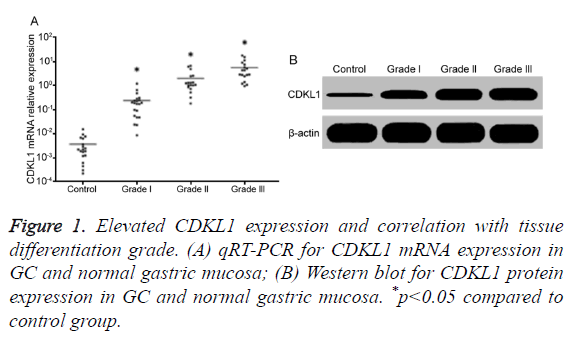
Elevated CDKL1 expression and correlation with tissue differentiation grade
qRT-PCR results showed that, compared with normal gastric mucosa, GC tissues had significantly higher CDKL1 mRNA expression, with highest level in lower differentiated grade III GC tissues, followed by moderately differentiated grade II, and highly differentiated grade I (Figure 1A). Consistently, Western blot results also showed that GC patient tumor tissues had significantly higher CDKL1 protein expression than normal gastric mucosa tissues, with even higher expression in those with advanced pathology grade (Figure 1B).
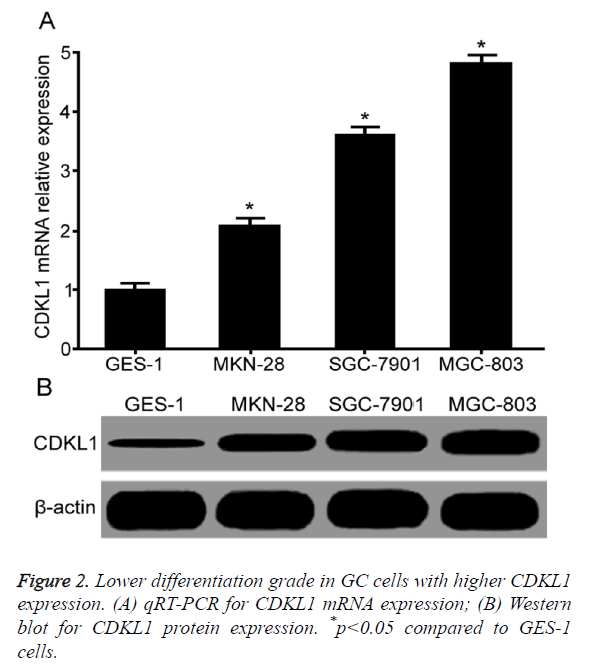
Lower differentiation grade correlated with higher CDKL1 expression
qRT-PCR results showed that compared with normal gastric mucosal epithelial cell GES-1, GC cell lines MKN-28, SGC-7901 and MGC-803 had significantly elevated CDKL1 mRNA expression level. With lower differentiation grade, CDKL1 mRNA expression was lower (Figure 2A). Western blot results showed significantly higher CDKL1 protein expression in GC cells MKN-28, SGC-7901 and MGC-803 than GES-1 cells, with highest expression level in MGC-803, followed by SGC-7901 and MKN-28 (Figure 2B).
