ISSN: 0970-938X (Print) | 0976-1683 (Electronic)
Biomedical Research
An International Journal of Medical Sciences
Research Article - Biomedical Research (2017) Volume 28, Issue 10
Efficacy and safety of percutaneous coronary intervention in patients with coronary heart disease undergoing hemodialysis
Zhuhua Ni, Lefeng Wang*, Xinchun Yang, Hongshi Wang, Li Xu, Weiming Li, Kun Xia, Yu Liu, Jifang He and Yonghui Chi
Department of Cardiology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
Accepted date: March 13, 2017
Percutaneous coronary intervention for patients with Coronary Heart Disease (CHD) accompanying End-Stage Renal Disease (ESRD) on hemodialysis therapy is complicated due to the presence of complex lesions and the associated severity of complications and high mortality rates. This study aimed to investigate the efficacy and safety and to simultaneously delineate optimal procedural algorithms and therapeutic strategies of PCI as the preferred revascularization approach in a limited population. A total of 18 patients with CHD accompanying ESRD on dialysis therapy who underwent PCI were analysed retrospectively. The procedural flowchart before and after PCI was described. The instant procedural success rate and peri- and post-procedural complications were analysed. Out-hospital follow-up data were also recorded. Twenty-eight PCI procedures were successfully performed in the 18 patients. The average number of stents was 2.2 ± 1.6 (1-6) per procedure, all of which were drug-eluting stents. Moderate to severe calcification in the coronary artery was found in 15 patients (83.3%), and rotational atherectomy was performed in four patients. The median follow-up duration was 18.9 (4-34) months. The degree of angina pectoris reduced in all patients, with no recurrence in 10 patients; the degree of angina pectoris reduced by one to two scales in eight patients after PCI. In-stent restenosis occurred in four patients (22.2%). One patient died after the fourth PCI procedure. PCI can be performed safely with high success rates in carefully selected patients with CHD accompanying ESRD on dialysis therapy and the symptoms would be alleviated effectively after optimizing procedural algorithms and therapeutic strategies.
Keywords
Coronary heart disease, End-stage renal disease, Hemodialysis, Percutaneous coronary intervention
Introduction
Cardiovascular diseases account for nearly half of all mortalities in patients with End-Stage Renal Disease (ESRD) [1,2]. More than two-third of patients receiving dialysis have prevalent coronary artery diseases, among whom three-fourth are symptomatic and have a multi-vessel disease [3]. Coronary revascularization is often performed in select patients who are sufficiently fit for the procedure or are symptomatic despite maximal and optimal medical therapy. The most appropriate method of revascularization is debatable [4-7]. Patients with severe or end-stage Chronic Kidney Diseases (CKD) may have less benefits from Coronary Artery Bypass Graft (CABG) surgery given its most invasive nature. Indeed, a higher peri-procedural mortality and morbidity reduces the net clinical benefit and advantages of bypass surgeries in the long-term; as a result, such a fragile population may benefit most from the less invasive Percutaneous Coronary Intervention (PCI) attempts, despite a higher recurrence of ischemic events requiring more re-interventions in the long-term [8].
Thus, PCI is an increasingly attractive treatment option for this cohort of patients with single- or even multi-vessel coronary artery diseases. With advancements in technical skills, instrumentation, and technology in PCI, the risk of complications has tremendously declined and the success rate has greatly improved [9].
Some studies have demonstrated that the introduction of Drug- Eluting Stents (DESs) has dramatically reduced restenosis rates and that use of these stents is associated with lower mortality, Myocardial Infarction (MI), and repeat revascularization compared with Bare Metal Stents (BMSs) in patients with ESRD on dialysis [10-12]. Therefore, it was reasonable to utilize DESs in this cohort of patients. DESs were used in all patients included in this trial.
Domestically, this cohort of patients is treated sub-optimally because of not only the complexity of the disease itself but also the cardiologist’s fear of the unacceptably high perioperative complications resulting from PCI. Because of the complexity and fragility of these patients, there exists vast differences in the preparations before PCI, considerations during PCI, and management after PCI compared with those after the same procedure in the general population. Thus, the aims of the study were not only to investigate the efficacy and safety of PCI in these patients but also to simultaneously explore the optimal procedural algorithms and therapeutic strategies of PCI, which was used as the revascularization method in the selected population.
