ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
- Biomedical Research (2011) Volume 22, Issue 2
Effects of Smilax myosotiflora on testicular 11?-hydroxysteroid dehydro-genase oxidative activity and plasma hormone levels in rats
Damayanthi D1*, Azman MAB2, Aminuddin AHK3, Hamid A4 and Nwe KHH4
1Faculty of Medicine, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia
2Department of Biomedical Sciences, Faculty of Allied Health Sciences, Universiti Kebangsaan Malaysia, 50300 Kuala Lumpur, Malaysia
3Department of Physiology, Faculty of Medicine, Universiti Kebangsaan Malaysia, 50300 Kuala Lumpur, Malaysia
4Department of Human Anatomy, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia
- *Corresponding Author:
- Damayanthi Durairajanayagam
Faculty of Medicine
Universiti Teknologi MARA
40450 Shah Alam, Selangor
Malaysia.
Accepted date: December 11 2010
Smilax myosotiflora, a popular local aphrodisiac is known to increase sexual libido. How-ever, the aphrodisiac efficacy of S. myosotiflora has not yet been scientifically established. Present investigation describes the effects of S. myosotiflora on testicular 11β-hydroxysteroid dehydrogenase (11β-HSD) oxidative activity and the levels of plasma corticosterone and tes-tosterone. Male Wistar rats (200-250g) were given either 8 mg/kg BW/day of S. myosotiflora or 120 μg/kg BW/day of Mifepristone (RU486, a glucocorticoid receptor (GR) antagonist) or both together (RU486+S. myosotiflora), for seven consecutive days. Results between groups were analyzed using analysis of variance (ANOVA) and Student’s t test. Differences were considered significant at P<0.05. Rats given S. myosotiflora showed no significant changes in 11β-HSD activity but had increased corticosterone (P<0.01) and decreased testosterone (P<0.01) levels compared to controls. Administration of RU486 alone decreased 11β-HSD activity (P<0.001), but increased testosterone levels (P<0.05) compared to controls. Con-versely, rats with RU486+S. myosotiflora showed increased 11β-HSD activity (P<0.001) but decreased testosterone levels (P<0.05) compared to RU486-treated rats. In the rats treated with RU486+S. myosotiflora, none of the parameters differed significantly from controls. Plasma corticosterone levels were found to be lowered in rats treated with RU486+S. my-osotiflora compared to S. myosotiflora-treated rats (P<0.05) towards control values. In this study, S. myosotiflora at a dose of 8 mg/kg BW/day was found to increase corticosterone with a corresponding decrease in testosterone levels. Previous studies show that RU486 acts through GR in affecting 11β-HSD activity and hormone levels. Since RU486+S. myosotiflora rats show opposite effects from RU486 rats, we therefore suggest that the actions of S. my-osotiflora on these parameters are possibly mediated through the GR. S. myosotiflora and RU486 probably competitively inhibit each other at the GR level.
Keywords
Smilax myosotiflora, Testicular 11β-HSD, Plasma corticosterone, Plasma testosterone
Introduction
Smilax myosotiflora is a slender herbaceous creeper from the family Smilacaceae. Found throughout the tropics and in the northern warm temperate regions, S. myosotiflora generally grows in the lowlands and foothills of Peninsu-lar Malaysia, Java (Indonesia) and Southern Thailand [1, 2]. It is known by several names locally, such as Ubi Jaga (most common), Ubi Besi, Itah Besi, Akar Ali, Akar Ding, Akar Tanding, Akar Restong, Akar Kerating, Keleh, Manto and Similax [1, 3, 4, 5].
In Malay traditional medicine, S. myosotiflora is used for several purposes. The leaves and fruit of the S. myosoti-flora are used for treating syphilis and rheumatism [5, 6, 7]. Externally, S. myosotiflora is used in the treatment of skin ailments including wounds, inflammations, boils and ulcers [8].