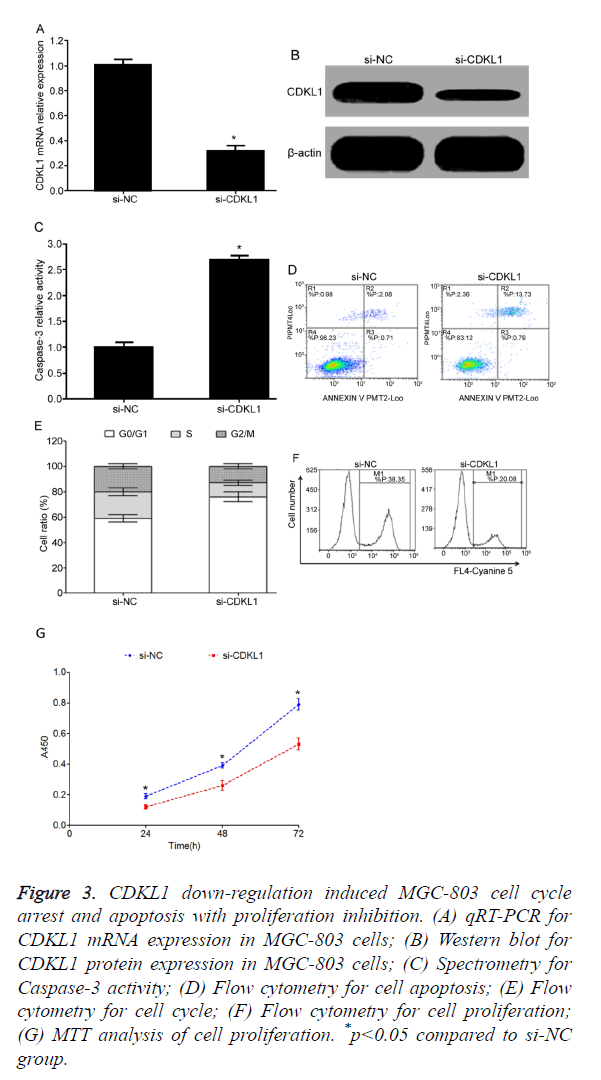
CDKL1 down-regulation induced MGC-803 cell cycle arrest and apoptosis with inhibition of cell proliferation
Test results showed that, compared with si-NC transfection group, si-CDKL1 transfection group had significantly decreased CDKL1 mRNA (Figure 3A) or protein (Figure 3B) expression level in MGC-803 cells, indicating high interference efficiency that can meet the requirement of further studies. Transfection of si-CDKL1 significantly up-regulated Caspase-3 activity in MGC-803 cells (Figure 3C), and enhanced cell apoptosis (Figure 3D). Flow cytometry results showed that after siRNA interference on CDKL1 expression, MGC-803 cells had significantly elevated G0/G1 phase ratio, and lower S phase or G2/M phase ratio (Figure 3E), plus reduced cell proliferation potency (Figure 3F). Consistently, MTT assay also demonstrated reduced proliferation of MGC-803 cell after transfection of si-CDKL1 (Figure 3G).
Figure 3. CDKL1 down-regulation induced MGC-803 cell cycle arrest and apoptosis with proliferation inhibition. (A) qRT-PCR for CDKL1 mRNA expression in MGC-803 cells; (B) Western blot for CDKL1 protein expression in MGC-803 cells; (C) Spectrometry for Caspase-3 activity; (D) Flow cytometry for cell apoptosis; (E) Flow cytometry for cell cycle; (F) Flow cytometry for cell proliferation; (G) MTT analysis of cell proliferation. *p<0.05 compared to si-NC group.
Discussion
Gastric Cancer (GC) is a common malignant tumor in digestive tract, and ranked as the third popular and second deadly cancer of the whole body [1]. China is an epidemic region of GC, with about 40%~50% GC cases occurring in China [20]. For most GC patients, surgical resection is still the major treatment approach. However, due to insidious onset and asymptomatic early phase, most GC patients do not show symptoms at early stage and are already at terminal stage at the time of diagnosis, thus making high frequency of distal metastasis and loss of optimal window for surgery, plus compromising sensitivity for chemo or radiotherapy, leading to inefficiency of treatment and worse prognosis [21]. Although combined therapy of surgical resection, chemo/radio-therapy, gene and immune treatment has made major progress and can extent patient’s lifespan to certain extents, unfavourable prognosis still exists as the post-op 5-year survival rate is only 15~20% [22].
Dysregulated growth of tumor cells is an important biological process and feature during tumor onset. Cell apoptosis and cell cycle/proliferation are two important aspects in cell growth features. Velocity of these two functions largely determines cell growth speed. When apoptosis is suppressed and cell cycle or proliferation is accelerated, cell growth speed was abnormally accelerated, leading to tumorigenesis. Regular mediation of cell cycle has importance in maintaining normal division, proliferation, differentiation, aging and internal homeostasis of cells. When cell cycle regulation showed abnormality, malignant proliferation is accompanied, with disruption of gene or chromosome inheritance stability for clearing and repairing blockades, resulting in facilitation of tumor formation.