Materials and Methods
Patients
A total of 18 patients with Coronary Heart Disease (CHD) accompanying ESRD on dialysis therapy who underwent PCI between January 2012 and December 2014 in the cardiology department of Beijing Chaoyang Hospital were enrolled retrospectively. There were 15 men and 3 women. Their mean age was 50.0 ± 6.1 (50-69) years, and the mean hemodialysis time was 7.5 ± 3.6 (2-15) years. The median follow-up duration was 18.9 (4-34) months. This study was conducted in accordance with the Declaration of Helsinki and with approval from the Ethics Committee of Beijing Chaoyang Hospital. Written informed consent was obtained from all participants. The included patients had unstable angina pectoris (including three old MIs) with grades III-IV according to the CCS classification, except for one patient with acute MI (AMI). For the patient with AMI in whom angina also often occurred with mild exertion, chest pain occurred 30 minutes after hemodialysis was initiated, with electrocardiogram (ECG) indicating a posterior wall MI and a drop in blood pressure to <90/60 mmHg. There were three other patients in whom dialysis had to be stopped because of severe chest pain and a drop in blood pressure to <90/60 mmHg during dialysis. For the remaining patients, angina pectoris occurred during hemodialysis, at rest, or with mild and moderate stress. No absolute contraindication for PCI existed in any of the patients.
Preparation before PCI and algorithm optimization for the procedure
We should fully communicate with the hemodialysis department before PCI, and the procedure should preferably be performed on the day of the routine dialysis; if PCI has to be performed on another day, then an additional hemodialysis is needed. Under this circumstance, the length of the dialysis was generally shortened to three hours. After PCI, all patients were directly sent to the dialysis department for hemodialysis. The patients who could not tolerate hemodialysis before PCI were transferred to the CCU or Urology Department for continuous renal replacement therapy (CRRT, also called hemofiltration) in the form of postdilution continuous venovenous hemofiltration with an effluent flow of 25 ml/kg/h (lower intensity) [13] for at least 48 h. The femoral artery was punctured in all patients, except in one patient in whom radial artery was punctured to protect the upper limb arteries, which had an arteriovenous fistula. Another upper limb artery may be needed in the future for this patient. For the convenience of femoral artery suturing after PCI, the puncture point was slightly moved up. The dosage of unfractionated heparin was 100 U/kg, which was the same as that used for PCI in patients whose renal function was normal. If the procedures lasted for more than one hour, then another 1000 U of heparin was administered every hour. The symptom-related coronary artery was identified via coronary angiogram and ECG, and only the symptom-related vessel was treated in one procedure. An extra support guiding catheter was selected for those patients who had heavy calcifications in the coronary artery, and a 7 F sheath and guiding catheter were sometimes needed for more support. For heavily calcified coronary lesions, a non-compliant post-dilation balloon was needed for pre-dilation, and if the lesions could still not dilate fully, a rotational atherectomy (rotablater) was performed at the beginning of the procedure at the discretion of the operators. DESs were implanted as the first choice for all patients after their coronary artery was fully dilated.
A contrast medium was used as little as possible in the procedure. No special emphasis was needed on the class of the contrast medium. The currently available non-ionic low-osmolar (iopromide and iopamidol) or iso-osmolar (iodixanol) contrast medium was selected at the operators’ discretion.
For convenience of hemodialysis, the sheath was pulled out immediately, and the femoral artery was sutured using the Perclose Proglide Suture-Mediated Closure System (Abbott Laboratories, Abbott Vascular Inc.) after PCI.
Hemodialysis algorithms and anticoagulation strategy
All patients underwent routine maintenance hemodialysis three times every week before PCI. After PCI, all the 18 patients were sent for hemodialysis within four hours (15 patients for routine dialysis for four hours and three patients who did not undergo PCI on the day of the routine dialysis for an additional dialysis for three hours). If the hemodialysis was initiated within two hours after PCI, then an Activated Clotting Time (ACT) measurement was needed. The dosage of unfractionated heparin was determined by the value of the ACT. If the hemodialysis was initiated beyond two hours after PCI for which the ACT measurement was not required, then the dosage of unfractionated heparin was administered as per routine. The target level of anticoagulation during hemodialysis was 80% ACT level elevation on baseline (200-250 s) and 40% elevation at the end of the hemodialysis. Generally, heparin modeling can be performed using an initial bolus of 4000 IU followed by an intermittent infusion of heparin (1000-2000 IU) on the arterial end of the fistula if the ACT value was <180% of the baseline level in the measurement taken every 30 minutes to maintain an ACT of 200-250 s (normal: 90-140 s).