S. myosotiflora is popular as an aphrodisiac amongst the Malays and aborigines [5, 7, 9]. S. myosotiflora rhizomes are used as a sexual tonic to improve the male libido [5]. In fact, S. myosotiflora has even a greater reputation among the Malays as a sexual tonic than S. callophylla (Itah Tembaga) [4]. However, the aphrodisiac property of S. myosotiflora has not yet been scientifically established.
Traditionally, S. myosotiflora rhizomes are either chewed (with betel) or made into a decoction as a sex-tonic [6, 7]. Habitual betel quid chewing is said to give euphoria, short-term increase in heart rate and blood circulation as well as an overall feeling of well-being [5]. Traditional preparation of the sexual tonic involves either boiling the S. myosotiflora rhizome alone or together with Tongkat Ali roots and other herbs (such as horny goat weed etc.) to enhance the efficacy of the decoction [5].
Testicular 11β-hydroxysteroid dehydrogenase (11β-HSD) is believed to regulate intracellular glucocorticoid recep-tor concentration and prevent glucocorticoid-associated inhibition of luteinizing hormone (LH)-induced steroido-genesis [10]. Profound stressful conditions increase plasma corticosterone (B) levels, decrease testicular 11β-HSD oxidative activity and plasma testosterone levels in rats [11].
The present investigation describes the effects of S. my-osotiflora on testicular concentrations of 11β-HSD and the levels of plasma corticosterone and testosterone in rats. The possible mechanism of action of S. myosotiflora on the parameters studied is also explored.
Material and Methods
Plant Material
Smilax myosotiflora rhizomes were collected from Gua Musang, Kelantan, Malaysia and a voucher specimen was deposited in the herbarium at the Forest Research Institute of Malaysia (FRIM). The S. myosotiflora rhizomes were washed, cleaned, then air-dried under shade at room tem-perature, and finally the whole rhizome was ground to a fine powder. The S. myosotiflora rhizomes used in this investigation was supplied in a fine powder form by FRIM and stored in tinted glass bottles under refrigeration (40C) at our laboratory.
To make 8 mg/kg BW/day of S. myosotiflora, 160 mg of its rhizome powder was reconstituted at room temperature (just prior to treatment) in 10 ml of 0.9% saline. The mix-ture was then vortexed until well-mixed and sonicated at room temperature for an hour. The suspension of S. my-osotiflora in 0.9% saline was given (gavaged) to the ex-perimental rats.
Chemicals
Corticosterone (B), 11-dehydrocorticosterone (A), bovine serum albumin (BSA) and Mifepristone (RU486) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Bio Rad-protein assay standard II (1mg/ml) and dye reagent concentrate were purchased from Bio-Rad Laboratories (Hercules, California, USA).
Animals and Treatments
Adult male Wistar rats (200-250g BW) were used in this study. Animals were allowed free access to rat chow and drinking water and kept under controlled temperature (27-290C) and lighting (12:12 hours light-dark cycle) sched-ule.
The rats were randomly divided into 3 groups, A, B and C. Animals were treated daily for seven consecutive days. Rats in Group A were gavaged with 0.5 ml of 8 mg/kg body weight/day S. myosotiflora. Rats in Group B were given 0.1 ml of 120 μg/kg BW/day Mifepristone (RU486) as intramuscular (i.m.) injections. RU486 at a dosage of 120 μg/kg BW/day was prepared by mixing 3 mg of Mifepristone in 1 ml ethanol and 9 ml olive oil.
The animals in Group C received both 0.5 ml of S. my-osotiflora orally and 0.1ml of RU486 i.m. The control group for Group A was gavaged with 0.5 ml of 0.9% sa-line as the vehicle, while the control animals for Group B were given 0.1 ml of olive oil i.m. The controls in Group C received both 0.5 ml of 0.9% saline orally as well as 0.1 ml olive oil given i.m. Rats were sacrificed twenty four hours after the last dose of treatment.