CDKs are a family of cell cycle phase regulating kinase, and can bind to cells at different phases to regulate cyclin activity, thus mediating cell cycle [23,24]. CDKL1, also named as KKIALRE, is encoded by gene located at 14q22.1 and belongs to CDC-2 related serine/threonine protein kinase family [18]. CDKL1 has a high homology with CDKs family, with 43% homology with CDK1 and 40% homology with CDK2, sharing similar functional domain of CDKs [25]. Previous study showed that CDKL1 could interact with cyclin, and mediates cell cycle [18]. Some studies showed that CDKL1 was widely expressed in human liver, kidney, ovary and brain [19,26]. In breast cancer tissues, CDKL1 was significantly up-regulated, indicating potential tumor facilitating role of CDKL1 [27]. Currently little is known regarding CDKL1 protein, nor does its role in tumor pathogenesis. This study aimed to use RNA interference (RNAi) approach to manipulate CDKL1 expression in gastric cancer cells, to investigate the role of CDKL1 in mediating GC cell cycle, proliferation and apoptosis, as well as its correlation with GC pathogenesis.
Results of this study showed that, compared with normal gastric mucosa, GC tissues had significantly elevated CDKL1 expression, with higher level in tumor tissues with more advanced histology grade. As tumor pathology grade can reflect cell differentiation grade and proliferation potency, with higher pathology grade indicating lower differentiation grade and stronger proliferation ability, our results thus demonstrated possible involvement of CDKL1 up-regulation in facilitating GC occurrence. In addition, correlation of CKDL1 expression with cell proliferation enhancement was also observed, indicating the role of CDKL1 up-regulation in facilitating GC onset. Tang et al. showed significantly elevated CDKL1 expression in breast cancer tissues, indicating tumor facilitating role by CDKL1 up-regulation [27]. In this study, GC tissues had significantly elevated CDKL1 expression, which was consistent with a previous study conducted by Tang et al. [27]. Compared with normal gastric mucosal epithelial cell line GES-1, GC cells had significantly elevated CDKL1 expression, whose level was higher in those cells with lower differentiation grade, suggesting that abnormally elevated CDKL1 was an important factor facilitating GC occurrence and potentiating its malignant biological properties. Therefore, this study used MGC-803 cell line, which has the most advanced malignancy, as the research subject, to further investigate the role of CDKL1 in regulating GC cell cycle, proliferation and apoptosis. Our results showed that siRNA interference significantly down-regulated CDKL1 expression in MGC-803 cells, and potentiated intracellular Caspase-3 activity to enhance cell apoptosis, as well as induced of G0/G1 phase arrest to reduce GC proliferation ability. Tang et al. also showed that after using shRNA to suppress CDKL1 expression, breast cancer MCF-7 cells had significantly depressed proliferation potency, plus enhanced sensitivity against chemotherapy drugs including epirubicin, 5- fluorouracil and docetaxel [27]. Song et al. showed that siRNA down-regulation of CDKL1 expression significantly suppressed proliferation of melanoma cell line A375 or MV3, and reduced their clonal formation ability to induce G1 phase arrest for facilitating cell apoptosis [25]. In this study, downregulation of CDKL1 significantly inhibited malignant biological properties of GC cell line MGC-803, which was consistent with previous studies performed by Tang et al. [25,27]. However, the limitation of this study mainly was the lack of mechanistic study on the role of CDKL1 in mediating GC cell cycle, proliferation and apoptosis, which should be addressed in further studies.
Conclusion
CDKL1 up-regulation is correlated with GC occurrence. Down-regulation of CDKL1 could induce cycle arrest of GC, inhibit cell proliferation and facilitate cell apoptosis.
References
- Sano T. Gastric cancer: Asia and the world. Gastric Cancer 2017.
- Waldum HL, Sagatun L, Mjones P. Gastrin and gastric cancer. Front Endocrinol (Lausanne) 2017; 8: 1.
- Hudler P. Outlook on epigenetic therapeutic approaches for treatment of gastric cancer. Curr Cancer Drug Targets 2017.
- Ye W, Li Y, Fan L, Zhao Q, Yuan H, Tan B, Zhang Z. Effect of annexin A7 suppression on the apoptosis of gastric cancer cells. Mol Cell Biochem 2017.
- Apicella M, Corso S, Giordano S. Targeted therapies for gastric cancer: failures and hopes from clinical trials. Oncotarget 2017.
- Liang TJ, Liu SI, Chen YC, Chang PM, Huang WC, Chang HT, Chen IS. Analysis of gallstone disease after gastric cancer surgery. Gastric Cancer 2017.
- Patel TN, Roy S, Ravi R. Gastric cancer and related epigenetic alterations. Cancer Med Sci 2017; 11: 714.