Perioperative complications and follow-up
The perioperative complications included coronary artery perforation, no-flow and slow-flow phenomena, major bleeding, procedure failure, heart failure, and mortality. The short-term follow-up (in-hospital and within 30 days) included observations on major bleeding, stroke, AMI, heart failure, and mortality. The long-term follow-up (>30 days) included the following observations: 1) recurrence of angina pectoris; 2) re-hospitalization due to angina pectoris; 3) re-PCI due to in-stent restenosis; 4) MI; 5) AMI; and 6) mortality. The improvement in angina pectoris was also documented in the long-term follow-up. After discharging the patients from the hospital, they were followed up via an outpatient or telephone interview. The longest follow-up duration was 34 months.
Statistical analysis
Data were analysed using the SPSS Statistical Package, version 13.0. Categorical variables were expressed as frequencies (n (%)); continuous variables were presented as means ± Standard Deviations (SDs) and analysed using the two-tailed Student’s t-test. A p value of < 0.05 was considered statistically significant.
Results
The baseline characteristics of the patients are shown in Table 1.
| Variables | Results |
|---|---|
| Age (years) | 50.0 ± 6.1 (50-69) |
| Male (%) | 15 (83.3%) |
| Risk factors of CHD | |
| Hypertension | 18 (100%) |
| Diabetes mellitus | 14 (77.8%) |
| Hyperlipidemia | 15 (83.3%) |
| CCS classification of angina pectoris | |
| Class III | 4 (22.2%) |
| Class IV | 14 (77.8) |
| Myocardial infarction | |
| OMI | 3 (16.7%) |
| AMI | 1 (5.6%) |
| EF value (%) | 61.5 ± 7.5 (43-72) |
| Note: CHD: Coronary Heart Disease; CCS: Canadian Cardiovascular Society; OMI: Old Myocardial Infarction; AMI: Acute Myocardial Infarction. | |
Table 1: Baseline characteristics of patients (n=18).
Twenty-eight PCI procedures were successfully performed in the 18 patients (once in 12 patients, twice in six patients, and four times in one patient).
Characteristics of lesions, category of stent, and number of rotablater needed
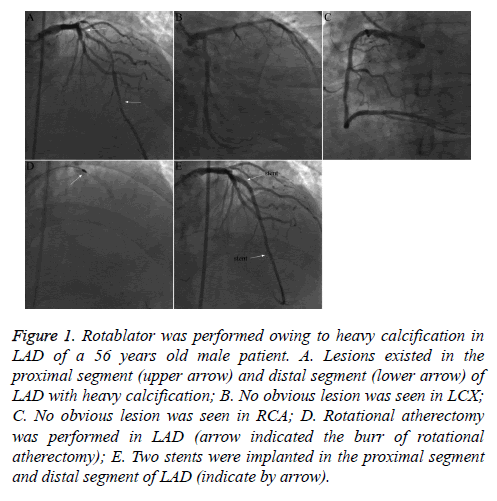
The majority of patients had multi-vessel lesions, and the number of stent implantations varied greatly per procedure [1-6], all of which were DESs. Moderate to severe coronary calcifications existed in most patients, and rotational atherectomy (rotablater) was performed in four patients (Table 2 and Figure 1).
| Variables | Results |
|---|---|
| Degree of calcification | |
| No or mild | 2 (11.1%) |
| Moderate to severe | 16 (88.9%) |
| Number of lesion vessels | |
| Single | 1 (5.6%) |
| Two or three | 17 (94.4%) |
| Rotational atherectomy | 4 (14.3%) |
| Number of stent implanted (each procedure) | |
| One | 10 (35.7%) |
| Two | 12 (42.9%) |
| Three | 3 (10.7%) |
| More than three | 3 (10.7%) |
Table 2: Characteristics of lesions and results (28 PCI procedures of 18 patients).