Bioassay of 11β-Hydrosysteroid Dehydrogenase Oxida-tive Activity
At sacrifice, the testes were rapidly removed then ho-mogenized in Krebs-Ringer bicarbonate buffer solution containing glucose (Sigma Chemical Co., St. Louis, MO, USA) on ice. Subsequently, 250μl of tissue homogenate was incubated at 370C in a shaking water bath with 12nM (1,2,6,7-3H)-corticosterone (specific activity: 24 Ci/mmol) (Amersham Life Science, Buckinghamshire, England) as the substrate, in the presence of 200μM NADP (Sigma Chemical Co., St. Louis, MO, USA). The amount of protein incubated was such that it would pro-vide a 40-50% conversion of (3H)-corticosterone to (3H)11-dehydrocorticosterone in control rats during 10 minutes of incubation, which has been reported as 200μg/ml for the testis [12, 13]. The reaction was termi-nated and steroids extracted by adding ethyl acetate. The steroids were then separated chromatographically on thin layer plates (Merck, Darmstadt, Germany), following which the steroid bands were identified under ultraviolet light and radioactivity was measured with a Liquid Scin-tillation Counter Wallac 1409 (Wallac Oy, Turku, Finland) counting 3H-B activity. 11β-HSD oxidative ac-tivity was determined as the percentage conversion of (3H)-corticosterone to (3H)11-dehydrocorticosterone [14].
Hormone Assay
Under anaesthesia, blood samples were collected from the heart using heparinized syringes and centrifuged at 40C.
The plasma was removed and kept frozen at -200C until used. Plasma total testosterone and corticosterone levels were assayed by radioimmunoassay (RIA) using com-mercially-available kits (Diagnostic Products Corpora-tion, LA, California, USA). The intra- and inter assay variation coefficients for total testosterone were within 10%.
Statistical Analysis
Data were analyzed using the Statistical Package for So-cial Sciences (SPSS Inc., Chicago, Illanois, USA). 11β-HSD activity was expressed as mean ± standard error of mean (SEM) while plasma testosterone levels were stated as mean ± 95% confidence interval (CI). Plasma corticos-terone levels were stated as mean ± range. For enzyme activity and plasma testosterone levels, differences be-tween experimental and control groups were evaluated by analysis of variance (ANOVA) and Student’s t-test. Mann Whitney test was employed for plasma corticosterone levels. Differences were considered significant at P<0.05.
Ethical Matters
This research was approved by the Medical Research and Ethics Committee of the Faculty of Medicine, UKM.
Results
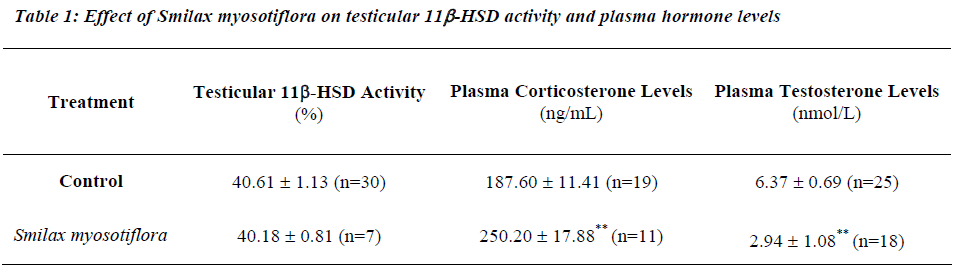
Effects of Smilax myosotiflora on testicular 11β-HSD activity and plasma hormone levels
Rats receiving 8mg/kg BW of S. myosotiflora for seven consecutive days did not show any significant changes in testicular 11β-HSD oxidative activity compared to con-trols (Table 1). However, in this group of rats, plasma corticosterone levels were increased (P<0.01) with a cor-responding decrease in plasma testosterone (P<0.01) as compared to controls (Table 1).
This increase in corticosterone with a corresponding de-crease in testosterone could possibly be mediated through the glucocorticoid receptors. RU486 (Mifepristone), an established anti-glucocorticoid [15,16], was therefore administered concurrently with S. myosotiflora to see whether RU486 could effectively block the testicular en-docrine profile caused by S. myosotiflora.