- Qin Y, Zhao L, Wang X, Tong D, Hoover C, Wu F, Liu Y, Wang L, Liu L, Ni L, Song T, Huang C. MeCP2 regulated glycogenes contribute to proliferation and apoptosis of gastric cancer cells. Glycobiol 2017.
- Wang HS, Nie X, Wu RB, Yuan HW, Ma YH, Liu XL, Zhang JY, Deng XL, Na Q, Jin HY, Bian YC, Gao YM, Wang YD, Chen WD. Downregulation of human Wnt3 in gastric cancer suppresses cell proliferation and induces apoptosis. Onco Targets Ther 2016; 9: 3849-3860.
- Hou X, Qiao H. Effect of miR-340 on gastric cancer cell proliferation and apoptosis. Int J Clin Exp Pathol 2015; 8: 13108-13113.
- Liu YF, Qu GQ, Lu YM, Kong WM, Liu Y, Chen WX, Liao XH. Silencing of MAP4K4 by short hairpin RNA suppresses proliferation, induces G1 cell cycle arrest and induces apoptosis in gastric cancer cells. Mol Med Rep 2016; 13: 41-48.
- Feng J, Chen X, Wang Y, Du Y, Sun Q, Zang W, Zhao G. Myricetin inhibits proliferation and induces apoptosis and cell cycle arrest in gastric cancer cells. Mol Cell Biochem 2015; 408: 163-170.
- Wang X, Yu Q, Zhang Y, Ling Z, Yu P. Tectonic 1 accelerates gastric cancer cell proliferation and cell cycle progression in vitro. Mol Med Rep 2015; 12: 5897-5902.
- Hochegger H, Takeda S, Hunt T. Cyclin-dependent kinases and cell-cycle transitions: does one fit all? Nat Rev Mol Cell Biol 2008; 9: 910-916.
- Wesierska-Gadek J, Krystof V. Selective cyclin-dependent kinase inhibitors discriminating between cell cycle and transcriptional kinases: future reality or utopia? Ann N Y Acad Sci 2009; 1171: 228-241.
- Whittaker SR, Mallinger A, Workman P, Clarke PA. Inhibitors of cyclin-dependent kinases as cancer therapeutics. Pharmacol Ther 2017.
- Barbacid M, Ortega S, Sotillo R, Odajima J, Martín A, Santamaría D, Dubus P, Malumbres M. Cell Cycle and Cancer: Genetic analysis of the role of cyclin-dependent kinases. Cold Spring Harbor Symposia Quant Biol 2005; 70: 233-240.
- Meyerson M, Enders GH, Wu CL, Su LK, Gorka C, Nelson C, Harlow E, Tsai LH. A family of human CDC2-related protein kinases. EMBO J 1992; 11: 2909-17.
- Yen SH, Kenessey A, Lee SC, Dickson DW. The distribution and biochemical properties of a Cdc2-related kinase, KKIALRE, in normal and Alzheimer brains. J Neurochem 1995; 65: 2577-2584.
- Lan H, Zhu N, Lan Y, Jin K, Teng L. Laparoscopic gastrectomy for gastric cancer in China: an overview. Hepatogastroenterol 2015; 62: 234-239.
- Zong L, Abe M, Seto Y, Ji J. The challenge of screening for early gastric cancer in China. Lancet 2016; 388: 2606.
- Liu KH, Hung CY, Lu CH, Hsu JT, Yeh TS, Lin YC, Hung YS, Chou WC. Survival outcomes of geriatric patients with clinically resectable gastric cancer: to operate or not. J Surg Res 2016; 206: 481-489.
- Hydbring P, Malumbres M, Sicinski P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat Rev Mol Cell Biol 2016; 17: 280-92.
- Santo L, Siu KT, Raje N. Targeting cyclin-dependent kinases and cell cycle progression in human cancers. Semin Oncol 2015; 42: 788-800.
- Song Z, Lin J, Sun Z, Ni J, Sha Y. RNAi-mediated down-regulation of CDKL1 inhibits growth and colony-formation ability, promotes apoptosis of human melanoma cells. J Dermatol Sci 2015; 79: 57-63.
- Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 2009; 28: 2925-2939.
- Tang L, Gao Y, Yan F, Tang J. Evaluation of cyclin-dependent kinase-like 1 expression in breast cancer tissues and its regulation in cancer cell growth. Cancer Biother Radiopharm 2012; 27: 392-398.