Figure 1: Rotablator was performed owing to heavy calcification in LAD of a 56 years old male patient. A. Lesions existed in the proximal segment (upper arrow) and distal segment (lower arrow) of LAD with heavy calcification; B. No obvious lesion was seen in LCX; C. No obvious lesion was seen in RCA; D. Rotational atherectomy was performed in LAD (arrow indicated the burr of rotational atherectomy); E. Two stents were implanted in the proximal segment and distal segment of LAD (indicate by arrow).
Complications and management during and after PCI
No-flow phenomenon occurred in one patient. After direct administration of nitroglycerin and sodium nitroprusside into the coronary artery and placement of an Intra-Aortic Balloon Counterpulsation Pump (IABP), the anterior blood flow was restored to TIMI III grade eventually, and the patient was transferred to the CCU for CRRT. Slow-flow phenomenon occurred in one patient, and after direct administration of nitroglycerine into the coronary artery, the anterior blood flow was restored to TIMI III grade. Severe dissection occurred in four patients, and stents were implanted immediately and successfully; thus, a calamitous consequence was avoided. Acute heart failure occurred in one patient immediately after PCI, and the symptoms were relieved in 30 minutes after administration of furosemide, cedilanid, morphine, etc., and the internal jugular vein was punctured instantly at the bedside to create a CRRT access; thereafter, the patient was transferred to the CCU for hemofiltration. One patient died of acute heart failure and malignant arrhythmia on the fifth day after PCI (details are provided below). No other major complications occurred during hospitalization and the 30 days of follow-up in other patients.
Number and cause of additional hemodialysis
Three patients in whom PCI was not performed on the day of the routine maintenance dialysis received an additional hemodialysis for three hours.
Number and causes of intolerance to dialysis after PCI and its management
There were three patients in whom dialysis had to be stopped because of severe chest pain accompanied by a drop in blood pressure. After shifting to CRRT at bedside for ~72 h, PCI was completed successfully in these three patients; CRRT at bedside was continued for an additional 48 h before shifting to hemodialysis again.
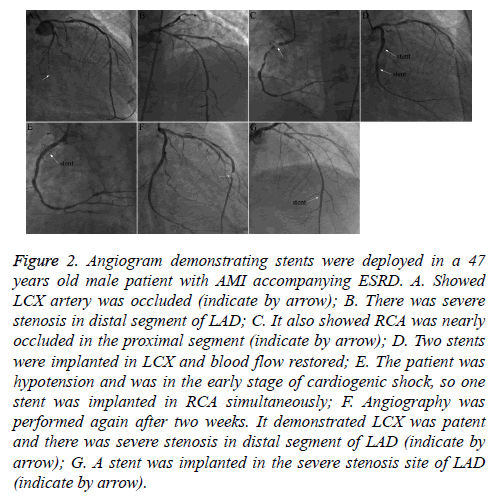
The patient who had AMI during dialysis was also transferred to the CCU for CRRT for 48 h after primary PCI (Figure 2) and then shifted to maintenance hemodialysis again.
Figure 2: Angiogram demonstrating stents were deployed in a 47 years old male patient with AMI accompanying ESRD. A. Showed LCX artery was occluded (indicate by arrow); B. There was severe stenosis in distal segment of LAD; C. It also showed RCA was nearly occluded in the proximal segment (indicate by arrow); D. Two stents were implanted in LCX and blood flow restored; E. The patient was hypotension and was in the early stage of cardiogenic shock, so one stent was implanted in RCA simultaneously; F. Angiography was performed again after two weeks. It demonstrated LCX was patent and there was severe stenosis in distal segment of LAD (indicate by arrow); G. A stent was implanted in the severe stenosis site of LAD (indicate by arrow).
The patient in whom no-flow phenomenon occurred during PCI was transferred to the CCU for CRRT for five days; on the fifth day after PCI, the IABP catheter was pulled out, and the patient was shifted to hemodialysis on the sixth day.
Number and management of patients with no or alleviated symptoms during follow-up after PCI
Angina pectoris improved to different degrees in nine patients who were free of angina and three patients in whom the degree of angina decreased by 1-2 scales (Canadian Cardiovascular Society (CCS)) and who were sensitive to anti-ischemic drugs during follow-up. For these 12 patients, no further interventional therapeutics were performed. The symptoms disappeared immediately after primary PCI in the patient with AMI; PCI was performed again for a severe lesion in the Left Anterior Descending (LAD) two weeks after, and no angina occurred during follow-up.