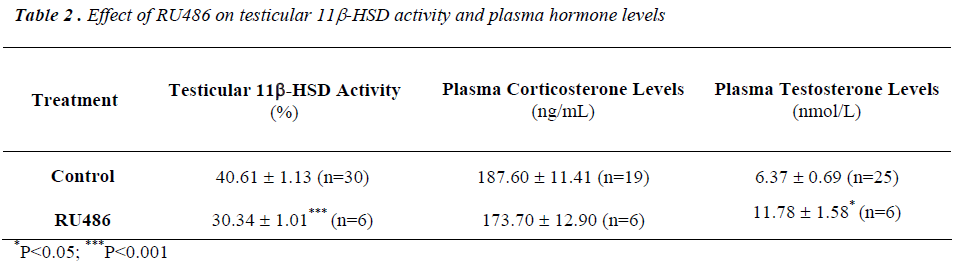
Effect of RU486 on testicular 11β-HSD activity and plasma hormone levels
Rats treated with RU486 alone had reduced testicular 11β-HSD oxidative activity (P<0.001) with an increase in plasma testosterone (P<0.05) compared to control (Table 2). However, no change in plasma corticosterone was de-tected (Table 2). The reduction in testicular 11β-HSD activity as observed in these animals could possibly be due to glucocorticoid receptor affinity of RU486.
Moreover, by blocking the effect of corticosterone at the receptor level, RU486 possibly augmented plasma testos-terone levels to almost double in concentration.
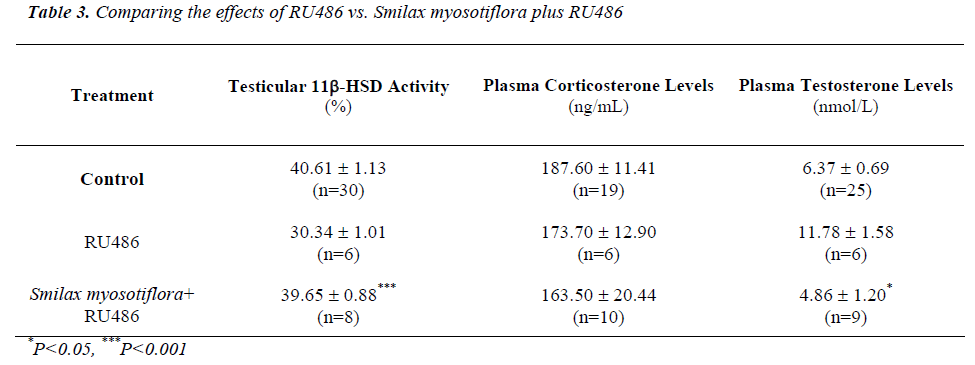
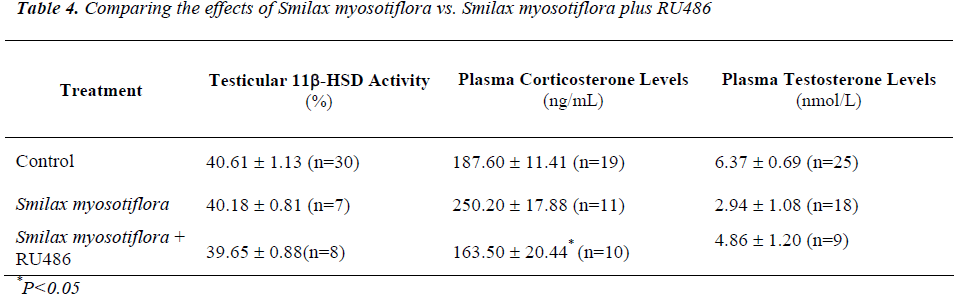
Effects of Smilax myosotiflora and RU486 on testicular 11β-HSD activity and plasma hormone levels
Testicular 11β-HSD oxidative activity in rats receiving S. myosotiflora concurrently with RU 486 was increased (P<0.001) compared to RU486 alone (Table 3), to that of control levels (Table 3). Thus, it seems that the effect of RU486 on 11β-HSD oxidative activity could be mini-mized by S. myosotiflora.
Animals receiving 8mg/kg BW of S. myosotiflora and RU486 had corticosterone levels that were lower (P<0.05) than that of S. myosotiflora alone (Table 4), and which did not significantly differ from control levels (Table 4). Thus, it seems that RU486 could reverse the effect of S. myosotiflora on corticosterone levels.
Similarly, plasma testosterone levels in rats receiving S. myosotiflora and RU486 were reduced (P<0.05) com-pared to that of rats given RU486 alone (Table 3) to con-trol values (Table 3). Again here it seems that S. myosoti-flora and RU486 cancelled out each other’s effect on tes-tosterone levels, leading it towards normal values.
Discussion
An aphrodisiac is a substance that is able to excite libido or aggravate sexual instinct [17]. Aphrodisiacs may exert its effect through three different modes of action i.e. by increasing libido or sexual desire, by increasing potency or effectiveness of erection and by increasing sexual pleasure [17]. Aphrodisiacs that enhance libido act at the level of the central nervous system by altering specific neurotransmitter or sex hormone concentrations [17]. Most of this type of aphrodisiac acts through an increase in testosterone concentration and is, therefore, male-specific [17].
Malaysian plants such as Smilax myosotiflora (Ubi Jaga) and Eurycoma longifolia Jack (Tongkat Ali) are used in Malay traditional medicine for its reputed aphrodisiac properties [18] that increase testosterone levels and give added vigor to sexual health and strength [5]. S. myosoti-flora rhizome is claimed to increase testosterone produc-tion in aging males, thereby improving spermatogenesis and sperm viscosity [5]. As such, it is traditionally believed that consumption of E. longifolia Jack and S. my-osotiflora in combination gives a more pronounced aph-rodisiac effect.
Studies show that E. longifolia Jack enhances the libido of sexually experienced male rats [19] and increases the sperm count and plasma testosterone levels in rats [20]. A potential phytoandrogen, i.e. a 4.3kDa bioactive peptide has been isolated from E. longifolia Jack and is reported to increase testosterone levels in rat [21]. A bioactive pep-tide similar to that of E. longifolia Jack has also been de-tected in the 50% ethanol extract of S. myosotiflora [17]. However, substantial scientific evidence documenting the purported aphrodisiac properties of S. myosotiflora re-mains lacking.
If S. myosotiflora does indeed cause an increase in sexual libido, then it is anticipated that S. myosotiflora consump-tion would lead to an increase in testosterone levels. However, in our present study, S. myosotiflora given in its crude form at a dose of 8 mg/kg BW/day for seven con-secutive days caused a decrease in plasma testosterone levels. Instead, we found that S. myosotiflora at this dose elevated the levels of corticosterone. Circulating gluco-corticoid levels rise sharply in response to stress, resulting in a significant drop in testosterone secretion, diminished libido and fertility [22]. Stress-induced depletion of plasma testosterone is due in part to a direct receptor-mediated effect of glucocorticoid in Leydig cells [23,24,25], which subsequently suppress testicular response to gonadotropins, LH and FSH [24].
On the other hand, the inhibitory effect of the elevated glucocorticoid on testosterone production could possibly be mediated by depressing testicular 11β-HSD oxidative activity. In the rat testis, 11β-HSD and glucocorticoid receptors are present in the rat Leydig cells [26]. Testos-terone production is maintained in the presence of normal plasma concentration of corticosterone, and is inhibited when 11β-HSD oxidative capacity is exceeded due to high levels of corticosterone [27]. Lowered 11β-HSD oxidative activity increases glucocorticoid-dependent in-hibition of testosterone production in Leydig cells [26]. However, in the rats given S. myosotiflora, testicular 11β-HSD oxidative activity remained unaffected. It may be possible that S. myosotiflora acts through a different ster-oidogenic enzyme other than 11β-HSD in affecting tes-tosterone levels. Alternately, by acting through glucocor-ticoid receptors, S. myosotiflora possibly exerts its effect on plasma levels of testosterone. To test this hypothesis, we administered S. myosotiflora together with the gluco-corticoid receptor blocker, RU486.