Number, causes, and management of PCI performed more than once
There were five patients in whom PCI had to be performed for the second time because of exacerbation of angina after the first PCI, although the degree of angina also decreased by 1-2 scales after the first PCI in a period of 5-18 months. A target lesion located on the in-stent restenosis site was tackled in three patients, and a target lesion located on the other vessels was also tackled in three patients (including the patient with AMI whose lesion in the LAD was also severe).
PCI was performed four times in one patient within six years (including twice when the procedure was completed before the date of this study) with a total of 13 implanted stents. The three prior procedures of stent implantation were performed in different coronary arteries, while the fourth procedure was performed in the right coronary artery where the in-stent restenosis occurred. The patient was transferred to the CCU for CRRT after PCI, and the patient’s condition was relatively stable. However, on the fifth day after PCI, the patient died suddenly of acute heart failure and malignant arrhythmia.
Thus, a total of four cases with in-stent restenosis were documented during follow-up, accounting for 22.2% of all cases (Table 3).
| Variables | Results |
|---|---|
| Follow-up duration (month) | 18.9 ± 8.3 (4-32) |
| Symptom relief after PCI | 18 (100%) |
| Symptom recurrence | 6 (33.3%) |
| Rehospitalization owing to | |
| Symptom recurrence | 6 (33.3%) |
| In-stent restenosis | 4 (22.2%) |
| Acute myocardial infarction | 0 |
| Stent thrombosis | 0 |
| Major bleeding | 0 |
| Heart failure | 0 |
| Stroke | 0 |
| Death | 1 |
| Stent thrombosis (EF value (%) | 59.7 ± 9.2 (46-74)* |
| *Comparing with the baseline (Table 1), there was no statistical significance (P=0.265). | |
Table 3: Follow-up results (n=18).
Different anticoagulation strategies during dialysis after PCI
ACT was measured for 12 procedures for which hemodialysis began within two hours (seven procedures with an ACT of >180 s and five procedures with an ACT of <180 s) after PCI. Heparin was routinely administered to the patients with an ACT of <180 s, and the first bolus of heparin was not administered to the patients with an ACT of >180 s; thereafter, heparin was routinely administered. For the other 16 procedures for which hemodialysis were initiated between two and four hours after PCI, ACT was not routinely measured. Heparin was administered in accordance with their past anticoagulation strategy (because all patients were undergoing maintenance hemodialysis, all of them had their own anticoagulation strategy, which was documented in the hemodialysis department). Bedside CRRT was performed in three cases before PCI and in four cases after PCI; heparin was administered in accordance with the value of the ACT.
Number of anticoagulant complications
No bleeding and thromboembolic complications occurred in any of the patients between the first dialysis after PCI and the subsequent dialysis.
Discussion
The lesions in the patients with CHD accompanying ESRD on dialysis therapy are often diffuse, particularly with heavy calcifications. Severe angina, even life-threatening AMI, and hemodynamic abnormality may occur frequently, and hemodialysis has to be stopped, which would greatly decline the quality of life of patients and increase their risk of mortality enormously. Domestically, revascularization in patients with CHD accompanying ESRD on dialysis therapy, especially CABG surgery, is often not feasible owing to the complexity and high mortality associated with such surgeries [14,15] and the reluctance of patients and their family numbers. On the contrary, PCI is well accepted by patients and their family members because of lesser trauma, rapid recovery, wide application, and technical sophistication. However, some particular difficulties exist in this cohort of patients: 1) the patients’ general condition is often poor, frequently accompanied with functional abnormalities in multiple organs and systems (e.g., anemia and electrolyte disorder), thus leading to decreased functional reserves in those organs and systems. For these patients, their condition may deteriorate suddenly in a certain organ or system and cascade reactions may occur, thus causing grave consequences if too much contrast medium is used or is not discharged immediately; 2) heavy calcification in the coronary artery makes it more difficult for interventional therapies (e.g., the operation time is longer; the dosage of a contrast medium used is higher; and the risks of surgical complications, such as dissection, slow-flow or no-flow phenomenon), and failure of stent crossing lesions or even stent dislodgment will increase dramatically; 3) if the dosage of the anticoagulant is not carefully calculated and considered during dialysis after PCI, the risk of bleeding or thromboembolic events will increase significantly. To overcome these disadvantages, overall considerations, focusing on details, optimization of algorithms of the therapy, and careful identification of indications are all necessary for a successful PCI, and only by considering these factors, the increase in the success rate and the decrease in the perioperative complications can be achieved.
The main reasons why PCI was performed successfully in this study were as follows: 1) careful identification of indications for PCI; all patients were in relatively good general conditions, i.e., most patients were relatively young and with good cardiac functions (most patients had an Ejection Fraction (EF) of >55% and an NYHA class I to II heart failure); 2) thorough preparation before PCI; for the patients who could not tolerate hemodialysis, bedside CRRT was needed to discharge excessive water and metabolic products before PCI to avoid acute heart failure during or after the procedure. For either PCI or CABG, CRRT is an indispensable stand-by measure for this cohort of patients. Generally, a lower intensity CRRT pattern was applied, i.e., the effluent flow did not exceed 25 ml/kg/h. Because the effluent flow was markedly lower than that in hemodialysis, the patients could tolerate the procedure well without experiencing angina or hemodynamic changes. Owing to the limited capacity of filtration, CRRT must be continued for a sufficient duration (generally >48 h) to filter excessive water and metabolic products as much as possible. For the patients with excessive water retained in the body, an additional dialysis was performed besides the routine dialysis. This strategy was employed in three patients in this study; 3) full communication with the hemodialysis department beforehand; PCI should be performed as much as possible on the day of the routine dialysis. The exact time point of PCI should be determined by the time of the dialysis so that hemodialysis could be initiated within four hours after PCI; 4) accurate formulation of the PCI strategy; the first important thing was to identify the ischemia-related artery via symptom determination, ECG, and angiography. Only the ischemia-related artery was tackled, and we did not focus on complete revascularization each time, but to resolve the most urgent problem with the least dosage of contrast medium in the shortest time. Rotational atherectomy was sometimes necessary for heavy calcified lesions. Rotational atherectomy may be performed at the beginning of the PCI according to the operator’s judgement or may be applied when the lesions do not fully dilate using the non-compliant balloon. Otherwise, it may lead to severe dissection or failure of stent crossing the lesions and even stent dislodgement. It was because those difficulties were fully considered in advance and correct measures were adopted beforehand that the perioperative complications mentioned above did not occur frequently in our investigation. In this observational study, rotational atherectomy was performed at the beginning of the procedure, and the arteries were dilated using the semi- or non-compliant balloon in three cases; rotational atherectomy was performed when the lesions could not fully dilate using the non-compliant balloon in one case; after the rotational atherectomy stents were all implanted successfully, 5) we focused on the anticoagulation strategy for dialysis or CRRT after PCI. The dosage and administration patterns of heparin were determined by the interval between the time point of PCI completion and the time point of initiating hemodialysis. The ACT should be measured routinely if hemodialysis was started within 2 h, and heparin was administered in accordance with the value of the ACT. Regular dosage and administration approaches of heparin were matched for those patients on maintenance dialysis if the interval was beyond 2 h, and the routine ACT measurement was not needed. This observation demonstrated that the anticoagulation strategy was safe and effective. No bleeding and thromboembolic events occurred in any of the cases; 6) stent selection; DESs were implanted in all patients in this study. The primary advantage of DESs is attributed to the dramatically decreased risk of restenosis with up to 60% relative risk reduction [11,16-18]. DESs appear to be a very attractive revascularization option in patients with ESRD; they are expected to reduce restenosis and the need for repeated procedures compared with traditional PCIs using BMSs. Notably, the data on DES use in dialysis patients are scarce, observational in nature, and based on retrospective analyses of small cohorts [19-21]. Although all patients were implanted with DESs, this study also showed that the stent restenosis rate in the dialysis patients was significantly higher than that in the general population for which the stent restenosis rate was ~5-8% during follow-up [22]. Because there were no mandating angiographic follow-up results, the actual stenosis rate was probably higher. Heavy calcifications existing in the coronary artery and increased inflammatory factors in most patients with ESRD may partly account for this phenomenon. Although there was a trial demonstrating that the clinical efficacy of Sirolimus-Eluting Stents (SESs) was better than that of Paclitaxel-Eluting Stents (PESs) [23], there is no robust evidence to come to a definite conclusion. In clinical practice, stent selection (SES or PES) could be determined at the doctor’s discretion; and 7) focusing on other details; puncture of the femoral artery as much as possible to protect the upper limb artery, which may be used for future arteriovenous fistulizations if one arteriovenous fistulization does not work. The femoral artery puncture point should be moved up slightly in favor of a vessel suture with stitching instrument (Perclose Proglide) for convenience of dialysis instantly after PCI.