As reported previously, we have also recorded that RU486 treatment lowered 11β-HSD oxidative activity but increased testosterone levels in rats [28]. However, in the present study, RU486 prevented S. myosotiflora-induced corticosterone increase and thus the inhibition of plasma testosterone levels in rats receiving both S. myosotiflora and RU486. At the same time, S. myosotiflora prevented the RU486-induced reduction in 11β-HSD activity as well as the increase in testosterone levels. It seems that both S. myosotiflora and RU486 competitively inhibit each other at the receptor sites to exert its effect on the parameters tested. Thus, we suggest that S. myosotiflora possibly acts through the same receptor as RU486 i.e. the glucocorti-coid receptors. However, the sexual potency and fertility of the test animals are under investigation.
Conclusion
The local herb S. myosotiflora is well known for its aph-rodisiac effects. The herb acts like a stressor by causing elevated corticosterone levels in male rats. It seems likely that S. myosotiflora and RU486 competitively inhibit each other at the glucocorticoid receptor sites. Further research is needed to evaluate the aphrodisiac mechanism of S. myosotiflora.
Acknowledgements
The present research was supported by the IRPA Grant 06-02-02-0081. The authors would like to sincerely thank Professor Amar Chatterjee, Senior Professor and Consult-ant Physiologist of the Faculty of Medicine, Universiti Teknologi MARA (UiTM), for his critical review of this manuscript.
References
- Burkill IH. A dictionary of the economic products of the Malay Peninsula Vol II (I-Z). Oxford University Press, Great Britain. 1935; pp. 2036-2039.
- Zhari I, Norhayati I, Jaafar L. Malaysian Herbal Monograph, Vol I. Malaysian Monograph Committee, Kuala Lumpur. 1999; pp. 67-70.
- Gimlette JD. A dictionary of Malayan medicine. Ox-ford University Press, Kuala Lumpur. 1971; pp. 87.
- Gimlette JD, Thomson HW. A Dictionary of Malayan Medicine. Oxford University Press, Malaysia. 1983; pp. 87 & 122.
- Wan Hassan WE. Healing Herbs of Malaysia. Federal Land Development Agency (FELDA), Kuala Lumpur. 2006; pp. 120 & 125.
- Burkill IH, Mohamed H. Malay Village Medicine. The Gardens’ Bulletin, S. S., 1930; 6: 1532-1533.
- Gimlette JD & Burkill IH. The Malayan Book of Medi-cine. The Gardens’ Bulletin, SS, Vol 6, Singapore. April 1930; pp. 542.
- Hasnah Osman, Shaida Fariza Sulaiman. Sterols from the rhizomes of Malaysian Smilax myosotiflora A. DC.(abstract). Proc. Medicinal Herbs: Ensuring Safety and Efficacy through Research and Development (Interna-tional Conference on Traditional / Complementary Medicine), 13th–15th November, 2000. Legend Hotel, Kuala Lumpur. P20
- Burkill IH. A dictionary of the economic products of the Malaysia. Ministry of Agriculture, Kuala Lumpur. 1966.
- Monder C, Miroff Y, Marandici A, Hardy MP. 11ß-hydroxysteroids dehydrogenase alleviates glucocorti-coid-mediated inhibition of steroidogenesis in rat Ley-dig cells. Endocrinology 1994; 134: 1199–1204.
- Monder C, Hardy MP, Blanchard RJ, Blanchard DC. Comparative aspects of 11ß-hydroxysteroid dehydro-genase. Testicular 11ß-hydroxysteroid dehydrogenase: development of a model for the mediation of Leydig cell function by corticosteroids. Steroids 1994; 59: 69-73.