The following other issues will also be discussed: 1. why no in-stent thrombosis was found in our investigation. In general, the rate of in-stent thrombosis in one year is ~1%, and the yearly rate following one year is ~0.5% per year [24]. In our investigation, no definite or probable stent thrombosis was found. The probable reasons were as follows: 1) no mandating angiography was performed to identify whether thrombosis existed because this cohort of patients was often excluded from emergent angiography, and 2) the sample size was small; 2. Why the heart function did not improve significantly after PCI during follow-up. Because of the very high risk of PCI procedure for this cohort of patients with ESRD, we only selected those with a relatively normal heart function (NYHA classification: I-II grade; baseline EF: 61.5 ± 7.5) for interventional therapy. The EF value at follow-up was 59.7 ± 9.2; when this value was compared with the baseline, there was no statistically significant difference (p=NS). The reasons why the heart function did not improve significantly were as follows: 1) the majority of the enrolled patients have a good heart function; with the progress of ESRD, the heart function did not deteriorate rapidly, probably partially owing to improvements in myocardial ischemia after PCI, and 2) the sample size was small; with increasing clinical experience, the patients with ESRD whose heart function was damaged (lower EF value) would be selected for PCI, and it is estimated that those patients would benefit from such a procedure. Certainly, this issue needs further investigations.
In conclusion, the primary therapeutic target for patients with CHD accompanying ESRD on dialysis therapy is to relieve the symptoms to tolerate dialysis and to improve angina pectoris in the long-term follow-up. For most of these patients, a complete revascularization was not always necessary. If a full preparation was made before the procedure, optimal therapeutic algorithm was applied, and correct therapeutic strategy was formulated, PCI for these patients would be safe and effective.
Certainly, there were several limitations in this investigation. The sample size was relatively small; it was not a randomized controlled study; and the length of follow-up was relatively short. To date, randomized controlled studies for this cohort of patients worldwide were seldom conducted; thus, studies with larger sample sizes and longer follow-up durations and randomized controlled trials are needed to clarify the safety and efficacy of PCI in this population in the future.
Conflict of Interest
All authors have no conflict of interest regarding this paper.
References
- de Lemos JA, Hillis LD. Diagnosis and management of coronary artery disease in patients with end-stage renal disease on hemodialysis. J Am Soc Nephrol 1996; 7: 2044-2054.
- Ohtake T, Kobayashi S, Moriya H, Negishi K, Okamoto K, Maesato K, Saito S. High prevalence of occult coronary artery stenosis in patients with chronic kidney disease at the initiation of renal replacement therapy: an angiographic examination. J Am Soc Nephrol 2005; 16: 1141-1148.
- Joki N, Hase H, Nakamura R, Yamaguchi T. Onset of coronary artery disease prior to initiation of haemodialysis in patients with end-stage renal disease. Nephrol Dial Transplant 1997; 12: 718-723.
- Edwards NC, Steeds RP, Ferro CJ, Townend JN. The treatment of coronary artery disease in patients with chronic kidney disease. QJM 2006; 99: 723-736.
- Bangalore S, Guo Y, Samadashvili Z, Blecker S, Xu J, Hannan EL. Revascularization in patients with multivessel coronary artery disease and chronic kidney disease: everolimus-eluting stents versus coronary artery bypass graft surgery. J Am Coll Cardiol 2015; 66: 1209-1220.
- Hsua CY, Chen YS. Coronary artery revascularization in hemodialysis patients with multivessel coronary artery disease. Formosan J Surg 2016; 49: 45-48.
- Ribichini F, Vassanelli C. Drug-eluting stent or coronary artery bypass graft surgery in hemodialysis patients? J Nephrol 2014; 27: 7-9.
- Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D. Guidelines on myocardial revascularization. Eur Heart J 2010; 31: 2501-2555.
- Malenka DJ, Leavitt BJ, Hearne MJ, Robb JF, Baribeau YR, Ryan TJ, Helm RE, Kellett MA, Dauerman HL, Dacey LJ, Silver MT, VerLee PN, Weldner PW, Hettleman BD, Olmstead EM, Piper WD, OConnor GT. Comparing long-term survival of patients with multivessel coronary disease after CABG or PCI: Analysis of BARI-like patients in northern New England. Circulation 2005; 112: 371-376.
- Chang TI, Montez-Rath ME, Tsai TT, Hlatky MA, Winkelmayer WC. Drug-eluting versus bare-metal stents during pci in patients with end-stage renal disease on dialysis. J Am Coll Cardiol 2016; 67: 1459-1469.
- Athappan G, Ponniah T. Clinical outcomes of dialysis patients after implantation of DES: meta-analysis and systematic review of literature. Minerva Cardioangiol 2009; 57: 291-297.
- Abdel-Latif A, Mukherjee D, Mesgarzadeh P, Ziada KM. Drug-eluting stents in patients with end-stage renal disease: meta-analysis and systematic review of the literature. Catheter Cardiovasc Int 2010; 76: 942-948.
- Renal Replacement Therapy Study Investigators, Bellomo R, Cass A, Cole L, Finfer S. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 2009; 361: 1627-1638.
- Herzog CA, Ma JZ, Collins AJ. Long-term outcome of dialysis patients in the United States with coronary revascularization procedures. Kidney Int 1999; 56: 324-332.
- Herzog CA, Ma JZ, Collins AJ. Comparative survival of dialysis patients in the United States after coronary angioplasty, coronary artery stenting, and coronary artery bypass surgery and impact of diabetes. Circulation 2002; 106: 2207-211.
- Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnąr F, Falotico R. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002; 346: 1773-1780.
- Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, OShaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003; 349: 1315-1323.
- Stone GW, Ellis SG, Cox DA, Hermiller J, OShaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, Popma JJ, Russell ME; TAXUS-IV Investigators. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med 2004; 350: 221-231.
- Okada T, Hayashi Y, Toyofuku M, Imazu M, Otsuka M, Sakuma T, Ueda H, Yamamoto H, Kohno N. One-year clinical outcomes of dialysis patients after implantation with sirolimus-eluting coronary stents. Circ J 2008; 72: 1430-1435.
- Das P, Moliterno DJ, Charnigo R, Mukherjee D, Steinhubl SR, Sneed JD, Booth DC, Ziada KM. Impact of drug-eluting stents on outcomes of patients with end-stage renal disease undergoing percutaneous coronary revascularization. J Invasive Cardiol 2006; 18: 405-408.
- Hassani SE, Chu WW, Wolfram RM, Kuchulakanti PK, Xue Z, Gevorkian N, Suddath WO, Satler LF, Kent KM, Pichard AD, Weissman NJ, Waksman R. Clinical outcomes after percutaneous coronary intervention with drug-eluting stents in dialysis patients. J Invasive Cardiol 2006; 18: 273-277.
- Aoyama T, Ishii H, Toriyama T, Takahashi H, Kasuga H, Murakami R, Amano T, Uetani T, Yasuda Y, Yuzawa Y, Maruyama S, Matsuo S, Matsubara T, Murohara T. Sirolimus-eluting stents vs bare metal stents for coronary intervention in Japanese patients with renal failure on hemodialysis. Circ J 2008; 72: 56-60.
- Tsujita H, Hamazaki Y, Nishikura T, Yokota H, Kondo S, Hosokawa S, Tsukamoto S, Mutou M, Sakurai M, Nishimura H, Kobayashi Y. Sirolimus-eluting stents versus paclitaxel-eluting stents for coronary intervention in patients with renal failure on hemodialysis. Cardiovasc Interv Ther 2013; 28: 9-15.
- Wenaweser P, Daemen J, Zwahlen M, van Domburg R, Jüni P, Vaina S, Hellige G, Tsuchida K, Morger C, Boersma E, Kukreja N, Meier B, Serruys PW, Windecker S. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J Am Coll Cardiol 2008; 52: 1134-1140.