- Nwe KHH, Morat PB, Khalid BAK. Opposite effects of sex steroids on 11ß-hydroxysteroid dehydrogenase activity in the normal and adrenalectomized rat testis. Gen Pharmac 1997; 28(5): 661-664.
- Nwe KHH, Hamid A, Morat PB, Khalid BAK. Differ-ential regulation of the oxidative 11ß-hydroxysteroid dehydrogenase activity in testis and liver. Steroids 2000; 65: 40–45.
- Edwards CRW, Stewart PM, Burt D, Brett L, McIntyre MA, Sutanto WS, DeKloet ER, Monder C. Localisation of 11ß-hydroxysteroid dehydrogenase tissue specific protector of the mineralocorticoid receptor. Lancet 1988; ii: 986-989.
- Philibert D. RU 38486: An original multifaceted anti-hormone in vivo. In: Agarwal M.K. (Ed). Adrenal ster-oid antagonism. Walter de Gruyter & Co., Berlin. 1984; pp. 77-101.
- Baulieu EE. Contragestion and other clinical applica-tions of RU486, an antiprogestagen at the receptor. Sci-ence 1989; 345: 1351–1357.
- Sandroni P. Aphrodisiacs past and present: a historical review. Clinical Autonomic Research 2001; 11: 303-307.
- Asiah O, Nurhanan MY, Mohd Ilham A. Determination of bioactive peptide (4.3kDa) as an aphrodisiac marker in six Malaysian plants. Journal of Tropical Forest Sci-ence 2007; 19: 61-63.
- Ang HH, Sim MK. Eurycoma longifolia Jack enhances libido in sexually experienced male rats. Experimental Animal 1977; 46: 287-290.
- Chan KL, Low BS, Teh CH, Das PK. The effect of Eurycoma longifolia on sperm quality of male rats. Nat Prod Commun 2009; 4(10):1331-1336.
- Sambandan TG, Ong BK, Norhaniza A, Mohd Puad A, Rha CK. Quantitative measurement of chemical or bio-active constituents of Eurycoma longifolia (Tongkat Ali). Poster presented at Herbal Asia 2004. 25th-28th March 2004, Kuala Lumpur.
- Phillips DM, Lakshmi V, Monder C. Corticosteroid 11ß-dehydrogenase in rat testis. Endocrinology 1989; 125: 209–216.
- Hales DB, Payne AH. Glucorticoid mediated repression of P450scc MRNA and de novo synthesis in cultured Leydig cells. Endocrinology 1989; 124 (5): 2099-2104.
- Orr TE, Mann DR. Role of glucocorticoid in the stress-induced suppression of testicular steroidogenesis in adult male rats. Horm Behav 1992; 26: 350-363.
- Orr TE, Taylor MF, Bhattacharyya AK, Collins DC, Mann DR. Acute immobilization stress disrupts testicu-lar steroidogenesis in adult male rats by inhibiting the activities of 17a-hydroxylase and 17,20-lyase without affecting the binding of LH/hCG receptors. J Androl 1994; 15: 302-308.
- Monder C, Sakai RR, Miroff Y, Blanchard DC, Blanchard RJ. Reciprocal changes in plasma corticos-terone and testosterone in stressed male rats maintained in a visible burrow system: Evidence for a mediating role of testosterone 11ß-hydroxysteroid dehydrogenase. Endocrinology 1994; 134: 1193–1198.
- Ge RS, Gao HB, Nacharaju VL, Gunsalus GL, Hardy MP. Identification of kinetically distinct activity of 11ß-hydroxysteroid dehydrogenase in rat Leydig cells. Endocrinology 1997; 138: 2435-2442.
- Khatiza Haida Ali @ Htay Htay Nwe. Changes in oxi-dative activity of testicular 11ß-hydroxysteroid dehy-drogenase and plasma testosterone levels following stress related steroids treatment in rats. Ph.D. Thesis. Universiti Kebangsaan Malaysia. 2002.